Abstract
Purpose
Despite the existence of various treatment options, the prognosis for patients with metastatic castration-resistant prostate cancer (mCRPC) remains unfavorable. One potential therapeutic approach is the use of [225Ac]Ac-PSMA-617, a targeted alpha therapy (TAT) that administers alpha-particle radiation specifically to prostate cancer cells expressing PSMA. In this study, we report the long-term survival outcomes of this novel therapy in a series of patients with mCRPC who have exhausted all standard treatment options.
Methods
The study enrolled patients with mCRPC who had shown resistance to standard lines of therapies, including next-generation anti-androgen therapies and taxane-based chemotherapies. These eligible patients received treatment with [225Ac]Ac-PSMA-617 at 100-150 kBq/kg doses administered every 8 weeks. The primary objective of the study was to assess overall survival (OS), while secondary objectives included evaluating radiological progression-free survival (rPFS), monitoring serum prostate-specific antigen (PSA) levels as a measure of biochemical response, and assessing adverse events using the CTCAE v5.0 grading system.
Results
Among the 63 initially enrolled patients, a total of 56 patients who had completed at least two cycles of [225Ac]Ac-PSMA-617 were included in this study. The mean age was 67 years (range, 39-87) and patients received a total of 204 cycles of [225Ac]Ac-PSMA-617 TAT. 91% of patients exhibited any PSA decline, with 67.8% experiencing a decline of 50% or more. The median follow-up was of 22 months (range: 6-59 months). Imaging-based disease progression was observed in 68% of patients, and 66% of patients succumbed to the disease. The median OS was 15 months (95% CI: 10-19). In univariate analysis, factors such as lack of >50% PSA decline (P=0.031), Eastern Cooperative Oncology Group (ECOG) performance status of 2 or higher (P=0.048), and radiological progression (rPD) (P<0.001) were found to be predictors of poor OS. However, in multivariate analysis, only rPD emerged as an independent prognostic factor with a hazard ratio (HR) of 8.264 (95% CI: 1.429-16.497, P=0.004). The estimated median rPFS was 9 months (95% CI: 7-15). Moreover, patients who demonstrated any PSA decline had a median rPFS of 10 months compared to only 3 months in patients without any PSA decline (multivariate HR: 6.749; 95% CI: 1.949-23.370; P=0.002). Fatigue was one of the most common treatment-emergent adverse events, with grades 1/2 occurring in 70% of patients and grades 3 or higher in 3.5% of patients. This fatigue was transient and resolved before the next treatment cycle. Additionally, approximately one-third of patients experienced xerostomia (grades 1/2: 32.1%).
Conclusion
[225Ac]Ac-PSMA-617 targeted alpha therapy, was found to be well-tolerated with acceptable adverse events and effective in the treatment of patients with end-stage mCRPC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate carcinoma (PCa) is a prevalent cancer among men and typically exhibits a slow-growing nature, responding well to standard therapy options [1]. However, approximately 10-20% of patients experience a breach in this indolent course, leading to aggressive metastasis after an initial response to androgen deprivation therapy, known as metastatic castration-resistant prostate carcinoma (mCRPC) [2]. Although multiple systemic therapies are available for mCRPC, the survival benefit remains limited to a few months [3]. Recent studies investigating radionuclide therapy targeting prostate-specific membrane antigen (PSMA) have shown promising results, both with beta and alpha emitters [2].
Even in patients with advanced disease, beta radionuclide therapy utilizing [177Lu]Lu-PSMA-617 has shown promising results, demonstrating encouraging biochemical and radiographic responses while maintaining low toxicity levels. The VISION trial, which showcased improved overall survival (OS) and progression-free survival (PFS), resulted in the FDA approval of [177Lu]Lu-PSMA-617 radioligand therapy for the treatment of PSMA-positive metastatic castration-resistant prostate cancer (mCRPC) [4]. However, the translation of these promising research outcomes into clinical practice has not been fully realized, as evidenced by a recent meta-analysis reporting non-response and disease progression in approximately 37.1% of patients [5]. Furthermore, even among responders, disease progression can still occur, and patients with extensive skeletal metastases may experience bone marrow failure [6]. These limitations have prompted the compassionate use of alpha-based radioligand therapy (RLT) as an alternative for patients who are refractory to beta RLT, owing to the favorable radiobiological characteristics associated with alpha emitters.
