Abstract
Proper interpretation of PET-CT images requires knowledge of the normal physiological distribution of the tracer, frequently encountered physiological variants, and benign pathological causes of FDG uptake that can be confused with a malignant neoplasm. In addition, not all malignant processes are associated with avid tracer uptake. A basic knowledge of the technique of image acquisition is also required to avoid pitfalls such as misregistration of anatomical and scintigraphic data. This article reviews these potential pitfalls as they apply to the abdomen and pelvis of patients with cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Since the introduction of combined positron emission tomography-computed tomography (PET-CT) in approximately 2003, there has been a rapid increase in its use for imaging abdominal malignancy. Many radiologists with relatively little formal training in radionuclide imaging are now involved in reporting PET-CT studies and many others radiologists have frequent exposure to PET-CT at multidisciplinary meetings. Therefore, a working knowledge of PET-CT and its potential pitfalls is now essential for the safe practice of abdominal radiology. When PET was introduced in the late 1990s, many of the potential pitfalls related to difficulties with localization of 18F-fluorodeoxyglucose (FDG) uptake. Tracer localization was performed on the basis of its relation to normal structures that take up FDG and by side-by-side comparison with CT or magnetic resonance images. This approach was generally accurate, but most radiologists who were involved in reporting studies during the transition from PET to PET-CT would admit that lesion localization became much more precise and hence diagnostic confidence much greater with the combined modality. In particular, differentiation of physiological activity from pathological uptake is easier with PET-CT. A review of the PET-CT literature showed a consequent incremental increase in diagnostic accuracy across a range of tumors of 10%–15% compared with PET alone [1]. In patients with colorectal cancer, Cohade et al. demonstrated a 50% decrease in lesion characterization as “equivocal or probable”, a 30% increase in “definite” lesion characterization, and a 25% increase in “definite” lesion localization [2]. However, the introduction of PET-CT has brought new potential pitfalls. In this review we describe and illustrate some of these pitfalls of abdominal PET-CT, considering first those that relate to the technology itself and then some issues that are specific to different organs and tumor types.
Technology-specific pitfalls
Misregistration
Misregistration refers to the incorrect superimposition of PET and CT data on a fused image, resulting in the potential for an abnormality to be ascribed to the wrong structure. This can be due to breathing, patient motion, bowel motility, or distension of the bladder and can cause false-positive or false-negative PET findings when radiotracer is superimposed on the wrong anatomical structure [3].
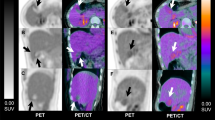
Respiratory motion during scanning causes the most prevalent artifact in PET-CT imaging. Because of the long acquisition time of a PET scan, it is acquired while the patient is freely breathing. The final image is hence an average of many breathing cycles. On the other hand, a CT scan is usually acquired during a specific stage of the breathing cycle. This difference in respiratory motion between PET scans and CT scans can cause misregistration on PET-CT images. This affects structures close to the diaphragm more than those in the lower abdomen and pelvis. In clinical practice, it most often causes difficulties in differentiating activity in the liver and adjacent structures. In one study of 300 patients imaged for a variety of cancers, 6 patients had hepatic lesions incorrectly reported as right basal lung lesions [4]. When this type of misregistration is recognized as a possibility, it can be very helpful to view the PET images alone (and in particular the non-attenuation-corrected images that have no input from the CT component of the study) to determine the relationship of focal uptake to the normal uptake in the liver (Fig. 1). The presence or absence of any basal pulmonary nodule or extrahepatic mass on the CT component of the study is also useful for distinguishing hepatic or extrahepatic abnormalities.
A On the axial PET-CT image there appears to be an area of increased uptake within the caudate lobe. B However, there is no anatomical abnormality seen on the CT. C When the sagittal non-corrected PET images are reviewed, the area of increased uptake is inferior to the liver. D This area corresponds to a lymph node (arrow) inferior to the caudate lobe.
