Abstract
The type, incidence, and severity of complications of balloon-occluded retrograde transvenous obliteration (B-RTO) for gastric varices should be precisely estimated. Complications were evaluated in 38 patients who had fundic gastric varices and 43 B-RTO procedures during injection of ethanolamine oleate (phase 1), within 4 h after injection (phase 2), 24 h after injection (phase 3), and from 24 h to 10 days after injection (phase 4). Endoscopic evaluation at 8 weeks showed resolution of gastric varices in 35 of 38 patients (92%) and smaller varices in the remaining three (8%). B-RTO caused transient hypertension in 35% of patients, hemoglobinuria in 49%, and fever in 33% during phases 1, 2, and 3, respectively. Pleural effusion, pulmonary infarction, ascites, gastric ulcers with unique appearance, localized mosaic-like change of gastric mucosa, and hemorrhagic portal hypertensive gastropathy were noted in phase 4. There were no fatalities. Lactate dehydrogenase, aspartate aminotransferase, and bilirubin increased on day 1. Each datum was retrieved within 7 days. The severity of lactate dehydrogenase elevation correlated significantly with the volume of infused ethanolamine oleate. Thus, B-RTO is a safe and effective management of fundic varices. However, short-term hemodynamic change after B-RTO may cause gastric mucosal damage. Pulmonary infarction and pleural effusion are potential complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Bleeding from gastric varices is a serious complication of portal hypertension and a serious medical emergency. Gastric varices bleed more profusely and are more likely to rebleed than esophageal varices [1]. The natural history of patients who have cirrhosis and fundic varices is adversely influenced by hemorrhage from fundic varices. Thus, in addition to urgent and elective treatment, prophylactic obliteration of high-risk large fundic varices has been recommended [2]. However, in most guidelines, there is no clear indication on whether and how to treat this problem. The optimal approach for fundic varices was not codified before the introduction of balloon-occluded retrograde transvenous obliteration (B-RTO) as a management procedure [3]. There are many optional procedures, such as endoscopic variceal ligation [4], transjugular intrahepatic portosystemic shunt (TIPS) [5], percutaneous transhepatic obliteration [6], transileocecal obliteration [6], and endoscopic sclerotherapy with [7] or without [8] cyanoacrylate. Nevertheless, B-RTO has been extensively applied in the past decade for the management of fundic gastric varices in several specialized centers. The technique provides good control of gastric varices with gastrorenal or gastrocaval collaterals and has been considered to be a feasible alternative to TIPS, although the nomenclature has varied among the reporters [3, 9, 10, 11]. Further, B-RTO may be applicable for gastric varices even in patients who have poor hepatic function and hepatic encephalopathy [11, 12, 13]. B-RTO increases hepatic portal blood flow, which may increase liver function [14, 15]. Development of esophageal varices after occlusion of the gastrorenal shunt has been reported as a long-term complication of B-RTO [16]. In addition, hemoglobinuria and fever are short-term complications [12]. One case of cardiogenic shock, caused by a mixture of ethanolamine oleate and iopamidol, has been reported [11]. Chikamori et al. [13] reported one case treated with this procedure that later developed gastritis with hemorrhage. Thus, no other warning or conspicuous systemic complications have been reported thus far [3, 9, 10, 11, 12, 13, 16].
The procedure of B-RTO consists of obliteration of the major portosystemic shunt in patients who have portal hypertension. This may lead to a marked change in the hemodynamics in the gastric mucosa, esophagus, spleen, intestine, liver, and systemic circulation. B-RTO can worsen portal hypertension by obliterating portosystemic shunts [15]. B-RTO creates a large thrombus not only in gastric varices but also in their in-flow and out-flow shunts. This thrombus may form very close to the major venous system such as the left renal vein or inferior caval vain and close to the major portal system including the splenic vein and portal trunk. These events may result in major complications, although actual clinical complications are indistinct in this aspect. The procedure of B-RTO is still under evaluation and has not been clearly defined in its details of technical procedure, and potential risks could be various and severe. Available reports have not stated important side effects, excluding those cited above, and prospective analyses of large series are unavailable. This study aims to fill this gap.
In the present study, we evaluated the incidence and severity of all complications encountered in the course of the procedure and during hospital stay after B-RTO.
