Abstract
The role of MR imaging in hilar cholangiocarcinoma is to confirm/reach a diagnosis and to assess resectability. Hilar cholangiocarcinoma shows the same signal intensity pattern of peripheral tumors both on T1- and T2-weighted images. On magnetic resonance cholangiopancreatography (MRCP) images, hilar cholangiocarcinoma appears as a moderately irregular thickening of the bile duct wall (5 mm) with symmetric upstream dilation of the intrahepatic bile ducts. The aim of preoperative investigation in Klatskin tumors typically requires the evaluation of the level of biliary obstruction, the intrahepatic tumor spread, and the vascular involvement; it also needs to show any atrophy–hypertrophy complex. Because of its intrinsic high tissue contrast and multiplanar capability, MR imaging and MRCP are able to detect and preoperatively assess patients with cholangiocarcinoma, investigating all involved structures such as bile ducts, vessels and hepatic parenchyma. The main reason for surgical/imaging discrepancy is represented by the microscopic diffusion along the mucosa and in the perineural space.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hilar cholangiocarcinoma, or Klatskin tumors, were initially defined as adenocarcinoma of the hepatic duct at the bifurcation within the porta hepatis. Now, they usually include cancers of the common hepatic duct (CHD) [1, 2].
Although surgery offers the only chance of cure, the majority of these tumors-because of their poor prognosis and advanced stage at the time of diagnosis have been managed with nonsurgical treatment such as percutaneous biliary drainage for palliation of jaundice [3].
Currently however, more patients may be candidates for extensive curable or palliative surgery because of rapid advances in surgical technique and diagnostic imaging.
The primary goal of preoperative examinations is to first exclude the established criteria for unresectable tumors, and then to define the tumor spread, to identify the level of obstruction, and to show any other combined findings. Extensive biliary and vascular involvements are considered the most important factors in determining unresectability [4]. For this reason the staging of hilar cholangiocarcinoma has depended on direct cholangiography, and in some cases, angiography.
In recent years, dynamic contrast medium-enhanced magnetic resonance (MR) imaging has proved to be useful in the characterization of liver lesions and MR cholangiopancreatography (MRCP) has proved to be very accurate in depicting various biliary and pancreatic disease entities [5–7].
In this article we discuss the role of MR and MRCP in the staging and treatment planning of hilar cholangiocarcinoma.
Role of MRI/MRCP in staging
The role of imaging is to differentiate benign from malignant causes of biliary structure, to determine resectability in patients with malignant disease, and to preoperatively stage those patients with potentially resectable tumors [8]. Thickening of the bile wall with obstruction at the hilum level is not pathognomonic of Klatskin tumor, because various other bile duct lesions may also appear with similar findings, e.g., neuroendocrine tumors, metastases, PSC or secondary sclerosing cholangitis resulting from autoimmune pancreatitis and recurrent cholangitis [9].
For futher characterization of benign or malignant stenoses, the morphology is important. While benign stenoses usually appear as regular, symmetrical and smooth-shaped luminal strictures, malignant biliary obstruction is suggested by the presence of an abrupt, irregular and uneven luminal narrowing. However, the clinical presentation and the medical history have to be taken into consideration in the differential diagnosis and a biopsy is requested to confirm the diagnosis.
If hilar cholangiocarcinoma is diagnosed, a correct local staging of the tumor is crucial for further patient treatment.
A pathological staging system has been developed by the American Joint Committee on Cancer Staging (AJCCS; Table 1) [10]. The major drawback of this staging system is that it does not correlate with resectability. On the other hand, the Memorial Sloan-Kettering staging system (Table 2) is based on the extent of biliary and vascular involvement, and correlates to resectability and survival [11]. However, the goals of diagnostic staging for hilar cholangiocarcinoma should be to determine the local and distant extent of disease for its impact on surgical resectability.
Prior to discussing the role of MR/MRCP in staging hilar cholangiocarcinoma it is important to know the macroscopic appearance and the characteristic ways that this tumor spreads.
Hilar cholangiocarcinoma produces a localized stricture, giving rise to its descriptive terms: focal stenosis or infiltrating stenosis. The frequent mode of spread is local extension from the biliary tree invading the liver. This appearance has implications for radiologic diagnosis and prognosis given the difficulty to obtain a surgical cure after transductal spread.
The Liver Cancer Study Group of Japan has proposed a new classification of cholangiocarcinoma based on growth characteristics, with tumors being identified as mass-forming, periductal-infiltrating, and intraductal-growing types [12]. This classification is considered to be the most useful because it describes the gross appearance, the growing characteristics, the biologic behavior, and the prognostic implications for patients.
