Abstract
Ultrasound (US)-guided percutanoeus radiofrequency (RF) ablation is difficult to perform for treating a hepatic tumor abutting the diaphragm due to a poor sonic window and high risk of diaphragmatic thermal injury. RF ablation with assistance of the use of artificial ascites is a simple and safe technique for treating a hepatic dome tumor abutting the diaphragm. One can improve the sonic window and separate the RF ablation zone from the diaphragm by downward displacement of the liver with the use of a simple and inexpensive technique. Dextrose water solution is an ideal fluid due to its nonionic nature. Complications related to the use of artificial ascites including hemoperitoneum are rare. Peritoneal adhesion and tumor location in the bare area are the limitations for the application of this technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Radiofrequency (RF) ablation has been widely accepted as an alternative treatment for unresectable primary and metastatic hepatic tumors. Recent studies have reported favorable survival results with excellent local control rates. The efficacy of RF ablation as the first-line treatment for early-stage hepatocellular carcinomas (HCCs) has also been demonstrated [1–5]. Ultrasound (US) is most widely used as a guiding technique for percutaneous RF ablation of hepatic tumor because of easy availability and real-time monitoring capability. However, when a tumor is located in the hepatic dome area and abuts the diaphragm, it may be difficult to target the tumor with US because of acoustic shadowing caused by the lung base or ribs, and there is a high risk of unwanted thermal injury to the diaphragm [6–9]. To overcome these limitations, we are currently using several techniques including [1] a different approach (laparoscopic or open) [2]; a different guiding modality (CT or MR) [3]; the use of an artificial fluid or air [4]; the use of a balloon catheter [5]; position change of the patient [6, 7]; pulling back or lifting the electrode [10, 11]. Among these approaches, the use of artificial ascites has gained a recent acceptance as a simple and effective technique for safe and complete ablation. In this pictorial essay, we introduce the techniques, indications, benefits, and pitfalls of the use of artificial ascites in percutaneous RF ablation for a HCC abutting the diaphragm.
Problems in percutaneous local treatment of an HCC abutting the diaphragm
There are two well-known limitations in the use of percutaneous US-guided thermal ablation of an HCC abutting the diaphragm. First, many HCCs abutting the diaphragm (or located in the hepatic dome) are often partially visible on US, even with a patient on deep inspiration state. If the tumor is partially visible or poor in conspicuity, it can be difficult to accurately target the index tumor with the RF electrode. Second, the risk of collateral thermal damage to the diaphragm is relatively increased as compared to that for treating an HCC that does not abut the diaphragm (Fig. 1). In this situation, a standardized ablation protocol cannot be performed due to severe pain of the patient during the ablation. Thus, any kind of technique to improve the sonic window and to separate the diaphragm from the RF ablation zone is necessary to perform a safe and complete ablation. The use of an artificial material including fluid or air is a good solution for this purpose. When we use US as a guiding modality, using fluid as artificial ascites is an ideal option (Fig. 2).
Diaphragmatic thermal injury after RF ablation of HCC abutting the diaphragm. (A) Contrast-enhanced Transverse CT scan obtained during arterial phase before RF ablation shows a 2.3-cm-diameter HCC (arrow) in liver segment 8. (B) Contrast-enhanced Transverse CT scan obtained during portal phase immediately after RF ablation shows a 3.0-cm-diameter RF ablation zone. The abutting diaphragm shows diffuse thickening due to collateral thermal injury (arrow).
Diagram to illustrate the concept of the introduction of artificial ascites in percutaneous US-guided RF ablation. When a tumor is located in the hepatic dome area and abuts the diaphragm, it is difficult to visualize the tumor on ultrasound (US) because of acoustic shadowing caused by the lung base or ribs, and there is a high risk of thermal injury to the diaphragm. One can improve the sonic window and separate the RF ablation zone from the diaphragm by downward displacement of the liver with the assistance of artificial ascites. Dark triangle represents an US field with poor window compared to transparent triangles with better sonic window. Ellipsoid lesions representing the index tumor abutting the diaphragm are seen more clearly after the downward shift of the diaphragm by artificial ascites.
Introduction of artificial ascites
How to select the peritoneal space
Many techniques can be used to introduce artificial ascites into the peritoneal spaces. Most operators are using 14–18G spinal, Chiba, or specially designed needles for selecting the peritoneal space. This technique seems to be the most popular technique for introduction of artificial ascites [12–15]. The puncture sites for introducing artificial ascites are the right subhepatic space or the left subhepatic space. An operator using a needle should monitor closely whether the tip of the needle is located in the peritoneal space until a satisfactory amount of artificial ascites is achieved, and remove the needle during RF ablation before targeting the index tumor. However, artificial ascites can be shifted away from the region of interest during the ablation. In that case, additional puncture is needed to introduce necessary amount of artificial ascites.