Targeted alpha therapy (TAT) utilizing [225Ac]Ac-PSMA has demonstrated clinical efficacy in previous studies, showing satisfactory biochemical responses and low toxicity profiles [7,8,9]. Several studies have already established the effectiveness and safety of TAT [10, 11]. A recent meta-analysis conducted by Lee et al. revealed that 61% of patients achieved a decline of more than 50% in serum prostate-specific antigen (PSA) levels, and 84% achieved any decline in PSA after undergoing [225Ac]Ac-PSMA-TAT [12]. These response rates were higher (46% and 75% for more than 50% decline and any decline in PSA, respectively) compared to the response rates observed in an earlier meta-analysis of [177Lu]Lu-PSMA-RLT therapy by Yadav et al. [5]. Even with this excellent biochemical response following [225Ac]Ac-PSMA-TAT, the median PFS and OS (8 and 12 months for [225Ac]Ac-PSMA-TAT and 11 and 14 months for [177Lu]Lu-PSMA-RNT were not that different from [177Lu]Lu-PSMA-RLT in recent meta-analyses [5, 12]. However, it should be noted that many of the studies included in the meta-analysis of [225Ac]Ac-PSMA-TAT had relatively short follow-up durations and involved patients who had previously received [177Lu]Lu-PSMA-RLT, which warrants caution when interpreting this comparison. Nonetheless, this raises the question of whether the clinical translation of the radiobiological properties of TAT into survival benefits may have been influenced by the toxicities associated with previous therapies, including the [177Lu]Lu-PSMA-RLT that patients had received.
In an effort to address this question, we aimed to investigate the long-term survival outcomes of patients who received [225Ac]Ac-PSMA-617 TAT as a compassionate treatment option, considering it as the final therapeutic approach available. It is worth noting that only a limited number of studies have examined the long-term survival outcomes associated with [225Ac]Ac-PSMA-617 TAT [13, 14]. Further, in order to assess the impact of prior [177Lu]Lu-PSMA-RLT on the outcomes of [225Ac]Ac-PSMA-617 TAT, we also conducted a comparison of the survival outcomes between a cohort of patients who were treatment-naïve for RLT and patients who had experienced treatment failure with prior [177Lu]Lu-PSMA-RLT.
MATERIALS AND METHODS
The retrospective cohort study conducted in this research was granted approval by the Institute's ethics review board under the reference number IEC-518/2018, RP-18/2018. The study was a collaborative effort between the Department of Nuclear Medicine and the Department of Medical Oncology at the All India Institute of Medical Sciences in New Delhi, India.
Patients
Each clinical case underwent a thorough discussion by the multidisciplinary tumor board, and it was collectively determined that referral to the Department of Nuclear Medicine for radionuclide therapy was appropriate. Prior to treatment, written informed consent was obtained from all patients, who were informed that the use of [225Ac]Ac-PSMA-617 was not yet approved for regular clinical practice and was being administered on compassionate grounds. This was done after conventional therapies had proven ineffective or at the patient's specific request. A summary of the treatment protocol is illustrated in Fig. 1.
Eligibility criteria for enrollment in the study
This study included patients diagnosed with prostate adenocarcinoma, confirmed through histological or cytological examination, who had undergone medical or surgical castration and experienced disease progression despite standard-line treatments. All patients had metastatic castration-resistant prostate cancer (mCRPC) and exhibited positive [68Ga]Ga-PSMA-PET with lesion uptake equal to or greater than that of the normal liver. Eligible patients met specific criteria, including adequate bone marrow, liver, and renal functions: a hemoglobin level above 8 g/dL, an absolute neutrophil count of at least 1.5 x 109/L, platelet count of 60 x 109/L or higher, bilirubin level below 1.5 times the upper normal limit, serum creatinine level below 1.6 mg/dL, and a glomerular filtration rate exceeding 40 mL/min/1.73 m2 (measured using the plasma sample method). Patients with Eastern Cooperative Oncology Group (ECOG) performance status ≤4 were included.
Progressive disease for study entry was defined as any one of the following:
-
1.
PSA progression: At least two subsequent PSA readings must be taken, with a minimum gap of one week between each. The values must have increased by at least 25%, and be a minimum of 2 ng/mL, from the initial baseline measurement.
-
2.
Soft tissue/visceral disease/bone metastases: The progression of the disease was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST 1.1) and Prostate Cancer Working Group 3 (PCWG3) criteria.
The study included patients who had undergone a minimum of two cycles of [225Ac]Ac-PSMA-617 therapy, administered at intervals of 8 weeks. The patients were divided into two groups based on their prior exposure to [177Lu]Lu-PSMA-617 radioligand therapy: Group I consisted of patients who had received prior [177Lu]Lu-PSMA-617 therapy, and Group II consisted of patients who had not been previously treated with radioligand therapy. Patients with severe medical conditions such as congestive heart failure, drug allergies, and cord compression, as well as those who declined to provide written informed consent, were excluded from the study.
