Abstract
Sinistral portal hypertension, a rare and localized form of portal hypertension, is the result of splenic vein thrombosis or obstruction and may cause gastrointestinal hemorrhages from the esophageal and gastric varices. This report presents two cases (69- and 10-year-old females) of bleeding gastric varices. The patients were diagnosed as having sinistral portal hypertension. Splenic artery embolization was performed in both patients to overcome intractable bleeding, and the clinical outcome was good.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Sinistral portal hypertension (SPH) is a clinical syndrome that results from splenic vein thrombosis (SVT). The main causes of isolated SVT are chronic pancreatitis and carcinoma of the pancreas. Occasionally, SVT has been described in relation to acute pancreatitis, renal neoplasm, pseudocysts, surgery, celiac and splenic artery aneurysms, retroperitoneal fibrosis, and retroperitoneal abscess [1]. SVT increases the pressure in a preocclusive area and may be complicated by bleeding gastroesophageal varices and splenomegaly. The diagnosis of SPH necessitates accompanying normal liver functions [2]. Splenectomy is the treatment option in patients with intractable bleeding. In contrast, in patients with high surgical risks, splenic artery embolization is an alternative to surgery, at least in emergency conditions. We report two patients with bleeding gastric varices due to SPH and discuss the clinical relevance.
Case reports
Case 1
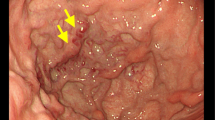
A 69-year-old woman was admitted to our hospital with a history of hematemesis and melena for 3 days. On admission, concomitant diseases were hypertension, coronary artery disease, hyperthyroidism, and morbid obesity. The patient was initially evaluated with esophageal gastroendoscopy to establish the bleeding point, which showed varicose structures in the esophagogastric junction, gastric cardia, and along the major curvature of the stomach (Fig. 1). Laboratory findings were normal for liver function tests, whereas hemoglobin was 9.7 g/dL, hematocrit was 29%, and platelet count was 147,000/mm3. Endoscopic ultrasonography (US) showed varices in the esophagogastric junction (Fig. 2). Abdominal computed tomography (CT) depicted normal liver parenchyma, lobulated lesions on the stomach wall, and vascular collaterals around the splenic hilus (Fig. 3). Magnetic resonance (MR) portography demonstrated vascular collaterals near the splenic hilus, splenorenal shunt, and SVT, with patent portal and superior mesenteric veins (Fig. 4A). The council of gastroenterology, general surgery, and radiology decided to treat the patient by an endovascular method instead of with splenectomy because of the patient’s poor clinical condition. Digital subtraction angiography showed SVT, gastric varices localized at the fundus of the stomach, drainage of splenic blood through short gastric veins, and splenorenal shunt (Fig. 4B). Portal and superior mesenteric veins were patent. The splenic artery was embolized with an 8-mm-diameter detachable balloon after perforating the pancreatic arteries through a catheter positioned in the splenic artery (Fig. 5). After embolization, there was no blood flow in the distal segment of the splenic artery. During the follow-up in the hospital, the patient had no episode of bleeding. She had only nausea and pain for a few days. On CT obtained 1 month later, the spleen was infarcted at a ratio of 40% (Fig. 6). Endoscopic examination showed that gastric varices were functioning at a significant decrease. The patient was followed by routine clinical visit and has been free of bleeding for 9 months.