Materials and methods
Patients
Forty-three B-RTO procedures were performed in 38 patients (21 male, 17 female) who had portal hypertension and gastric fundic varices between December 1995 and July 2000 in Maebashi Red Cross Hospital (Maebashi, Japan). Patients who had Child class C cirrhosis with ascites were excluded from this study because B-RTO may increase portal pressure [15]. Written informed consent was obtained from all patients. The main clinical characteristics of these patients are listed in Table 1. Liver cirrhosis was diagnosed in 37 patients. One patient without cirrhosis had left-side portal hypertension, which was caused by splenic vein obstruction after traumatic pancreatitis. Twenty-seven of 38 patients (71%) had associated esophageal varices and 13 patients had hepatocellular carcinoma. Computed tomography (CT) or ultrasonography excluded the presence of portal tumor thrombus in all patients who had hepatocellular carcinoma. The form of fundic varices was classified into three categories (Table 1): F1, tortuous varices; F2, moderately enlarged nodular varices; and F3, massive tumor-like varices. Nineteen patients had a history of variceal bleeding. Endoscopic variceal ligation was achieved in five of seven patients who had active bleeding at the time of urgent endoscopy. Clipping was performed in two patients. Hemostasis was secured in all seven patients. Injection sclerotherapy was not performed. Six patients underwent subsequent B-RTO within 24 h as emergent cases. This study also included 13 patients with elective B-RTO and 19 with prophylactic B-RTO.
B-RTO procedure
A 6.5-French balloon catheter (Create Medic, Yokohama, Japan) was inserted in the gastrorenal shunt, gastrocaval shunt, or both through the femoral or internal jugular vein under local anesthesia. Balloon-occluded retrograde transvenous varicerography was obtained in advance. Four thousand units of haptoglobin (Welfide, Osaka, Japan) was administered by drop infusion just before treatment. After identification of gastric varices and their associated in-flow and draining vessels, the balloon was inflated with CO2. Eighteen patients had two or more varices-associated in-flow shunts. Total numbers of in-flow shunts were 29 posterior gastric veins, 23 left gastric veins, and eight short gastric veins. Five percent solution of ethanolamine oleate with iopamidol (5% EOI) that contained equal amounts of 10% ethanolamine oleate (Grelan, Tokyo) and iopamidol 300 (Schering, Berlin, Germany) was slowly infused through the catheter into the gastric varices and their in-flow shunts in a retrograde manner during balloon occlusion. After 30 min, hemolyzed blood and excess EOI were collected through the catheter and then the balloon was deflated. In 12 patients, microcoils were used in addition to 5% EOI to embolize out-flow minor collaterals.
Evaluation of efficacy of B-RTO
We observed the site endoscopically at 1, 4, and 8 weeks after the procedure and evaluated the efficacy of B-RTO at 8 weeks in all 38 patients. Five patients (13%) who showed no improvement of varices by endoscopic investigation at 4 weeks after treatment underwent additional B-RTO procedures.
Subjective symptoms and objective findings
Complications, if any, were examined during the following intervals: phase 1, initial 30 min during balloon occlusion and injection of sclerosant; phase 2, within 4 h after balloon deflation; phase 3, within 24 h after B-RTO; and phase 4, from 24 h through 10 days. In phase 1, while blood flow of the varices was blocked by the balloon and 5% EOI was permeated through, arterial blood pressure was measured every 2 min and patients were asked to report any symptoms every 5 to 10 min. Blood pressure and body temperature was measured every 8 h for the following 72 h. Chest roentgenogram was performed before and on the day after B-RTO. Technical complications during the procedure were carefully noted.
Changes in laboratory data
Hepatic and renal function tests including total bilirubin (T-Bil), indirect bilirubin (I-Bil), alanine aminotransferase, aspartate aminotransferase (AST), lactate dehydrogenase (LDH), choline esterase (ChE), blood ammonia, serum creatinine, serum urea nitrogen, platelet count, and percentage of prothrombin activity were analyzed 1 day and 1 week after B-RTO. Patients who required blood transfusion within 48 h before B-RTO or urgent B-RTO were excluded from the laboratory data evaluation study. Twenty-six patients were entered into this study.
Statistical analysis
Results are expressed as mean ± standard deviation unless otherwise stated. The correlation between increased LDH and infused amount of 5% EOI was tested by calculating the Pearson correlation coefficient (r). Analysis of multiple comparisons was performed by repeated measure one-way analysis of variance followed by Fisher PLSD test. The level of statistical significance was set at p < 0.05.
Results
Efficacy of B-RTO
Endoscopic investigation at 8 weeks after B-RTO demonstrated the disappearance of gastric varices in 35 of 38 patients (92%), and smaller varices were observed in the remaining three (8%). No patient died and no patient manifested bleeding or rebleeding from gastric varices within the 2-month follow-up period.