[225Ac]Ac-PSMA-617 Dosing and administration
Patients in this study received [225Ac]Ac-PSMA-617 via intravenous infusion at intervals of 8 weeks. The administered dose ranged from 100-150 KBq/kg body weight (2.7-4.05μCi/Kg BW). To administer the dose, it was dissolved in 10 mL of 0.9% normal saline and given over a period of 5 minutes. Following the infusion, a flush with 20 mL of normal saline was performed. The treatment regimen was continued until the patients reached a cumulative dose of 74 megabecquerels (MBq) or 2000 microcuries (μCi).
Follow-up
Patients underwent regular monitoring and surveillance after each cycle of [225Ac]Ac-PSMA-617 therapy. Follow-up check-ups were scheduled at 2, 4, and 6-8-week intervals. During these check-ups, comprehensive assessments were conducted, including complete blood counts and liver and renal function tests, to closely monitor for potential side effects. Following the completion or discontinuation of therapy, monthly follow-ups were conducted. Additionally, [68Ga]Ga-PSMA PET/CT scans were performed after every 2-3 cycles or when deemed necessary. This could be in cases where new pain sites developed, there was a notable decrease or doubling of prostate-specific antigen (PSA) levels, or during follow-up visits after the completion or discontinuation of the [225Ac]Ac-PSMA-617 targeted alpha therapy (TAT) regimen.
Treatment outcome measures
The study's primary outcome measure was overall survival (OS). Secondary outcome measures included radiological progression-free survival (rPFS), the identification of factors predicting OS and rPFS, assessment of the PSA response rate (PSA-RR), evaluation of molecular response, determination of the radiological disease control rate (DCR), assessment of clinical response, and analysis of the adverse event profile.
Definitions of outcome measures
Overall survival: Time from the commencement of [225Ac]Ac-PSMA-617 therapy to death due to any cause or the date of the last evaluation.
Radiological progression-free survival: Duration between the start of treatment and either the documented progression of the disease as seen on imaging or the patient's death. Patients without any signs of tumor progression who were still alive at the time of analysis were censored at their latest tumor evaluation date.
Biochemical response: The treatment response was assessed using the PCWG3 criteria [15]. The PSA-RR (Prostate-Specific Antigen Response Rate) was calculated by determining the percentage of patients who experienced a decrease of at least 50% in their PSA levels from the baseline after completing 4 and 8 weeks of [225Ac]Ac-PSMA-617 RLT cycles. Additional evaluations were conducted at monthly intervals following the completion of the [225Ac]Ac-PSMA-617 treatment regimen.
Radiologic tumor response: Radiological tumor response assessment was conducted by a combination of soft tissue assessment as per RECIST 1.1 criteria and bone lesion assessment according to PCWG3 criteria using a comprehensive criterion “PCWG-modified RECIST 1.1” [16]. The proportion of patients who attained a complete response or partial response was defined as the objective response rate (ORR).
Radiological disease-control rate (rDCR): The percentage of patients who achieved either a response or stable disease according to the PCWG-modified RECIST1.1 criteria
Clinical response: Clinical response criteria were evaluated using Visual Analog Score (VAS) [17], Analgesic Score (AS) [17], Karnofsky Performance Status (KPS) [18], and Eastern Cooperative Oncology Group (ECOG) Performance Status. VAS was used to measure the intensity of pain and ranged from 0 (no pain) to 10 (intolerable pain) [17]. AS was measured according to the Urological Group of the European Organization of Research and Treatment of Cancer (EORTC, Protocol 30921), and was the product of two five-point scales (type of analgesic and frequency of administration) [17]. KPS ranged from 100 (no evidence of disease and no complaints) to 0 (dead) [18]. ECOG status ranged from 0 (fully active, able to carry out all pre-disease performance without restrictions) to 5 (dead) [18].
Toxicity: Treatment-related adverse events (AEs) were documented as per the National Cancer Institute-Common Terminology Criteria for Adverse Events, version 5.0 [19].
Discontinuation of [225Ac]Ac-PSMA-617 therapy
The administration of treatment was discontinued in the following circumstances: disease progression during the course of therapy, an unexpectedly rapid response prior to completing the treatment plan, or upon reaching the maximum prescribed cumulative dose of 74 MBq (2 mCi).
Statistical analysis
Categorical variables were represented as numbers and percentages, while continuous data were assessed for normality using the D'Agostino-Pearson test. Normally distributed continuous data were presented with mean, standard deviation, and range, while skewed data were described with median and interquartile range (IQR).