Case 2
A 10-year-old girl with intermittent hematemesis for 3 years was admitted to our hospital. The patient had no history of pancreatitis or surgery. Levels of protein C, factor V, and protein S were within normal ranges. Doppler US showed only splenomegaly in 2001. Because of a history of intermittent bleeding, barium studies and Doppler US were repeated. Barium studies showed lobulated filling defects due to gastric varices (Fig. 7), and Doppler US demonstrated widespread portosystemic shunt, perigastric collaterals, splenorenal shunt, and dilated coronary and umbilical vein with splenomegaly. Esophagoscopy and gastroscopy showed varices at the fundus and cardia of the stomach. Liver biopsy was normal. While in the hospital, the patient experienced life-threatening variceal bleeding. Therefore, emergency angiographic examination was done, which showed a high degree of splenic vein stricture with poststenotic dilatation and gastric varices (Fig. 8). Portal and superior mesenteric veins were patent (Fig. 9). Because of low blood levels and the patient’s poor condition, (hemoglobin, 8.9 g/dL; hematocrit, 26.5%; platelet count, 63,000/mm3), splenic artery embolization was performed by using push coils (Fig. 10). No procedure-related complications occurred. Bleeding stopped immediately after embolization. The patient’s clinical condition improved rapidly. The surgeon decided to remove spleen, and the patient underwent an uneventful splenectomy 10 days later. During the operation, a lymph node that compressed the splenic vein was found near the stenotic area. Histopathologic analysis of this lymph node demonstrated reactive hyperplasia. During clinical visits over the 6 months, she was free of bleeding.
Discussion
Esophageal varices due to portal hypertension are the common causes of upper gastrointestinal hemorrhage. Most varices are related to cirrhosis and intrahepatic fibrosis leading to sinusoidal obstruction or obstruction beyond the sinusoid. Portal hypertension and variceal hemorrhage due to extrahepatic obstruction is uncommon. A rare, often unrecognized, condition is splenic vein occlusion that may lead to a localized form of portal hypertension known as SPH. SVT often occurs without evidence of SPH, and SPH can occur in the absence of bleeding gastric varices. The exact incidence of each is unknown. Madsen et al. reported one of the largest series, with 209 cases of SPH, 115 of which showed upper gastrointestinal bleeding [3]. In our patients, one with splenic vein thrombosis and the other with splenic vein stenosis, caused SPH accompanied by gastric varices and bleeding.
Splenic vein obstruction may result from intrinsic thrombosis or, more commonly, from extrinsic compression of splenic vein by a variety of benign and malign conditions, including chronic pancreatitis (most common), acute pancreatitis, pancreatic carcinoma, pancreatic pseudocysts, metastatic disease, lymphoma, surgery, celiac and splenic arterial aneurysms, retroperitoneal abscess, and fibrosis [1, 3, 4, 5]. Intrinsic thrombosis of the splenic vein may be idiopathic or may result from polycythemia or other myeloproliferative disorder. Sakorafas et al. detected 34 SPHs (7%; 12 with gastric varices) in 484 patients with chronic pancreatitis [2]. One of our patients had no etiologic factor for SVT, which was considered idiopathic. The other patient had splenic vein stenosis due to reactive hyperplastic lymph node compression.
Splenic vein occlusion is often clinically silent but may cause localized venous hypertension that can result in splenomegaly, anemia, abdominal pain, and upper gastrointestinal hemorrhage due to gastric varices. SVT should be suspected in patients with splenomegaly and a history of gastrointestinal hemorrhage with normal liver tests.
In patients with splenic vein obstruction, increased pressure in the splenic vein before the obstruction leads to reversal of flow through the short gastric veins into the gastric fundus and lower esophageal veins. These patients have normal portal pressure; however, venous blood from the dilated fundal plexus can enter the portal venous system through the coronary vein without producing uphill esophageal varices. This leads to prominent varices in the fundus of the stomach but few in the lower esophagus. Unlike the classic feature of portal hypertension, SVT is characterized by isolated varices in the gastric fundus without associated varices in the esophagus (Fig. 11). Rarely, a second pathway may be seen that involves the portosystemic shunt and develops along the lower end of the esophagus to the azygous system. Other routes of collateral blood flow are hepatopetal flow from the left gastroepiploic vein to the right gastroepiploic vein and hepatopetal flow from the left gastroepiploic vein to the left colic vein [6].