Complications of the B-RTO procedure
Technical complications
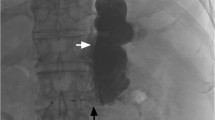
There were three technical complications in 43 B-RTO procedures. All occurred during 5% EOI infusion. The balloon ruptured in one patient when about 27 mL of 5% EOI had been just infused through the gastrorenal shunt. EOI was washed away immediately from the gastric varices through the left renal vein to the inferior vena cava. The patient complained of chest discomfort. Hemoglobinuria appeared within approximately 20 min and lasted for about 5 h. However, liver and renal functions remained unchanged (data not shown). B-RTO was repeated 4 weeks later. In another patient who had alcoholic liver cirrhosis, extravasation of 5% EOI was noted just after the injection, which spread gradually along the left diaphragm (Fig. 1). The patient complained of chest oppression and back pain. The varix was eradicated successfully despite this complication. In another patient, the balloon portion of the catheter slipped off the gastrorenal shunt and was displaced into the left renal vein when just after starting the 5% EOI injection. B-RTO was successfully completed on the same day by infusing 5% EOI more slowly. This patient had a prominent but tapering wedge-shaped gastrorenal shunt. The left renal vein was not affected and renal function did not change.
Clinical complications
Subjective symptoms reported within 10 days of B-RTO are listed in Table 2, and the objective findings are listed in Table 3. During phase 1, the major complaints were epigastralgia and chest pain. More than 20% of patients complained of nausea but vomiting was reported by only one patient. Just after the infusion of 5% EOI, 35% of patients developed transient hypertension. During phase 2, hemoglobinuria was observed in approximately 50% of the patients despite haptoglobin administration. During phase 3, the most frequent complication was fever. During phase 4, pleural effusion was noted on chest roentgenogram in five patients (Fig. 2) but disappeared within 7 days in all patients. One patient complained of mild dyspnea the next morning; arterial partial pressure of oxygen was low (53.8 mmHg), necessitating further investigation including chest CT. A small wedge-shape lesion was detected in the right lung, with pleural effusion (Fig. 3) suggestive of pulmonary infarction.
Ascites developed in three patients, which was mild in only two. Massive ascites with abdominal distention was noted in one patient, a 58-year-old male who had alcoholic cirrhosis. The patient complained of abdominal fullness and an inability to eat to satisfy his appetite for 10 days. However, 2 weeks after B-RTO, ascites began to decrease significantly by conservative treatment including a low-salt diet and administration of diuretics.
Follow-up endoscopic examination on postoperative day 7 displayed the development of small gastric ulcers in four patients (Fig. 4). These were located at the top of nodular gastric varices. Each ulcer was surrounded by a fine reticular pattern separating areas of raised congested mucosa (mosaic-like pattern). In one patient, ulceration was accompanied by a fresh blood clot in the stomach and a small clot at the bottom of the ulcer, which indicated gastric hemorrhage from the ulcer induced by B-RTO. Blood transfusion was not needed in this patient. All ulcers showed rapid healing without apparent fold convergency formation by conservative therapy in about 1 week. The localized mucosal changes with a mosaic-like pattern and congestion in the area of treated gastric varices were observed in 39 of 43 B-RTO procedures (91%) including the four ulcers described above. All 35 patients in whom gastric varices were completely eradicated showed the mosaic-like pattern on the treated varices. One patient complained of tarry stool 6 days after B-RTO. Endoscopic examination showed exacerbation of portal hypertensive gastropathy (PHG) and diffuse oozing in the antral region. There were no ulcers or erosions. Oozing was resistant to conservative treatment. Repeated series of sclerotherapy with polidocanol were needed for hemostasis.
Endoscopic examination demonstrating a small gastric ulcer (arrowhead) on the top of a successfully treated gastric varix on postoperative day 7. Fresh blood clots are present in the stomach. Note the small clot at the bottom of the ulcer. The ulcer was surrounded by mosaic-like pattern of mucosal change, i.e., localized PHG (arrows).
Changes in laboratory data
Laboratory data are presented in Table 4. LDH, AST, T-Bil, and I-Bil were increased on day 1 and returned to normal on day 7. LDH isozyme 1 + 2, which is influenced by hemolysis, was 67.4 ± 7.1% on day 1. Serum alanine aminotransferase remained unchanged on day 1. To further investigate the influence of hemolysis on LDH, we assessed the relation between LDH and the administered volume of 5% EOI, which is potent inducer of hemolysis [17]. The amount of infused 5% EOI was 27.6 ± 12.8 mL. The increase in LDH (LDHday 1 −LDHday 0) correlated significantly with the infused amount of 5% EOI (Fig. 5). Levels of serum creatinine, serum urea nitrogen, platelet count, ChE, and blood ammonia did not change in our patients who had been treated with B-RTO.