The periductal infiltrating type is most prevalent in the hilar portion of biliary tree, which has a characteristic growth pattern that infiltrates along the main and intrahepatic bile ducts [13].
Therefore, the exact evaluation of longitudinal tumor extension on the biliary duct is extremely important.
Less CBD carcinomas developing in the CBD appear as a rounded intraluminal mass, usually within the mid extrahepatic biliary tree at the distal CHD or at the CBD [12]. This polypoid of papillary variety should bring the patient to medical attention relatively early, thus improving the prognosis.
Microscopically, cholangiocarcinoma represents an adenocarcinoma with glandular appearance arising from the epithelium of the bile ducts. These tumors have a tendency to spread between the hepatocyte plates, along the duct walls, and adjacent to the nerves. Perineural invasion is frequent, found in as many as 81% of reported cases [13]. Results of mucin are nearly always positive, and mucin production is often abundant. The neoplastic cells provoke a variable desmoplastic reaction, therefore the tumor mass most often lies in a connective tissue stroma, but the degree of desmoplastic reaction among the cholangiocarcinomas varies considerably. The sclerosis and fibrosis of surrounding tissue may be difficult to differentiate from tumor, and even surgical exploration cannot always reveal the true extent of the tumor especially regarding bile and liver parenchyma [11, 14]. Satellite nodules are less commonly seen in hilar cholangiocarcinoma, compared to intrahepatic cholangiocarcinoma, which is in keeping with the much earlier manifestation of lesion situated at the hilum [14].
The radiological analysis should always include the tumor extent, presence or absence of liver infiltration, vascular involvement (especially hepatic artery and portal vein), lymph node metastases as well as distant metastasis, especially liver metastasis.
The imaging evaluation of patients with hilar cholangiocarcinoma has traditionally included computed tomography and sonography or a combination of the two techniques for diagnosis and preoperative assessment for resectability [18, 19]. However the final diagnosis usually relied on direct cholangiographies, such as endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography (PTC). Direct cholangiography has been regarded as the most accurate preoperative diagnostic modality for assessing the longitudinal extension of hilar cholangiocarcinoma [20]. However, injection of contrast medium through high-grade stenoses and subsequent drainage failure may result in severe complications, such as cholangitis and/or sepsis, associated with a significant morbidity rate up to 7% and mortality rate up to 1% [21]. Moreover direct cholangiographies are not able to assess the infiltrative growth pattern along bile ducts. For intraductal papillary type cholangiocarcinoma, cholangiography can lead to overestimating bile duct involvement because of intraductal necrotic material.
In recent years, magnetic resonance (MR) imaging, in conjunction with magnetic resonance cholangiopancreatography (MRCP), has proved helpful in diagnosing hilar cholangiocarcinoma and in determining resectability [22–24]. This is due to MR imaging and MRCP being able to investigate all different components: bile ducts, vessels, and invasion of adjacent liver parenchyma.
Two recently introduced technical improvements have contributed to further increasing the diagnostic value of MRI, including MRCP [22]. The first is parallel imaging (iPAT), and the second is respiratory independent sequences navigator triggering, which have substantially increased the spatial resolution as a critical parameter in biliary imaging.
Single-shot pulse sequences, such as half Fourier acquired single-shot turbo spin echo (HASTE), often suffer from image artefacts, which are related to their long echo trains with apparent blurring due to the off-resonances during the readout of the echo train. This problem can be reduced by applying iPAT to shorten the length of the echo train without loss of spatial resolution, resulting in decreased signal decay and reduced blurring.
It is important to use sequences with thin-slice thickness (3–4 mm) that provide sufficient signal to obtain good quality images and are sufficiently thin to detect subtle abnormalities.
For MRCP, the latest developments are 3D-triggered T2-weighted FSE sequences with a voxel size of approximately 1.5 mm, by which high quality MPR and maximum intensity projections can be obtained.
Hilar cholangiocarcinoma often shows circumferential growth and spreads along bile ducts with poor conspicuity on noncontrast MR images [22, 24].
Lobar atrophy of the liver combined with marked biliary dilatation should raise suspicion of cholangiocarcinoma, but this feature is not pathognomonic.
On T1-weighted MR images with or without fat suppression, cholangiocarcinomas appear mildly to moderately hypointense but may also be isointense relative to liver parenchyma (Fig. 1). On T2-weighted images, they are isointense or mildly hyperintense (Fig. 1).