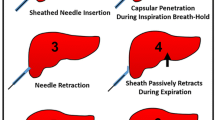
To overcome this disadvantage, we select the peritoneal space under US-guidance with a 5-Fr angiosheath using the Seldinger technique (Fig. 3). After local anesthesia at the skin of the puncture site, we insert an 18-gauage sheathed needle just into the peritoneum. To effectively select the peritoneal space, we instruct the patient to slightly inspirate to downward displace the level of the liver parenchyma and to hold his/her breath. We advance the sheath needle into the subcapsular portion of the liver parenchyma. After removal of the inner stylet of the sheath needle, we instruct the patient to fully expire and hold the breath again. At this moment, the tip of the sheath usually remains in the peritoneal space by being retracted from the upwardly displaced hepatic parenchyma with full expiration. At this moment, we quickly insert a 0.035-in guide-wire though the sheath and check whether the wire is located in the peritoneal space by US. Finally, we place a 5-Fr angiosheath over the guide-wire. After placement of the angiosheath in the peritoneal space, we open a three-way stopcock at the side arm of the angiosheath that is connected to 500 mL of a 5% dextrose in water solution (5% DW). If the entire boundary of the index tumor is visible and a safe RF electrode path can be achieved by downward displacement of the liver from US monitoring, we conclude that the introduction of artificial ascites was technically successful. Subsequently, we proceed with conventional RFA procedures. Our technique using an angiosheath may provide rapid introduction of artificial ascites due to larger bore of the route without the necessity of monitoring the tip of the needle during the ablation [16].
Technique to select the perihepatic peritoneal space using the Seldinger technique. After local anesthesia at the skin of the puncture site, we insert an 18-guage sheathed needle just into the peritoneum. Black solid arrows represent the stylet of sheath needle for angio-puncture. To effectively select the peritoneal space, we instruct the patient to slightly inspire to displace downward the level of the liver parenchyma, and to hold the breath. We advance the sheath needle into the subcapsular portion of the liver parenchyma. After removal of the inner stylet of sheath needle, we instruct the patient to fully expire and again hold the breath. At this moment, the tip of the sheath usually remains in the peritoneal space by being retracted from the upwardly displaced hepatic parenchyma with full expiration. At this moment, we quickly insert a 0.035-in guide-wire though the sheath and check whether the wire was located into the peritoneal space by US. Finally, we place 5 Fr angiosheath over the guide-wire.
Proper solution and amount of artificial ascites
Using computer simulations and phantom experiments, Brace et al. [17] determined that the use of 5% DW provides significantly more electrical isolation than the use of normal saline, which reduces unwanted heating of the adjacent tissue. The use of normal saline actually increased the amount of RF current in the adjacent tissue. Laeseke et al. determined the relative effectiveness of two fluids (5% DW vs. 0.9% normal saline) for protecting the diaphragm and lung during RF ablation in a swine model. Instillation of 5% DW into the peritoneal cavity before hepatic RF ablation decreases the risk and severity of diaphragm and lung injuries as compared with no pretreatment or pretreatment with 0.9% saline [18]. Thus, 5% DW appears ideal as a protective fluid in contrast to the use of normal saline. There is no established data regarding the minimum amount of artificial ascites to use to avoid thermal injury to the abutting organ. We try to separate the hepatic capsule at the RF ablation zone to at least 5 mm from the dangerous vital organ. However, a sheet (2–3 mm) of artificial ascites may have a role for preventing significant thermal damage. We can accumulate more fluid at the subphrenic space by altering the position of the patient, such as by the use of the Trendelenberg position.
Technical feasibility and the therapeutic efficacy of artificial ascites
We assessed the feasibility and efficacy of the use of percutaneous RF ablation with artificial ascites in 25 patients with HCC in the hepatic dome. Artificial ascites was successfully achieved in 22 (88%) of 25 patients with the administration of a mean volume of 348 mL 5% DW for an additional mean time of 9.3 min. There was substantial improvement in the visibility in 93.4% (15/16) of the partially visible tumors and in achieving a better path in 77.8% (7/9) of the tumors with a poor electrode path. The primary technique effectiveness rate for hepatic dome tumors was 96% (24/25) as seen on a one-month follow-up CT (Figs. 4, 5). Except in one case that showed a transient injury, there was no diaphragmatic thermal injury. No complication related to artificial ascites occurred during the follow-up period [16]. Hinshaw et al. demonstrated that pretreatment with intraperitoneal 5% DW before RF ablation of 20 patients with peripheral liver tumors decreased pain, narcotic use, and length of hospital stay [19].