Discussion
B-RTO is an effective therapy for gastric fundic varices [3, 9, 10, 11, 12, 13, 14, 15, 16]. We confirmed the efficacy of this procedure in 35 of 38 gastric varices (92%) and reported novel and significant complications in detail.
Pulmonary glue emboli may occur after gastric variceal sclerotherapy [18]. Gastric varices generally drain directly into a large vein such as the left renal vein or inferior vena cava and lack drainage into intervening small veins. Therefore, sclerosant or fragments of blood clots could flow out and lodge into the pulmonary artery from a gastrorenal shunt or gastrocaval shunt after balloon deflation. However, only one patient developed pulmonary infarction as demonstrated by symptoms and confirmed by CT. Fortunately, the infarct seen in the present study was small and located in the lung periphery, and the ventilatory impairment in this patient was reversible. Arterial partial pressure of oxygen recovered within 7 days to 78.5 mmHg, suggesting that, in addition to pulmonary infarction, some other mechanisms could be involved in ventilatory impairment. For instance, ethanolamine oleate might have impaired pulmonary gas exchange, possibly by alveolar wall edema and lung congestion [19], which was detected by our routine CT. Thus, the influence of B-RTO on ventilatory function needs further investigation including a proper assessment of the incidence of pleural effusion. Routine check of blood gas analysis and chest roentgenogram is recommended in all patients on the day after B-RTO.
Complications that occurred during phase 4, such as PHG and ascites, may be implicated in the increase in portal pressure. Previous studies have shown that obliteration and eradication of esophageal varices by endoscopic sclerotherapy can result in the development or worsening of the mosaic-like pattern of portal hypertensive gastric mucosa [20, 21, 22, 23]. As demonstrated by one patient who developed hemorrhagic PHG, obliteration-induced PHG is one of the undesirable outcomes of B-RTO. Bleeding from PHG is likely to be resistant to conservative therapy and may require subsequent endoscopic therapy or TIPS. The effects of B-RTO on PHG should be determined in future studies.
The incidence of gastric ulcers on treated gastric varices was estimated at 9% of B-RTO procedures (Table 3). These ulcers were small and round and surrounded by the mosaic-like mucosal changes and red marks. Endoscopically they resembled the lesions of PHG, although they were localized on the obliterated gastric varices and immediately surrounding mucosa. These “localized PHGs” and ulcers with unique appearance were likely due to obliteration of both gastric varices and their out-flow collaterals modifying the mucosal perfusion and causing congestion of regional mucosa. Features of localized PHG had disappeared before endoscopy at 8 weeks, indicating improvement of mucosal perfusion within several weeks, possibly by the development of novel collaterals to release the localized congestion, although the exact mechanism is unknown at present. Localized PHG was observed in all patients in whom fundic varices were subsequently eradicated. Thus, absence of this lesion might be considered a marker of unsuccessful treatment. Based on our experience, we recommend periodic follow-up endoscopy, especially at approximately day 7.
One group recently reported amelioration of ascites in two patients after B-RTO, possibly due to improvement of hepatic function reserve and hypoalbuminemia [24]. Similarly, in our study, the rate of ascites retention was only 7%. Only one patient had to stay longer in hospital due to ascites. Thus, despite obliteration of the major part of the portosystemic shunt, the incidence or severity of ascites after B-RTO was not high. B-RTO can improve hepatic encephalopathy through increasing portal blood flow [11, 13]. In the present series, one patient developed a transient grade II hepatic encephalopathy on the day after B-RTO, although this occurred because of high-grade fever and subsequent dehydration. Thus, the development of encephalopathy on the day after B-RTO could have been avoided by sufficient hydration.
Our results showed that increased LDH correlated with the amount of infused 5% EOI. Ethanolamine oleate may increase LDH by inducing intravascular hemolysis [17]. B-RTO resulted in worsening of T-Bil, I-Bil, and AST, which was associated with hemolysis. Renal function was not affected by the B-RTO procedure in our study. However, a close assessment of renal function is important because ethanolamine oleate causes a transient decrease in renal arterial blood flow, hemolytic nephropathy, and tubular necrosis [17], and because deterioration of renal function is frequently seen in cirrhotic patients [25].