Normal free breathing for the acquisition of CT scans has been found to improve image registration and reduce artifacts, but has poorer image correlation due to free-breathing artifacts and reducing lesion conspicuity on CT due to motion artifact [5]. However, the use of breath-hold imaging has distinct advantages in terms of CT scan quality, particularly in improved visualization of pulmonary abnormalities. Our current practice is to instruct the patient to breathe gently throughout both components of the study.
Patient motion is minimized by (a) instructing patient not to move during the study, (b) placing them in a comfortable position before the start of the study, and (c) having the patients empty their bladder before the start of the study. The imaging component of most PET-CT studies can now be completed in approximately 25 min. Most patients can keep their arms above their head for this length of time, but if there is doubt about this it is often best to let them leave their arms by their side, as artifacts due to this are typically less troublesome than those due to gross movement during the study. Bladder filling is inevitable during image acquisition, and this results in minor misregistration in many patients (Fig. 2), but this pitfall is readily recognized. Bowel peristalsis and positional change also occurs during image acquisition and this results in further potential for misregistration, particularly in the small bowel. Limited studies exist on the use of antiperistaltic agents such as N-butylscopolamine in PET imaging, but they have predominantly addressed its effect on FDG uptake rather than bowel motility and misregistration [6]. There is scope for further research in this area; at present we do not administer antiperistaltic agents.
Attenuation correction artifacts
Attenuation correction refers to manipulation of the PET data to produce an image that takes into account the greater attenuation of photons that arise from deeper structures in the body or pass through highly attenuating structures such as bone on their route to the detector. It is generally achieved by applying an attenuation map to the PET data, derived from the CT image (after correction for the different photon energies used in both modalities). Attenuation correction artifacts can occur where there are highly attenuating objects in the path of the CT beam, such as hip prostheses, metallic stents, high-density drainage tubes, and contrast-enhanced vessels. The CT-derived attenuation map corrects (or overcorrects) photopenic areas adjacent to high-attenuation structures at CT and makes them appear hypermetabolic on the attenuation-corrected PET images. These artifacts can easily be identified by comparing the attenuation-corrected images with the uncorrected images. If the uptake adjacent to a high-attenuating structure, e.g., a metal hip prosthesis, is an artifact, then the uncorrected images will show it as a photopenic region even though it may appear to have increased tracer uptake on the corrected images [7].
Truncation
Truncation artifacts in PET-CT are due to the difference in size of the field of view between the CT (50 cm) and the PET (70 cm) tomographs. These artifacts are frequently seen in large patients or in patients scanned with arms down, such as in the case of melanoma and head and neck indications. When the patient extends beyond the CT field of view, the extended part of the anatomy is truncated and consequently is not represented in the reconstructed CT image. This, in turn, results in no attenuation correction value for the corresponding region in the PET emission data, hence introducing a bias on the PET attenuation-corrected images that underestimates the standardised uptake values in these regions. Truncation also produces streaking artifacts at the edge of the CT image, resulting in an overestimation of the attenuation coefficients used to correct the PET data. This increase in attenuation coefficients creates a rim of high activity at the truncation edge, potentially resulting in misinterpretation of the PET scan [8]. In practice, these artifacts are avoided by recognizing that they may occur and by relatively effective image reconstruction algorithms that extrapolate the CT attenuation data to areas not included in the field of view.
Organ and tumor-specific pitfalls
FDG uptake is not specific for neoplastic processes; it accumulates physiologically in various normal organs, including the gastrointestinal tract, urinary tract, brown fat, and gonadal tissues. False-positive FDG uptake can also occur at PET-CT due to granulomatous disease, abscess, post-surgical changes, post-radiotherapy, foreign body reaction, or inflammation (e.g., in diverticulitis, gastritis, or arteriosclerosis).
There are also several neoplasms that are frequently not hypermetabolic and thus not FDG avid, leading to false-negative results. These cancers include certain renal cell cancers and lymphomas, hepatocellular carcinomas, cholangiocarcinomas, neuroendocrine tumors, colonic mucinous adenoacarcinomas, prostate carcinomas, and carcinoid tumors.