Successful ablation using artificial ascites in a 59-year-old woman with HCC. (A) Contrast-enhanced transverse CT scan obtained during arterial phase before RF ablation shows a 1.8-cm-diameter HCC (arrow) in the high dome of liver segment 8. (B) Planning ultrasonography shows a subtle hypoechoic nodule in the hepatic dome. However, there is no proper RF electrode path owing to an overlapped costochondral junction (arrow). (C) Ultrasound obtained after introducing artificial ascites via an 18 gauge spinal needle shows a more conspicuous index tumor (arrow) due to improvement of the sonic window. (D) Ultrasound during ablation shows a hyperechoic ablation zone including the index tumor (arrow). (E) Contrast-enhanced transverse CT scan obtained during arterial phase immediately after RF ablation shows a 3.0-cm-diameter RF ablation zone (arrow) with a proper ablative margin. (F) Contrast-enhanced coronal CT scan obtained during arterial phase immediately after RF ablation shows a 3.0-cm-diameter RF ablation zone (arrow) that is separated from the diaphragm. There is no thermal injury to the abutting diaphragm. (G) Contrast-enhanced transverse CT scan obtained during arterial phase at one month after RF ablation shows the RF ablation zone without residual unablated tumor progression (arrow). The artificial ascites has been completely absorbed.
Successful ablation using artificial ascites in a 51-year-old man with HCC. (A) Contrast-enhanced transverse CT scan obtained during arterial phase before RF ablation shows a 1.6-cm-diameter HCC (arrow) in the high dome of liver segment 8. The index tumor was not visible on planning ultrasonography. (B) Ultrasound after introducing artificial ascites via a 5-Fr introducer sheath demonstrates the index tumor clearly (arrow) due to the improved sonic window. (C) Ultrasound during targeting the index tumor shows the RF electrode traversing the tumor (arrow). (D) Ultrasound after ablation shows a hyperechoic ablation zone, including the index tumor (arrow).
Safety of artificial ascites
Traditionally, the ascitic fluid was thought to wash away thrombogenic material at the puncture site and decrease the “tamponade effect” from the opposing parietal peritoneum against the liver [20, 21]. However, our study of demonstrated that induction of artificial ascites was a safe procedure. No patient showed immediate or delayed hemoperitoneum or peritonitis. The artificial ascites was spontaneously absorbed in most patients within 1 week. This may result from coagulation of the electrode path when we removed the electrode. Furthermore, based on our results, we now doubt if the introduction of ascites should be regarded as a relative contraindication for percutaneous RF ablation in patients with a correctable coagulopathy. Recently, we perform RF ablation with artificial ascites if the prothrombin time ratio is greater than 50% (prothrombin time with international normalized ratio <1.7) and a platelet count is greater than 50,000 cells/mm3. However, the operator should carefully manipulate and monitor the needle tip if a spinal needle is used for introduction of fluid (Fig. 6). The needle for artificial ascites should be removed after achieving artificial ascites at the time of RF electrode insertion as it is difficult to monitor both the spinal needle and RF electrode simultaneously.
Hemoperitoneum related to introducing artificial ascites in a 47-year-old woman with HCC. (A) Contrast-enhanced transverse CT scan obtained during arterial phase before RF ablation shows a 1.3-cm-diameter HCC (arrow) in liver segment 7. (B) Planning ultrasonography shows a subtle hypoechoic nodule (arrow) in the hepatic dome that is barely noted only in the deep inspiration state. In addition, the tumor is abutting the diaphragm. (C) Ultrasound obtained after introducing artificial ascites via an 18 gauge spinal needle (arrow) shows the index tumor. However, the improvement of the sonic window is marginal due to the tumor location close to the bare area. (D)Ultrasound during targeting the index tumor shows a RF electrode traversing the tumor (arrow). During the ablation, we kept the spinal needle in the perihepatic space. (E) Contrast-enhanced transverse CT scan obtained during arterial phase immediately after RF ablation shows a hemoperitoneum (arrow) around the perihepatic space. (F) Hepatic arteriograpghy shows two sites of extravasations (arrows), suggesting arterial bleeding from the subcapsular area. (G) Hepatic arteriography after coil and gelfoam emblization (arrow) shows no evidence of extravasation from the bleeding sites.