In summary, we demonstrated in the present study that the major complications of B-RTO included transient increases in arterial blood pressure, hemoglobinuria, and fever. Further, small pulmonary infarction, pleural effusion, ascites, gastric ulcers with unique appearance and location, and severe PHG were noted in a minority of patients. Most of these complications were mild in nature and reversible. All data indicating deterioration on day 1 was reversed within 7 days. We confirmed the safety and efficacy of B-RTO in this series. If this rate and mildness of complications are confirmed in further series of patients with different etiologies and severities, this technique may become the standard treatment for fundic varices.
References
SK Sarin D Lahoti SP Saxena et al. (1992) ArticleTitlePrevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients Hepatology 16 1343–1349 Occurrence Handle1:STN:280:ByyD2sjht1M%3D Occurrence Handle1446890
N Akiyoshi H Shijo T Iida et al. (2000) ArticleTitleThe natural history and prognostic factors in patients with cirrhosis and gastric fundic varices without prior bleeding Hepatol Res 17 145–155 Occurrence Handle10.1016/S1386-6346(99)00072-8 Occurrence Handle10707008
H Kanagawa S Mima H Kouyama et al. (1996) ArticleTitleTreatment of gastric fundic varices by balloon-occluded retrograde transvenous obliteration J Gastroenterol Hepatol 11 51–58 Occurrence Handle1:STN:280:BymB28rkvVU%3D Occurrence Handle8672742
G Shiha SS El-Sayed (1999) ArticleTitleGastric variceal ligation: a new technique Gastrointest Endosc 49 437–441 Occurrence Handle1:STN:280:DyaK1M3hvVWhsg%3D%3D Occurrence Handle10202055
K Barange JM Peron K Imani et al. (1999) ArticleTitleTransjugular intrahepatic portosystemic shunt in the treatment of refractory bleeding from ruptured gastric varices Hepatology 30 1139–1143 Occurrence Handle10.1002/hep.510300523 Occurrence Handle1:STN:280:DC%2BD3c%2FgsVKjsw%3D%3D Occurrence Handle10534333
T Kakio T Ito K Sue et al. (1993) ArticleTitleHemostasis of gastric variceal hemorrhage by transileocoecal and transhepatic obliteration Acta Med Okayama 47 39–43 Occurrence Handle1:STN:280:ByyB3M3mtlI%3D Occurrence Handle8460553
YH Huang HZ Yeh GH Chen et al. (2000) ArticleTitleEndoscopic treatment of bleeding gastric varices by N-butyl-2-cyanoacrylate (Histocryl) injection: long-term efficacy and safety Gastrointest Endosc 52 160–167 Occurrence Handle10.1067/mge.2000.104976 Occurrence Handle1:STN:280:DC%2BD3cvlslyhuw%3D%3D Occurrence Handle10922085
SK Sarin (1997) ArticleTitleLong-term follow-up of gastric variceal sclerotherapy: an eleven-year experience Gastrointest Endosc 46 8–14 Occurrence Handle1:STN:280:ByiH3crmsFA%3D Occurrence Handle9260698
F Chikamori S Shibuya Y Takase et al. (1996) ArticleTitleTransjugular retrograde obliteration for gastric varices Abdom Imaging 21 299–303 Occurrence Handle10.1007/s002619900068 Occurrence Handle1:STN:280:BymB28bnslY%3D Occurrence Handle8661570
T Sonomura M Sato K Kishi et al. (1998) ArticleTitleBalloon-occluded retrograde transvenous obliteration for gastric varices: a feasibility study Cardiovasc Intervent Radiol 21 27–30 Occurrence Handle10.1007/s002709900206 Occurrence Handle1:STN:280:DyaK1c7ktFygsA%3D%3D Occurrence Handle9473542
S Hirota S Matsumoto M Tomita et al. (1999) ArticleTitleRetrograde transvenous obliteration of gastric varices Radiology 211 349–356 Occurrence Handle1:STN:280:DyaK1M3ksFWqtQ%3D%3D Occurrence Handle10228513
K Koito T Namieno T Nagakawa et al. (1996) ArticleTitleBalloon-occluded retrograde transvenous obliteration for gastric varices with gastrorenal or gastrocaval collaterals AJR 167 1317–1320 Occurrence Handle1:STN:280:ByiD28joslI%3D Occurrence Handle8911204