Liver
Liver activity is usually mildly intense with a uniformly mottled appearance. This quantum mottle is particularly pronounced on axial images and relates to the relatively small number of photons that generate a PET image compared with a CT image. It can sometimes be difficult to determine whether irregular uptake in the liver is due to quantum mottle or to metastatic disease. A useful rule-of-thumb in this regard is that focal areas of uptake that are clearly visible on the rotating maximum intensity projection (MIP) PET image are usually true lesions, while those that are not visible on this image are generally due to image noise (Fig. 3). In addition, absence of any corresponding abnormality on appropriately enhanced CT images, or more usefully on more sensitive hepatocyte-specific contrast enhanced MRI, is usually an indication that a questionable area of abnormal FDG uptake is spurious.
Another artifact that can cause false-positive FDG uptake in the liver is due to an image reconstruction artifact where two “bed positions” meet. The PET component of a PET-CT body study is usually acquired in 4–6 increments of about 15 cm each. The final whole body image is generated by software “stitching” of these images. This can result in focal areas of apparent FDG accumulation on axial images (Fig. 4). The key to identifying this artifact again lies in observing the rotating MIP image, which demonstrates the linear nature of the abnormality. The abnormality is also usually only visible on one axial section and does not correspond with any abnormality on anatomical imaging. It is thought to be due to increased image noise towards the edge of each volume of data.
A Apparent focal accumulation of tracer in the right lobe of the liver (arrow) on an axial fused PET-CT image is due to ‘straight-line artifact’ (arrowhead) on the MIP image (B). The linear activity extends beyond the liver. Similar linear artifacts are visible in the pelvis in this patient (arrows).
The sensitivity of PET-CT for detecting malignancy reduces with small lesions, particularly when they are less than 1 cm in diameter (Fig. 5). Therefore, a lack of FDG uptake in such lesions should not be interpreted as evidence that they are benign.
Up to 30%–50% of hepatomas are reported to lack FDG avidity [9]. For cholangiocarcinoma the sensitivity of PET was 85% for the detection of malignancies with a mass-forming morphology, but only 18% for the detection of cancers with an infiltrating morphology [10]. A more recent article using PET-CT found no significant difference in the accuracy of the technique for diagnosis of mass-forming, infiltrating and intraductal types of cholangiocarcinoma, with sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of PET-CT in primary tumor detection were 84.0%, 79.3%, 92.9%, 60.5%, and 82.9%, respectively [11]. In essence, the positive predictive value of PET-CT is high, but malignancy should not be confidently ruled out on the basis of negative FDG uptake for both hepatoma and cholangiocarcinoma.
Necrotic and mucinous metastatic adenocarcinomas are also known to be poorly FDG avid [3]. Hepatic colorectal metastases treated with chemotherapy (particularly with the newer antiangiogenic agents) frequently lack FDG uptake in spite of demonstration of residual viable tumor at the time of resection (Fig. 6). Therefore pre-operative planning for liver resection should be based on pre-chemotherapy imaging as well as post-therapy imaging, ideally with hepatocyte-contrast enhanced magnetic resonance imaging in our experience.
Spleen
There is usually slightly less FDG uptake in the normal spleen than in the liver. In general, splenic uptake that is greater than liver uptake indicates malignant disease in a patient with lymphoma or other known malignancy. Lymphomatous involvement of the spleen can cause either focal or diffuse increased uptake [12]. As with other abdominal organs, increased uptake in the spleen can occur with benign inflammatory disease such as sarcoidosis (Fig. 7). Increased splenic uptake is seen in patients treated with granulocyte colony-stimulating factor and has recently been described in a case report of a patient with infectious mononucleosis [13, 14]. Therefore thorough knowledge of the clinical history is important in order to avoid these false-positive interpretations.
Pancreas
Normally, metabolic activity within the adrenal glands and the pancreas is subtle to mild. Increased FDG uptake in the pancreas can occur in chronic active pancreatitis, autoimmune pancreatitis, and irradiated tissue, and may be indistinguishable from pancreatic malignancy [15, 16]. Focal FDG uptake due to portal vein thrombosis, hemorrhagic pseudocysts, and retroperitoneal fibrosis has also been reported [17]. Early stage pancreatic cancers can be falsely negative at FDG PET. Pancreatic tumors of endocrine origin, such as insulinoma, glucagonoma, and VIPoma, are usually not highly FDG avid. Overall, therefore, differentiation of benign and malignant pancreatic processes is not as straightforward with PET-CT as in many other abdominal organs.