Diagnostic procedures for SPH include barium studies, esophagogastroduodenoscopy, endoscopic US, Doppler US, CT, and MR imaging. Selective splenic arteriography is the gold standard for diagnosis of SVT [7]. Barium studies show gastric varices that appear as broad, serpentine, redundant filling defects or clusters of polypoid defects simulating thickened folds. Esophagogastroduodenoscopy and endoscopic US are useful for determining the gastric varices. CT, US, and MR imaging may also visualize deeper intramural and perigastric varices, normal liver parenchyma, splenomegaly, and thrombus in the splenic vein. Gastric varices are usually recognized on CT as enhancing, well-defined, round or tubular densities on the posterior medial wall of the gastric fundus. The angiographic diagnosis of SVT includes nonopacification of a segment or an entire length of splenic vein, despite visualization of the intrasplenic or hilar veins and portal vein. Delayed images show normal filling of a patent portal vein without evidence of esophageal varices because blood is diverted from the fundal plexus through the coronary vein to the portal venous system, thus bypassing the obstructed splenic vein. Splenomegaly, perigastric and perisplenic varices, and portosystemic shunts also can be visualized by angiography.
Therapeutic decisions should be based on the patients’ clinical, laboratory, and imaging findings. Whereas splenectomy is clearly indicated in symptomatic SVT, the role of prophylactic splenectomy in asymptomatic patients with gastric varices remains controversial [2]. In a study by Loftus et al., 25 of 37 patients with SPH underwent splenectomy [8]. The 3-year survival rates were similar between patients who did (78%) and did not (64%) undergo splenectomy. Four patients (16%) who had splenectomy rebled, but not due to SPH. Among those who did not undergo splenectomy, six patients (50%) presented with bleeding, and only three had bleeding due to SPH. This study did not demonstrate significant differences in overall survival rate and the number of bleeding episodes between patients who did and did not undergo splenectomy. This finding likely was related to the associated disease processes. They concluded that patients with minimal or no transfusion requirements, no prior episodes of gastrointestinal bleeding, and normal laboratory values can be followed expectantly. Sakorafas et al. found that 12 (35%) gastric or gastroesophageal varices in 34 patients with SPH was due to chronic pancreatitis [2]. In their study, splenectomy was performed in 23 patients. No patient with SVT treated with splenectomy developed gastrointestinal bleeding. In contrast, only one of the 11 patients not treated with splenectomy developed worsening gastric varices that bled and caused death. Although surgical splenectomy cures most of these patients, immediate risks of surgery can be considerable and, in the long term, the removal of the entire spleen can lead to a variety of immunologic deficits. Disadvantages of splenectomy include associated operative mortality and morbidity rates, recurrent bleeding, and the potential for overwhelming postsplenectomy sepsis. Splenectomy in the presence of SVT or obstruction can be difficult and fraught with the risk of severe hemorrhage. Large perigastric varices make ligation of the short gastric veins potentially difficult and dangerous. Some investigators have suggested immediate preoperative splenic artery embolization to decrease vascular inflow to the spleen [2]. Splenic artery embolization also has been reported as an alternative treatment for gastric variceal bleeding in the setting of SVT [2, 9, 10]. However, this technique can be complicated by pain, partial infarction of the stomach and pancreas, splenic abscess, overwhelming pneumonia, septicemia, hematoma, delayed rupture of the spleen, and death [11, 12]. When the splenic embolization method is performed with an aseptic technique, antibiotic prophylaxis, and limited volume embolization of spleen, rates of morbidity and mortality can be decreased significantly. Most complications occur when embolization is directed to obtain complete or near-complete infarction [12].
In case of splenic vein stenosis, another alternative treatment is percutaneous transhepatic stent placement to the stenotic splenic vein. According to our research, there have not been enough studies about this method in the literature. Cekirge et al. reported one case of splenic vein stent placement after TIPS procedure who had gastric variceal bleeding due to splenic vein stenosis and alcoholic liver disease [13]. The indications for splenic vein stent placement have not been clarified. Our second patient who had splenic vein stenosis was a child. There have been no reports in which investigators have assessed the long-term patency of venous intravascular stents in children. Investigators were hesitant to deploy stents for two reasons. First, because children are expected to grow and the stent is relatively fixed in size, the stent ultimately could function as a stenosis. Second, all stents are susceptible to intimal hyperplasia, which may lead to repeat stenosis [14]. For these reasons, we did not consider this treatment.