F Chikamori N Kuniyoshi S Shibuya (2000) ArticleTitleTransjugular retrograde obliteration for chronic portosystemic encephalopathy Abdom Imaging 25 567–571 Occurrence Handle10.1007/s002610000046 Occurrence Handle1:STN:280:DC%2BD3M%2FjsFCqug%3D%3D Occurrence Handle11029085
T Akahane T Iwasaki N Kobayashi et al. (1997) ArticleTitleChanges in liver function parameters after occlusion of gastrorenal shunts with balloon-occluded retrograde transvenous obliteration Am J Gastroenterol 92 1026–1030 Occurrence Handle1:STN:280:ByiA38%2Fmslc%3D Occurrence Handle9177524
F Chikamori N Kuniyoshi S Shibuya et al. (2000) ArticleTitleShort-term hemodynamic effects of transjugular retrograde obliteration of gastric varices with gastrorenal shunt Dig Surg 7 332–336 Occurrence Handle10.1159/000018874
A Matsumoto N Hamamoto T Nomura et al. (1999) ArticleTitleBalloon-occluded retrograde transvenous obliteration of high risk gastric fundic varices Am J Gastroenterol 94 643–649 Occurrence Handle10.1111/j.1572-0241.1999.00928.x Occurrence Handle1:STN:280:DyaK1M7osVCnuw%3D%3D Occurrence Handle10086645
H Wada M Hashizume H Yamaga et al. (1990) ArticleTitleHemodynamic and morphological changes in the dog kidney after injection of 5% ethanolamine oleate into the superior vena cava Eur Surg Res 22 63–70 Occurrence Handle1:CAS:528:DyaK3cXlvVCnt7Y%3D Occurrence Handle2384125
AA Palejwala HL Smart M Hughes (2000) ArticleTitleMultiple pulmonary glue emboli following gastric variceal obliteration Endoscopy 32 S1–S2 Occurrence Handle10.1055/s-2000-9180 Occurrence Handle1:STN:280:DC%2BD3c7ltlWnsg%3D%3D Occurrence Handle10691281
S Vallgren GH Sigurdsson G Moberger et al. (1988) ArticleTitleInfluence of intravenous injection of sclerosing agents on the respiratory function Acta Chir Scand 154 271–276 Occurrence Handle1:CAS:528:DyaL1cXltVKjsrY%3D Occurrence Handle3376686
K Kotzampassi E Eleftheriadis H Aletras (1990) ArticleTitleThe ‘mosaic-like’ pattern of portal hypertensive gastric mucosa after variceal eradication by sclerotherapy J Gastroenterol Hepatol 5 659–663 Occurrence Handle1:STN:280:By2D3czks1Y%3D Occurrence Handle2129836
SK Sarin HM Shahi M Jain et al. (2000) ArticleTitleThe natural history of portal hypertensive gastropathy: influence of variceal eradication Am J Gastroenterol 95 2888–2893 Occurrence Handle10.1111/j.1572-0241.2000.03200.x Occurrence Handle1:STN:280:DC%2BD3crgt12hsw%3D%3D Occurrence Handle11051364
H Boldys T Romanczyk M Hartleb et al. (1996) ArticleTitleShort-term effects of variceal sclerotherapy on portal hypertensive gastropathy Endoscopy 28 735–739 Occurrence Handle1:STN:280:ByiC2MbptV0%3D Occurrence Handle9007425
T Iwao A Toyonaga K Oho et al. (1997) ArticleTitlePortal-hypertensive gastropathy develops less in patients with cirrhosis and fundic varices J Hepatol 26 1235–1241 Occurrence Handle10.1016/S0168-8278(97)80457-6 Occurrence Handle1:STN:280:ByiA2MvjtFE%3D Occurrence Handle9210609
T Fukuda S Hirota S Matsumoto et al. (2004) ArticleTitleApplication of balloon-occluded retrograde transvenous obliteration to gastric varices complicating refractory ascites Cardiovasc Intervent Radiol 27 64–67 Occurrence Handle15109232
PY Martin RW Schrier (1997) ArticleTitlePathogenesis of water and sodium retention in cirrhosis Kidney Int 59 S43–S49 Occurrence Handle1:CAS:528:DyaK2sXktF2js7c%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimoda, R., Horiuchi, K., Hagiwara, S. et al. Short-term complications of retrograde transvenous obliteration of gastric varices in patients with portal hypertension: effects of obliteration of major portosystemic shunts. Abdom Imaging 30, 306–313 (2005). https://doi.org/10.1007/s00261-004-0270-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-004-0270-8