Adrenal glands
PET-CT allows determination of the attenuation and the metabolic activity of adrenal lesions with a single examination and in the majority of cases should provide diagnostic information. Lesions with activity that is less or much greater than that of the liver can be confidently diagnosed as benign or malignant, respectively [18]. In another study by Maurea et al. [19], adrenal FDG uptake was considered abnormal when tracer uptake was greater than the uptake of the blood pool and that of background activities with no similar uptake on the contralateral side. All malignant lesions showed abnormally increased uptake. In benign lesions, no abnormally increased FDG uptake was observed, except for one benign pheochromocytoma. Pheochromocytomas, either benign or malignant, are metabolically active and thus accumulate FDG, although uptake is found in a greater percentage of malignant than benign pheochromocytomas [20].
Increased FDG uptake in the suprarenal or retrocrural region due to brown fat, which may mimic an adrenal lesion or a retrocrural node, is another pitfall of PET [21]. Brown fat uptake is usually recognizable on the basis of its characteristic pattern of uptake throughout the thoracic paraspinal and supraclavicular regions, coregistration to areas of fat on CT, and lack of a corresponding adrenal or retrocrural mass (Fig. 8).
A On a fused PET-CT image there is increased uptake in the left retrocrural region, which could be mistaken for uptake within a retrocrural lymph node. However, there is no evidence of a mass in this region on the CT image (B) and there is a characteristic pattern of uptake throughout the thoracic paraspinal and supraclavicular regions on the PET image (C), consistent with brown fat.
Gastrointestinal tract
Radiotracer uptake within the gastrointestinal tract is highly variable in normal individuals. The origin of 18F-FDG uptake in the digestive tract is unknown; possible causes are active smooth muscle, metabolically active mucosa, swallowed secretions, or colonic microbial uptake [22].
Stomach
Homogenous mild-to-moderate increased uptake in the stomach wall and gastro-esophageal junction is relatively common. FDG uptake in the stomach has a J-shaped configuration on the MIP image. Gastric wall inflammation is a common entity. Diffuse increased radiotracer gastric uptake has been described in patients with gastritis [23]. In contrast, when focal uptake is identified within the stomach, PET-CT images should be carefully evaluated for the presence of an underlying mass (Fig. 9).
Small bowel
The small bowel typically demonstrates homogenous low-level FDG uptake; however, uptake that is more focally intense is seen quite frequently in normal individuals. Normal appearance of the small bowel on CT is a useful indication that FDG uptake is likely to be normal, particularly if it is diffuse or segmental. Mural thickening, particularly in combination with typical patterns of bowel wall enhancement seen at contrast-enhanced CT, allows diffuse FDG uptake within the small bowel to be ascribed to an infectious or inflammatory cause with greater certainty (Fig. 10). Both primary and metastatic malignancies can affect the small bowel, and both can demonstrate increased FDG uptake. Differentiation of such abnormalities from physiological small bowel uptake is aided by identification or exclusion of corresponding abnormalities on the CT component of the study (Fig. 11). Therefore administration of oral contrast medium prior to PET-CT is very helpful and is used routinely in our practice, when abdominal disease is considered likely or even possible.
Colon
Normal colonic activity is also variable, ranging from faint heterogeneous activity, to mild focal, segmental, or diffuse activity. Uptake in the cecum and right colon is usually higher than the rest of the colon because of the abundance of the lymphocyte cells, which are very glucose avid [22]. Diffusely increased FDG uptake has been described in inflammatory colitis [24, 25]. Most such patients have colonic mural thickening on the CT component of the study. Some patients have quite pronounced FDG uptake in the colon without any subsequent abnormality being identified. In these cases, there is usually involvement of a relatively long segment of colon (greater than 10 cm) and there is no mural abnormality on the CT images.