Advantages and limitations of the use of artificial ascites
The main advantage of the use of artificial ascites over artificial pleural effusion is to achieve two goals, including improving the sonic window and providing an insulating effect from thermal injury [22–24]. Artificial pleural effusion may be beneficial in cases of a tumor located in the bare area or by the use of a nonthermal ablative technique such as ethanol injection. US-guided RF ablation with artificial ascites is more safe and cost-effective as compared to CT-guided transthoracic ablation as it can be performed with a less expensive guiding modality (US) and requires a shorter procedure time.
There are several technical limitations in introducing artificial ascites. First, the main obstacle in introducing artificial ascites is peritoneal adhesion due to prior treatments (surgical resection, transarterial chemoembolization (TACE), or thermal ablation) (Fig. 7). In our study, two of three cases with technical failure had a history of previous surgery [16]. Therefore, an operator should keep in mind that these two factors are the most common causes of technical failure for successful induction of artificial ascites and consider an alternative approach or treatment. Second, the thickness of artificial ascites is usually variable depending on the location of liver. For example, the posterior part of the perihepatic space is usually narrower than the anterior or lateral part as the liver is attached as a bare area by triangular ligaments. If a tumor is located in the bare area of the liver, it is impossible to separate the tumor from the abutting diaphragm (Figs. 8, 9). Third, although the use of artificial ascites contributes to improving the sonic window, it is sometimes difficult to target the index tumor because the liver floating on the artificial ascites tends to be displaced during the insertion of the electrode. Finally, the interposed omentum may sometimes be an obstacle for successful induction of artificial ascites (Fig. 10).
Peritoneal adhesion as a limiting factor for successful ablation in a 75-year-old-man with HCC. (A) Contrast-enhanced transverse CT scan obtained during arterial phase before RF ablation shows a 1.2-cm-diameter HCC (arrow) in the high dome of liver segment 8. He has a history of previous TACE and RF ablation. (B) Ultrasound during introducing artificial ascites via an 18 gauge spinal needle shows multiple linear septations (i.e., adhesions) at the perihepatic space (arrow). (C) After introducing artificial ascites via 5 Fr introducer sheath across the adhesions, US shows more effective fluid collection at the hepatic dome. (D) Ultrasound after satisfactory induction of ascites shows an index tumor clearly. (E) Contrast-enhanced transverse CT scan obtained during arterial phase immediately after RF ablation shows a 3.0-cm-diameter RF ablation zone (arrow) with ablative margin.
Anatomical pitfall (left posterior subphrenic area and S7 bare area) in successful induction of artificial ascites in a 48-year-old-woman with massive ascites. (A) and (B) Contrast-enhanced transverse CT scans obtained during portal phase show uneven distribution of the ascitic fluid due to peritoneal attachment of the liver. (C) Contrast-enhanced sagittal CT scan obtained in the portal phase shows that the postero-superior aspect of the left lateral segment is very narrow. (D) Contrast-enhanced sagittal CT scan obtained during the portal phase shows the postero-superior aspect of the right lobe could not be separated by ascites due to the bare area.
Anatomical pitfall (left posterior subphrenic area) in a 63-year-old-man with HCC. (A) Contrast-enhanced transverse CT scan obtained during arterial phase before RF ablation shows a 1.0-cm-diameter viable HCC (arrow) posterior to the Lipiodolized nodule in liver segment. (B) Planning ultrasonography shows a hypoechoic nodule (arrow) posterior to the Lipiolized nodule. However, the tumor is abutting the diaphragm and heart. (C) Ultrasound obtained after introducing artificial ascites via an 18 gauge spinal needle shows no separation of the index tumor from the abutted diaphragm (arrow). (D) Contrast-enhanced sagittal CT scan obtained during portal phase immediately after RF ablation shows a 3.0-cm-diameter RF ablation zone including Lidiodolized nodule. Note the limited space (arrow) of the postero-superior aspect of the left subphrenic space.
Omental fat as a limiting factor in a 53-year-old-man with HCC. (A) Contrast-enhanced transverse CT scan obtained during arterial phase before RF ablation shows a 1.8-cm-diameter HCC in the high dome of liver segment 8. Note the interposed omentum (arrow) at the perihepatic space. (B) Ultrasound obtained after introducing artificial ascites via 5f introducer sheath shows a more conspicuous index tumor due to improving sonic window. However, the omentum (arrow) is still located along the RF electrode path. (C) Ultrasound during targeting the tumor shows more poor sonic window due to the overlapped omentum (arrow).