In our patients, splenectomy was not performed because it is high-risk surgery, so proximal splenic artery embolization was performed. The advantage of proximal embolization is that is avoids total splenic infarct and related complications. The spleen continues to supply blood from the short gastric arteries (branches of the left gastroepiploic artery) through the anastomoses between the right gastroepiploic and left gastroepiploic arteries, which may preserve some or all of the splenic parenchyma. These collaterals may develop over time and increase left-side portal pressure. There is not enough knowledge about this subject in the literature because only a few cases treated by endovascular route have been reported [2, 9, 10].
In conclusion, SPH is a relatively unusual cause of gastroesophageal varices that has similar clinical but characteristic imaging features when compared with classic portal hypertension. Accurate diagnosis is important because simple removal of splenic venous return by surgery or and an endovascular method may eliminate life-threatening possible variceal bleeding. Splenic artery embolization must be considered in selected patients, especially in case of emergency or poor surgical candidates.
References
GR Evans AE Yellin FA Weaver PC Stain (1990) ArticleTitleSinistral (left-sided) portal hypertension Am Surg 56 758–763
GH Sakorafas MG Sarr DR Farley (2000) ArticleTitleThe significance of sinistral portal hypertension complicating chronic pancreatitis Am J Surg 179 129–133
MS Madsen TH Peterson H Sommer (1986) ArticleTitleSegmental portal hypertension Ann Surg 204 72–77
AR Moosa MA Gadd (1985) ArticleTitleIsolated splenic vein thrombosis World J Surg 9 384–390
P Bernades A Baetz P Levy et al. (1992) ArticleTitleSplenic and portal venous obstruction in chronic pancreatitis. A prospective longitudinal study of a medical-surgical series of 266 patients Dig Dis Sci 37 340–346
AG Little AR Moossa (1981) ArticleTitleGastrointestinal hemorrhage from left-sided portal hypertension Am J Surg 141 153–158
KA Illing RM Spitzer TK Oates (1997) ArticleTitleOptimal diagnosis of splenic vein thrombosis: brief clinical report Am Surg 63 1005–1006
JP Loftus DM Nagorney D Ilstrup AR Kunselman (1993) ArticleTitleSinistral portal hypertension. Splenectomy or expectant management Ann Surg 217 35–40
VG McDermott RE England GE Newman (1995) ArticleTitleBleeding gastric varices secondary to splenic vein thrombosis successfully treated by splenic artery embolization Br J Radiol 68 928–930
T Sato K Yamazaki J Toyota et al. (2000) ArticleTitleGastric varices with splenic vein occlusion treated by splenic arterial embolization J Gastroenterol 35 290–295 Occurrence Handle10.1007/s005350050348
L Back C Bagwell B Greenbaum et al. (1987) ArticleTitleHazards of splenic embolization Clin Pediatr 26 292–295
B Palsson M Hallen AM Forsberg A Alwmark (2003) ArticleTitlePartial splenic embolization: long term outcome Langenbecks Arch Surg 387 421–426
SH Cekirge JP Weiss RG Foster GK McLean (1993) ArticleTitleStent placement in a splenic vein stenosis after TIPS creation Radiology 189 197–198
K Yamakado A Nakatsuka N Tanaka et al. (2001) ArticleTitleMalignant portal venous obstructions treated by stent placement: significant factors affecting patency J Vasc Interv Radiol 12 1407–1415 Occurrence Handle1:STN:280:DC%2BD3MjgtFWntg%3D%3D Occurrence Handle11742015
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cakmak, O., Parildar, M., Oran, I. et al. Sinistral portal hypertension; imaging findings and endovascular therapy. Abdom Imaging 30, 208–213 (2005). https://doi.org/10.1007/s00261-004-0231-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-004-0231-2