A Axial T1-weighted Spoiled Gradient Echo image shows a bulky mass of low signal intensity (arrow) located at the porta hepatis. B–C Axial and coronal T2-weighted FSE images show the high intensity of the lesion (arrows) with marked intra-hepatic bile duct dilatation extending into the left hepatic duct. D CPRM image demonstrates an abrupt stenosis of the primary biliary confluence (arrow) with interruption of the left secondary biliary confluence (short arrow) and dilatation of intra-hepatic bile ducts. E–F Axial T1-weighted, dynamic gadolinium-enhanced images. The lesion appears hypointense during the arterial phase (E) and hyperintense at delayed phase (F) (arrows).
Thickening of bile duct walls greater than 5 mm is highly suggestive of cholangiocarcinoma. However this measurement is not sensitive and at least 50% of tumors show thinner wall diameters [22].
Hilar cholangiocarcinomas do not show a unique enhancement pattern. The majority of the lesions are typically hypovascular tumors, compared to adjacent liver parenchyma, showing a heterogeneous enhancement that gradually increases to a peak on delayed images (Fig. 1) [5]. This pattern is consistent with the fibrous nature of the tumor. Other tumors show periductal enhancement, whereas a small percentage of hilar cholangiocarcinomas are hypervascular; note however that the immediate diffuse enhancement, seen with other hypervascular liver lesions, is rarely seen [24].
Bile duct invasion
The most common staging system describes the extent of tumor spread within the biliary system according to Bismuth and Corlette classification [25]. Both MRCP and direct cholangiography provide similar projectional images, and they demonstrate the tumor as a signal void or contrast-filling defect within the bile duct. However compared to ERCP, MRCP has the advantage of determining the suprahilar tumor extension more accurately, which may be difficult to assess with ERCP, because of insufficient contrast filling of the proximal bile duct system, due to severe stenosis of the confluence.
Furthermore, the combination of cross-sectional and projectional imaging (MRCP) allows the presence and the size of extraluminal extension of the tumor and liver parenchyma invasion to be evaluated, leading to a greater accuracy to preoperative staging. The presence of bile duct obstruction can be diagnosed with accuracies far greater than 90% [26].
The level of obstruction and extent of biliary duct involvement according to the classification of Bismuth-Corlette can be predicted with accuracies from 88% up to 96% [26–28] (Fig. 2).
In an article [29] comparing ERCP, PTC and MRCP in evaluating tumor extent, the statistical analysis showed PTC to be significantly superior to ERCP, but not to MRCP. The most common mistake for each imaging modality was an overestimated tumor extent, which may exclude patients from potentially curative surgery [29]. One cause of error in staging hilar cholangiocarcinoma is represented by lack of recognition of submucosal spread, resulting in understaging of the disease and consequent planning errors in treatment, resulting in unnecessary laparotomies [13]. The identification of submucosal spread is difficult and may be underestimated by both noninvasive and invasive diagnostic imaging modalities, representing a frequent cause of treatment failure.
A recent article evaluated the combined use of CT and direct cholangiography for preoperative assessment of hilar cholangiocarcinoma [30]. Despite the improved assessment achieved by using both imaging modalities, tumor invasion of the intrahepatic bile ducts was underestimated in 13% of the patients and overestimated in 4%. The underestimation of diffuse tumor infiltration extending to the hepaticoduodenal ligament was a common cause of prediction failure, using helical CT in this study [30]. This can be explained by the infiltrative growth pattern and the propensity of cholangiocarcinoma to grow axially along segmental ducts.
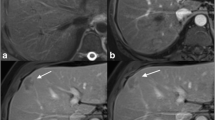
In our experience, the presence and the extent of bile duct wall enhancement on delayed images, can be an indicator of spread along the bile duct walls via the nerve and perineural tissue of Glisson’s capsule toward the porta hepatic (Fig. 3) [31]. Biliary stent placement results in mild inflammation of bile duct walls, which appears as an increased gadolinium enhancement with an appearance indistinguishable from superficial spread of cholangiocarcinoma.
A Axial Half Fourier RARE T2-weighted image shows a hyperintense infiltrating tissue at the primary biliary confluence (arrow). B Coronal Half Fourier RARE MRCP shows abrupt stenosis below the primary biliary confluence (arrow) and at the level of the secondary left biliary confluence (short arrow). C–D Coronal and axial delayed contrast-enhanced FSPGR T1-weighted images show extent of bile duct wall enhancement along the primary biliary confluence and the left hepatic and intrahepatic ducts, indicative of periductal spread (arrows).