Intense focally increased FDG uptake has been described for both malignant and benign processes. Non-neoplastic causes that have been described include appendicitis, diverticulitis, and focal abdominal or pelvic abscesses. PET-CT findings in these cases may be diagnostic since the CT manifestations of these entities are well described (Fig. 12) [23]. When focal intense activity is identified, further investigation with colonoscopy or CT colonography should usually be recommended, as significant polypoid or sessile tumors may not be visible on the CT images of the unprepared colon (Fig. 13).
A There are two focal areas of increased FDG uptake in the pelvis on the oblique MIP image lateral to the ureters. B Bowel wall thickening (arrow) corresponding with one of these is visible in the sigmoid colon on the CT image. No abnormality was seen on CT to correspond with the other area. These were two synchronous sigmoid adenocarcinomas on colonoscopy.
Urinary tract
Because FDG is filtered through the glomeruli, without reabsorption (unlike glucose), activity is seen with the collecting system, ureters, and urinary bladder. Although the intensity and location of urinary FDG uptake permits correct identification under most circumstances, pooling of the tracer in the renal calices or pelvis, dilated or redundant ureters, or bladder diverticula can be a confounding finding [26]. Focal FDG accumulation in the ureters can be misdiagnosed as pelvic lymph node metastasis or lymphoma. This pitfall can usually be avoided by viewing the MIP image, where ureteric activity has a characteristic vertical anatomical course (Fig. 14). The apparent size of an area of focal FDG uptake in the ureter is frequently significantly larger than the size of the ureter, particularly where the ureter crosses the common iliac vessels. Again, recognition of this characteristic location of apparent mismatch will avoid false-positive interpretation of images. Duplication of the renal pelvis and ureter is common, which can result in two separate columns of pooled activity in the retroperitoneum. If the presence of this variant is not known beforehand or is not recognized, it can be misinterpreted as adenopathy medial to a single ureter [27]. Unrecognized renal transplants and horseshoe kidneys may also lead to false-positive results.
In addition to the above difficulties with urinary activity, the role of PET-CT in the detection and staging of renal cell carcinoma is hampered by intrinsically low sensitivity in this tumor, with many primary renal tumors demonstrating low levels of FDG uptake. Renal cysts are characteristically photopenic (Fig. 15). Therefore, it is important to suggest further imaging when any FDG uptake is seen in a renal mass (Fig. 16).
Reproductive tract
Uterus
Normal uptake of FDG in the endometrium of pre-menopausal patients varies cyclically, being increased at the ovulatory and menstrual phase (Fig. 17) [28]. It may not be possible to differentiate this uptake pattern from uterine carcinoma. However, FDG uptake is usually more irregular, diffuse, and intense in uterine cancer. Uterine fibroids are also known to occasionally show increased FDG [29].
Ovary
Increased ovarian uptake is associated with malignancy in post-menopausal women, but may be physiological around the time of ovulation in pre-menopausal women (Fig. 18). Detecting a dominant ovarian follicle on CT and discussing the menstrual cycle phase with the patient may assist in differentiating physiological from malignant FDG ovarian uptake [28].
Prostate gland
The evaluation of primary prostate cancer with FDG PET is difficult due to hypometabolism of the tumor.
Testes
Moderate testicular uptake is normally seen and is homogenous and symmetrical.
Musculoskeletal
Bone
Healing bone is associated with elevated FDG uptake. The origin of such FDG uptake is unclear. Hematoma formation and the granulation tissue associated with resorption of the hematoma could account for the early phase of FDG uptake, but the uptake observed weeks into the healing phase suggests that the procallus itself is associated with elevated glycolytic metabolism [26]. Increased uptake in rib fractures overlying the liver should not be confused with liver metastases (Fig. 19).
The diffuse increase in bone marrow activity seen following chemotherapy and the administration of colony-stimulating factors is now well recognized [3].