Summary and conclusion
Radiofrequency ablation with the assistance of artificial ascites is a simple and safe technique for treating a hepatic dome tumor abutting the diaphragm. One can improve the sonic window and separate the RF ablation zone from the diaphragm by downward displacement of the liver using a simple and inexpensive technique. Complications related to artificial ascites including hemoperitoneum are very rare. Peritoneal adhesion and tumor location in the bare area are the main limitations for the application of this technique.
References
Lencioni R, Allgaier HP, Cioni D, et al. (2003) Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 228(1):235–240
Lin SM, Lin CJ, Lin CC, et al. (2004) Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma ≤4 cm. Gastroenterology 127(6):1714–1723
Lencioni R, Cioni D, Crocetti L, et al. (2005) Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 234(3):961–967
Tateishi R, Shiina S, Teratani T, et al. (2005) Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 103(6):1201–1209
Choi D, Lim HK, Rhim H, et al. (2007) Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol 7(3):684–692
Koda M, Ueki M, Murawaki Y (2003) Diaphragmatic perforation and hernia after hepatic radiofrequency ablation. AJR Am J Roentgenol. 180(6):1561–1562
Rhim H (2005) Complications of radiofrequency ablation in hepatocellular carcinoma. Abdom Imaging 30:409–418
Kim YS, Rhim H, Sung JH (2005) Bronchobiliary fistula after radiofrequency thermal ablation of hepatic tumor. J Vasc Interv Radiol 6(3):407–410
Rhim H, Dodd GD III, Chintapalli KN, et al. (2004) Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics 24(1):41–52
Machi J, Uchida S, Sumida K, et al. (2001) Ultrasound-guided radiofrequency thermal ablation of liver tumors: percutaneous, laparoscopic, and open surgical approaches. J Gastrointest Surg 5(5):477–489
Raman SS, Ku DS, Vodopich DJ, et al. (2002) Minimizing diaphragmatic injury during radio-frequency ablation: efficacy of subphrenic peritoneal saline injection in a porcine model. Radiology 222(3):819–823
Ohmoto J, Tsuzuki M, Yamamoto S (1999) Percutaneous microwave coagulation therapy with intraperitoneal saline infusion for hepatocellular carcinoma in the hepatic dome. AJR Am J Roentgenol 172(1):65–66
Ohmoto J, Yamamoto S (2001) Percutaneous microwave coagulation therapy using artificial ascites. AJR Am J Roentgenol 176(3):817–818
Kapoor BS, Hunter DW (2003) Injection of subphrenic saline during radiofrequency ablation to minimize diaphragmatic injury. Cardiovasc Intervent Radiol 26(3):302–304
Kondo Y, Yoshida H, Shiina S, et al. (2006) Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg 93(10):1277–1282
Rhim H, Lim HK, Kim YS, et al. (2008) Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol 190(1):9108
Brace CL, Laeseke PF, Prasad V, Lee FT (2006) Electrical isolation during radiofrequency ablation: 5% dextrose in water provides better protection than saline. Conf Proc IEEE Eng Med Biol Soc 1:5021–5024
Laeseke PF, Sampson LA, Brace CL, et al. (2006) Unintended thermal injuries from radiofrequency ablation: protection with 5% dextrose in water. AJR Am J Roentgenol 186(5 Suppl):S249–S254
Hinshaw JL, Laeseke PF, Winter TC III, et al. (2006) Radiofrequency ablation of peripheral liver tumors: intraperitoneal 5% dextrose in water decreases postprocedural pain. AJR Am J Roentgenol 186(5 Suppl):S306–S310
Perrault J, McGill D, Ott BJ, et al. (1978) Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology 74(1):103–106
Murphy FB, Barefield KP, Steinberg HV, et al. (1988) CT or sonography guided biopsy of the liver in the presence of ascites: frequency of complications. AJR Am J Roentgenol 151:485–486
Koda M, Ueki M, Maeda Y, et al. (2004) Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. AJR 183:583–588
Minami Y, Kudo M, Kawasaki T, et al. (2003) Percutaneous ultrasound-guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. J Gastroenterol 38(11):1066–1070
Shibata T, Iimuro Y, Ikai I, et al. (2002) Percutaneous radiofrequency ablation therapy after intrathoracic saline solution infusion for liver tumor in the hepatic dome. J Vasc Interv Radiol 13(3):313–315
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rhim, H., Lim, H.K. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: the value of artificial ascites. Abdom Imaging 34, 371–380 (2009). https://doi.org/10.1007/s00261-008-9408-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-008-9408-4