For this reason, it is recommended to image patients suspected of biliary tumor before stent placement to avoid the problem of misstaging the tumor because of inflammatory changes secondary to the presence of the stent. In addition, when patients with biliary drainage are referred for MRI, the biliary tree has often collapsed and the evaluation of biliary pathologies is virtually impossible by MRCP. Ideally, therefore MR and MRCP should be performed before biliary drainage and stent whenever possible.
Vessel invasion
Precise evaluation of the hepatic artery and portal vein is important in the preoperative planning.
Conventional X-ray arteriography has been largely performed for this purpose, but its well-known disadvantages, like invasiveness and the use of iodinated contrast materials, work against its use, as does the inherent difficulty in documenting the relationship of a mass to adjacent vessels if the tumor is avascular [32].
Dynamic contrast enhanced MR imaging is comparable to angiography in assessing the portal vasculature invasion in patients with cholangiocarcinoma (Fig. 4).
With MR angiography, narrowing of the vessel wall, abrupt cut-off, and focal irregular indentation were well visualized.
In general, MR angiography suppresses, either partially or completely, the signal from the background tissue; consequently, it provides little information about primary cancer surrounding the vessels. We recommend visualizing MR enhanced axial and coronal images as well as MR angiography to determine serosal invasion or tumor adhesion.
In particular dynamic imaging in the coronal oblique plane is particularly useful for distinguishing vessels from bile ducts and for showing the relationship of the lesion to the portal veins, because the whole of the portal veins are typically seen on one or two sections, hilar lesions are more easily localized, and the coronal anatomy is similar to that seen on surgery (Fig. 4).
3D steady state sequences (FIESTA, True FISP, Balanced FFE) were accurate in determining the extent of portal vein infiltration but were inferior in specificity in distinguishing hepatic arterial invasion [33].
Liver parenchyma invasion
Invasion of adjacent liver parenchyma is important in determining tumor resectability. Cross sectional MR images are able to detect a mass that frequently grows beyond the duct, and invades the adjacent liver parenchyma (Fig. 5).
A combination of early and late fat-suppressed gadolinium-enhanced images is very helpful to identify liver parenchimal invasion and the presence of liver metastasis [23]. Fat suppression also reduces the signal of fatty tissue in the porta hepatis, which improves the conspicuity of cholangiocarcinomas and facilitates the evaluation of the extent of tumor and infiltration into adjacent tissues and organs.
Lobar atrophy, due to longstanding severe biliary obstruction and/or severe portal vein occlusion, is an important predictive factor for unresectability, but does not affect the overall patient prognosis proportionately [34]; the assessment of the atrophy/hypertrophy complex helps in surgical treatment planning, considering that no liver resection should be performed that leaves an atrophic remnant (Fig. 6).
Other findings
Lymphadenopathy with portocaval and porta hepatis nodes is an associated finding in up to 73% of patients with cholangiocarcinoma [14]. This aspect is best demonstrated with a combination of T2-weighted fat suppressed and T1-weighted post gadolinium images on a late phase. On these delayed postgadolinium images, fine tumor strands are frequently observed and 5 mm or smaller lymph nodes are consistent with tumor extension if three or more of them are clustered in the tumor region.
In advanced cholangiocarcinoma, intraperitoneal tumor spread may occasionally be found and is also best seen on late postgadolinium fat suppressed images [23].
Role of MR and MRCP in treatment planning
Approximately 40% of patients with hilar cholangiocarcinoma may undergo surgical resection with a curative intent, which has increased 5-year survival rates overall from 1% to 20% after surgery [34, 35].
Hepatectomy for cholangiocarcinoma can be a challenging procedure and has a long convalescence period. Morbidity rates are rather high, with 44% of patients suffering a major postoperative complication and nearly 10% overall mortality rate [35].
Surgical exploration should only be performed when preoperative examination has shown curative resection to be possible, because the risks of palliative surgery for malignant obstructive jaundice are high, with surgical mortality rates of 20–30% [35].
The prognosis for mass-forming and periductal-infiltrating cholangiocarcinomas is generally unfavorable, whereas the prognosis for intraductal-growing cholangiocarcinoma is much better (or excellent) after surgical resection. MR can differentiate between the different appearances of growth patterns of cholangiocarcinoma [22].