Bone metastases maybe difficult to diagnose with CT alone. Sclerotic lesions on CT may represent progressive metastatic disease as well as a blastic reparatory response of the skeleton to treatment. Sclerotic lesions may also be due to benign skeletal disease, such as Paget’s disease or fibrous dysplasia. The presence and degree of FDG uptake in these suspicious but equivocal skeletal sites can assist in the differential diagnosis. Bone metastases involving the marrow are often more conspicuous on PET. Sometimes after G-CSF, metastases appear as relatively cold defects.
Joints
Degenerative or inflammatory joint disease can give rise to elevated FDG uptake (Fig. 20). Osteophytes can demonstrate FDG uptake, which depends on the degree of metabolic activity. Although osteophytes can occur at any level of the vertebral column, those that are located anteriorly may be confused with the paravertebral lymph nodes unless the CT images are evaluated on bone window settings.
Muscles
Moderate to high FDG uptake is visible in the muscles that contribute to breathing in patients with chronic obstructive pulmonary disease due to difficulty in breathing and the use of accessory muscles to facilitate breathing (Fig. 21). Occasionally, asymmetrical muscle uptake is seen after unaccustomed physical exertion in the previous 24 h.
Lymph nodes
A major attribute of FDG PET is the ability to depict malignant neoplasms in lymph nodes when the nodes are not pathologically enlarged. However, FDG uptake in lymph nodes is not specific for malignant neoplasm. Active granulomatous disease such as tuberculosis and sarcoidosis, infection or recent instrumentation can cause high FDG uptake in involved nodes.
Peritoneum
Increased FDG uptake is frequently seen in peritoneal metastases that are either nodular or diffuse (Fig. 22), although small volume peritoneal disease may not be associated with any scintigraphic abnormality [30]. As with pleural effusions, malignant ascites does not take up FDG, although it may be assumed to be malignant if nodular uptake is seen in the peritoneum adjacent to the fluid. Increased uptake may also be seen in the peritoneum post-operatively due to inflammation (Fig. 23).
Conclusion
PET-CT using FDG as the radiotracer is a very useful modality for imaging abdominal malignancies. Combined PET-CT imaging allows for easier recognition of normal variants and better localization and characterization of subtle findings. Although the addition of anatomic CT information does aid in the interpretation of PET, artifacts can be introduced as a result of the dual-modality nature of the examination. To optimise interpretation, PET-CT images should be reviewed with the help of non-attenuation-corrected images, rotating MIP images, all other available imaging and knowledge of the patient’s history.
References
Czernin J, Allen-Auerbach M, Schelbert HR (2007) Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006. J Nucl Med 48(Suppl 1):78S–88S.
Cohade C, Osman M, Leal J, Wahl RL (2003) Direct comparison of (18)F-FDG PET and PET/CT in patients with colorectal carcinoma. J Nucl Med 44(11):1797–803.
Blake MA, Singh A, Setty BN, et al. (2006) Pearls and pitfalls in interpretation of abdominal and pelvic PET-CT. Radiographics 26(5):1335–1353.
Osman MM, Cohade C, Nakamoto Y, et al. (2003) Clinically significant inaccurate localization of lesions with PET/CT: frequency in 300 patients. J Nucl Med 44(2):240–243.
Vogel WV, van Dalen JA, Wiering B, et al. (2007) Evaluation of image registration in PET/CT of the liver and recommendations for optimized imaging. J Nucl Med 48(6):910–919.
Emmott J, Sanghera B, Chambers J, Wong WL (2008) The effects of N-butylscopolamine on bowel uptake: an 18F-FDG PET study. Nucl Med Commun 29(1):11–16.
Kapoor V, McCook BM, Torok FS (2004) An introduction to PET-CT imaging. Radiographics 24(2):523–543.
Sureshbabu W, Mawlawi O (2005) PET/CT imaging artifacts. J Nucl Med Technol 33(3):156–161. quiz 63–64.
Trojan J, Schroeder O, Raedle J, et al. (1999) Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol 94(11):3314–3319.
Anderson CD, Rice MH, Pinson CW, et al. (2004) Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg 8(1):90–97.
Kim JY, Kim MH, Lee TY, et al. (2008) Clinical role of 18F-FDG PET-CT in suspected and potentially operable cholangiocarcinoma: a prospective study compared with conventional imaging. Am J Gastroenterol 103(5):1145–1151.
Rini JN, Leonidas JC, Tomas MB, Palestro CJ (2003) 18F-FDG PET versus CT for evaluating the spleen during initial staging of lymphoma. J Nucl Med 44(7):1072–1074.
Sugawara Y, Zasadny KR, Kison PV, Baker LH, Wahl RL (1999) Splenic fluorodeoxyglucose uptake increased by granulocyte colony-stimulating factor therapy: PET imaging results. J Nucl Med 40(9):1456–1462.
Lustberg MB, Aras O, Meisenberg BR (2008) FDG PET/CT findings in acute adult mononucleosis mimicking malignant lymphoma. Eur J Haematol 81(2):154–156.
Nakamoto Y, Saga T, Ishimori T, et al. (2000) FDG-PET of autoimmune-related pancreatitis: preliminary results. Eur J Nucl Med 27(12):1835–1838.
Bares R, Klever P, Hauptmann S, et al. (1994) F-18 fluorodeoxyglucose PET in vivo evaluation of pancreatic glucose metabolism for detection of pancreatic cancer. Radiology 192(1):79–86.
Friess H, Langhans J, Ebert M, et al. (1995) Diagnosis of pancreatic cancer by 2[18F]-fluoro-2-deoxy-d-glucose positron emission tomography. Gut 36(5):771–777.
Yun M, Kim W, Alnafisi N, et al. (2001) 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. J Nucl Med 42(12):1795–1799.
Maurea S, Mainolfi C, Bazzicalupo L, et al. (1999) Imaging of adrenal tumors using FDG PET: comparison of benign and malignant lesions. AJR Am J Roentgenol 173(1):25–29.
Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC (1999) Pheochromocytomas: imaging with 2-[fluorine-18]fluoro-2-deoxy-d-glucose PET. Radiology 212(1):35–41.
Yeung HW, Grewal RK, Gonen M, Schoder H, Larson SM (2003) Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med 44(11):1789–1796.
Abouzied MM, Crawford ES, Nabi HA (2005) 18F-FDG imaging: pitfalls and artifacts. J Nucl Med Technol 33(3):145–155. quiz 62–63.
Prabhakar HB, Sahani DV, Fischman AJ, Mueller PR, Blake MA (2007) Bowel hot spots at PET-CT. Radiographics 27(1):145–159.
Skehan SJ, Issenman R, Mernagh J, Nahmias C, Jacobson K (1999) 18F-fluorodeoxyglucose positron tomography in diagnosis of paediatric inflammatory bowel disease. Lancet 354(9181):836–837.
Hannah A, Scott AM, Akhurst T, et al. (1996) Abnormal colonic accumulation of fluorine-18-FDG in pseudomembranous colitis. J Nucl Med 37(10):1683–1685.
Shreve PD, Anzai Y, Wahl RL (1999) Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics 19(1):61–77. quiz 150-151.
Vesselle HJ, Miraldi FD (1998) FDG PET of the retroperitoneum: normal anatomy, variants, pathologic conditions, and strategies to avoid diagnostic pitfalls. Radiographics 18(4):805–823. discussion 23–24.
Lerman H, Metser U, Grisaru D, et al. (2004) Normal and abnormal 18F-FDG endometrial and ovarian uptake in pre- and postmenopausal patients: assessment by PET/CT. J Nucl Med 45(2):266–271.
Subhas N, Patel PV, Pannu HK, et al. 2005. Imaging of pelvic malignancies with in-line FDG PET-CT: case examples and common pitfalls of FDG PET. Radiographics 25(4):1031–1043.
Turlakow A, Yeung HW, Salmon AS, Macapinlac HA, Larson SM (2003) Peritoneal carcinomatosis: role of (18)F-FDG PET. J Nucl Med 44(9):1407–1412.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McDermott, S., Skehan, S.J. Whole body imaging in the abdominal cancer patient: pitfalls of PET-CT. Abdom Imaging 35, 55–69 (2010). https://doi.org/10.1007/s00261-008-9493-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-008-9493-4