Percutaneous and endoscopic biliary drainage are the preferred methods for palliation of jaundice in these patients, because of their lower morbidity and mortality rates, compared to those for palliative surgery. The criteria for unresectability include involvement of the main portal vein, of both branches of the portal vein, of the portal vein of one lobe of the liver combined with involvement of the hepatic artery to the contralateral lobe, combination of vascular involvement of one lobe of the liver with extensive ductal involvement of the contralateral lobe, bilateral neoplastic extension to the segmental branches of the intrahepatic bile ducts, and hepatic or nodal metastases [36].
Since MR and MRCP are able to obtain an accurate preoperative staging of biliary, liver, vascular involvement, this aspect is critical in choosing the best treatment option, in terms of radical surgery or palliative biliary drainage.
The precise localization and the level of the involved duct determine the choice of treatment modality; for instance, infiltration beyond the secondary branches on both sides of the liver was generally considered unresectable. Recently, it has been demonstrated that complete resection inclusive partial hepatectomy and regional lymphadenectomy might be the surgical treatment of choice for selected patients. Since there is great variability in bile duct anatomy—that is, the secondary confluence of the bile duct is only a few millimeters from the hepatic hilum in some patients, while it is several centimeters from the hepatic hilum in others—not all Bismuth type IV cholangiocarcinomas are surgically unresectable [37].
Simultaneous extended hepatectomy, removal of extra hepatic bile ducts with or without resection, and anastomosis of the portal vein are considered potentially curative even for Bismuth type IV hilar cholangiocarcinoma.
Consequently, defining the extent of biliary duct invasion and constructing the exact road map of the biliary tree in such patients are crucial for planning and choosing the appropriate treatment.
The MRCP can be very useful in visualizing the exact biliary tree map, in a non-invasive manner.
Moreover delayed periductal enhancement is useful in assessing perineural spread and this sign can improve diagnostic accuracy in identifying resectable tumors.
In conclusion, MRCP and dynamic MRI are able to provide all informations for pretherapeutic staging of hilar cholangiocarcinomas, because of their intrinsic high soft tissue contrast and multiplanar capability, and should be considered a real one-stop-shop noninvasive technique.
References
Klatskin G (1965) Adenocarcinoma of the hepatic ducts at its bifurcation with the porta hepatis: an unusual tumor with distinctive clinical and pathological features. Am J Med 38:241–245
Alexander F, Rossi RL, O’Bryan M, et al. (1994) Biliary carcinoma: a review of 109 cases. Am J Surg 147:503–509
Launois B, Wemyss-Holden S, Maddern GJ (2002) Current and future trends in management and treatment of Klatskin tumour. Int J Clin Oncol 7:91–102
Gibson RN, Yeung E, Thompson JN, et al. (1986) Bile duct obstruction: radiologic evaluation of level, cause, and tumor resectability. Radiology 160:43–47
Guthrie JA, Ward J, Robinson PJ (1996) Hilar cholangiocarcinoma: T2-weighted spin-echo and gadolinium enhanced FLASH MR imaging. Radiology 201:347–351
Fulcher AS, Turner MA (1997) HASTE MR cholangiography in the evaluation of hilar cholangiocarcinoma. AJR Am J Roentgenol 169:1501–1505
Reinhold C, Bret P, Atri M, et al. (1996) MR cholangiopancreatography:potential clinical application. Radiographics 16:309–320
Adam A, Benjamin IS (1992) Review. The staging of cholangiocarcinoma. Clin Radiol 46:299–303
Vogl TJ, Schwarz WO, Heller M, et al. (2006) Staging of Klatskin tumours (hilar cholangiocarcinomas): comparison of MR cholangiography, MR imaging, and endoscopic retrograde cholangiography. Eur Radiol 16(10):2317–25
American Joint Committee on Cancer (2005) AJCC cancer staging. New York: Springer, pp 1–150
Slattery JM, Sahani DV (2006) What is the current state of the art imaging for detection and staging of cholangiocarcinoma? Oncologist 11:913–922
Nakanuma Y, Minato H, Kida T, et al. (1994) Pathology of cholangiocarcinoma. In Primary liver Cancer in Japan. Tokyo, Japan: Springer, pp 39–50
Bhuiya MR, Nimura Y, Kamiya J, et al. (1992) Clinicopathologic studies on perineural invasion of bile duct carcinoma. Ann Surg 215:344–349
Lim JH, Park CK (2004) Pathology of cholangiocarcinoma. Abdom Imaging 29:540–547
Lim JH (2003) Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol 181:819–827
Malhi H, Gores GJ (2006) Review article: the modern diagnosis and therapy of cholangiocarcinoma. Aliment Pharmacol Ther 23:1287–1296
Choi BI, Lee JM, Han JK (2004) Imaging of intrahepatic and hilar cholangiocarcinoma 29:548–557
Looser C, Stain SC, Baer HU, Triller J, Blungart LH (1992) Staging of hilar cholangiocarcinoma by ultrasound and duplex sonography: a comparison with angiography and operative findings. Br J Radiol 65:871–877
Tillich M, Mischinger HJ, Preisegger KH, Rabl H, Szolar DH (1998) Multiphasic Helical CT in Diagnosis and Staging of Hilar Cholangiocarcinoma. AJR Am J Roentgenol 171:651–658
Wilkinson M (1996) The art of diagnostic imaging: the biliary tree. J Hepatol 25:5–19
Bilbao MK, Dotter CT, Lee TG, et al. (1976) Complications of endoscopic retrograde cholangiopancreatography (ERCP): a study of 10.000 cases. Gastroenterology 70:314–320
Zech JC, Schoenberg SO, Reiser M, Helmeberger (2004) Cross-sectional imaging of biliary tumors:current clinical status and future developments. Eur Radiol 14:1174–1187
Manfredi R, Barbaro B, Masselli G, Vecchioli A, Marano P (2004) Magnetic Resonance imaging of cholangiocarcinoma. Seminar Liv Dis 24:155–164
Manfredi R, Masselli G, Maresca G, et al. (2003) MR imaging and MRCP of hilar cholangiocarcinoma. Abdom Imaging 28:319–325
Bismuth H, Corlette MB (1975) Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 140:170–178
Lopera JE, Soto JA, Munera F (2001) Malignant hilar and perihilar biliary obstruction: use of MR cholangiography to define the extent of biliary ductal involvement and plan percutaneous interventions. Radiology 220:90–96
Zidi SH, Prat F, Le Guen L, Rondeua Y, Pelletier G (2000) Performance characteristics of magnetic resonance cholangiography in the staging of malignant hilar strictures. Gut 46:103–106
Manfredi R, Brizi MG, Masselli G, et al. (2001) Malignant Biliary hilar stenosis. MR cholangiopancreatography compared with endoscopic retrograde cholangiography. Radiol Med 102:48–54
Otto G, Romaneehsen B, Bittinger F, et al. (2004) Preoperative imaging of hilar cholangiocarcinoma:surgical evaluation of standard practices. Z Gastroenterol. 42(1):9–14
Lee HY, Kim SH, Lee JM, et al. (2006) Preoperative assessment of respectability of hepatic hilar cholangiocarcinoma:combined CT and cholangiography with revised criteria. Radiology 239:113–121
Masselli G, Casciani E, Polettini E, et al. (2006) MR imaging and MRCP in the preoperative evaluation of hilar cholangiocarcinoma: correlation with surgical and pathologic findings[Abstract]. Radiol Suppl RSNA 2006:273
Mcfarland EG, Kaufman JA, Saini S, et al. (1996) Preoperative staging of cancer of the pancreas: value of MR angiography versus conventional angiography in detecting portal venous invasion. AJR Am J Roentgenol 166:37–43
Lee GM, Park KB, Shin YM, et al. (2003) Preoperative evaluation of hilar cholangiocarcinoma with contrast enhanced 3D Fast Imaging with steady-state precession magnetic resonance angiography: comparison with intraarterial digital subtraction angiography. World J Surg 7:278–283
Lygidakis NJ, Van der Henden MN, Houthoff HJ (1988) Surgical approaches to the management of primary biliary cholangiocarcinoma of the porta hepatis. The decision making dilemma. Hepatogastroenterology 35:261–267
Baer HU, Stain SC, Dennison MD, et al. (1992) Improvement in survival by aggressive resections of hilar cholangiocarcinoma. Ann Surg 217:20–27
Rosai J (1996) Ackerman’s Surgical Pathology, 8th edn. St. Louis: Mosby 914–915, 960
Lygidakis NJ, Sgourakis GJ, Dedemadi GV, Vlachos L, Safioleas M (2001) Long term results following resectional surgery for Klatskin tumors: a twenty-year personal experience. Hepatogastroenterology 48:95–101
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masselli, G., Gualdi, G. Hilar cholangiocarcinoma: MRI/MRCP in staging and treatment planning. Abdom Imaging 33, 444–451 (2008). https://doi.org/10.1007/s00261-007-9281-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-007-9281-6