The chi-square test or Fisher-exact test was used to analyze categorical variables between different patient groups, and unpaired samples t-test (parametric) or Mann-Whitney U test (non-parametric) was used for continuous variables. Paired-samples t-test (parametric) or Wilcoxon signed-rank test (non-parametric) was employed to compare pre-and post-therapy parameters. Kaplan-Meier survival curves were generated, and the log-rank test was used to compare overall survival (OS) and progression-free survival (PFS) between the two groups. Cox proportional-hazards regression model was used to identify the factors affecting survival outcomes; all variables with P-value <0.1 on univariate Cox-regression analysis were included in the multivariate model. All statistical analyses were conducted with MedCalc software, and P-value <0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
A total of 63 consecutive patients with metastatic castration-resistant prostate cancer (mCRPC) were initially enrolled for [225Ac]Ac-PSMA-617 targeted alpha therapy (TAT) treatment between April 2018 and November 2022. However, 7 patients were excluded from the study as they received only a single cycle of [225Ac]Ac-PSMA-617 therapy for various reasons. Among these 7 patients, two, unfortunately, passed away after the first cycle, three were lost to follow-up, and two patients withdrew their consent after the first cycle of treatment. Eventually, the analysis included 56 patients who had received at least two cycles of [225Ac]Ac-PSMA-617 treatment. Among the 56 patients, 27 patients (48.2%) had experienced disease progression after prior treatment with [177Lu]Lu-PSMA-617 radioligand therapy, constituting Group I (Gr-I), while 29 patients (51.8%) chose upfront [225Ac]Ac-PSMA-617 therapy as their initial treatment option and constituted Group II (Gr-II).
The demographic characteristics, clinical profiles, and administered activity of [225Ac]Ac-PSMA-617 treatment were found to be comparable between the two groups, as indicated in Table 1. The majority of the patients (50 out of 56; 89%) had a Gleason score higher than 7. All patients had received more than two lines of standard treatment (LoTs), with 89.2% (50 out of 56) of patients having received at least one line of chemotherapy, and 25% (14 out of 56) of patients receiving two lines of chemotherapy. Residual or recurrent disease in the primary site was observed in 85.7% (48 out of 56) of patients. Bone metastases were present in 94.6% (53 out of 56) of patients, while 43% (24 out of 56) had visceral metastases. The pre-treatment levels of prostate-specific antigen (PSA) and the extent of disease observed on [68Ga]Ga-PSMA-PET/CT scans were similar between the groups of patients refractory to [177Lu]Lu-PSMA-617 therapy and those who were treatment-naïve, indicating a comparable extent of disease (P>0.05).
The last cut-off date for the follow-up was February 24, 2023. The median duration of follow-up from the initiation of [225Ac]Ac-PSMA-617 targeted alpha therapy (TAT) was 22 months, ranging from 6 to 59 months. In the group of patients who were refractory to [177Lu]Lu-PSMA-617 therapy, the median follow-up was 21 months (95% CI, 19.8-23.0), while in the [177Lu]Lu-PSMA-617-naïve group, the median follow-up was 22 months (95% CI, 18.3-24.8).
At the time of enrollment, none of the patients exhibited grade 3 toxicities. The average hemoglobin level was 10.8 g/dL (range: 9.9-11.7), the median platelet count was 219 lakhs/mm3 (range: 172.5-284.5), the average white blood cell (WBC) count was 6668.4/mcL (range: 6083-7252), and the average alkaline phosphatase level was 656.4 IU/mL (range: 42-5501). The median creatinine level was 0.76 mg/dL (range: 0.3-1.4).
Treatment
The median cumulative radioactivity administered during the treatment was 26.4 MBq (IQR: 15-39.9) [715 μCi, IQR: 403-1078 μCi]. A total of 204 cycles of [225Ac]Ac-PSMA-617 targeted alpha therapy (TAT) were given: 26 patients received up to 3 cycles, while the remaining 30 patients received 4 to 9 cycles (Table 2). The median injected cumulative activity of [225Ac]Ac-PSMA-617 TAT did not differ significantly between the two groups [Group I: 574 μCi (IQR: 430.7-877.2) vs. Group II: 792 μCi (IQR: 403-1078); P=0.215].
PSA response assessment
After receiving [225Ac]Ac-PSMA-617 treatment, 51 out of 56 patients (91%) experienced any decline in PSA levels, and 38 out of 56 patients (67.8%) showed a PSA decline of 50% or more. Subgroup analysis revealed that the PSA response rate (PSA-RR) was similar between the [177Lu]Lu-PSMA-617-naïve group and the [177Lu]Lu-PSMA-617-refractory group (20/29, 69% vs. 18/27, 66.6%, respectively; P=0.854).
Overall and radiological progression-free survival
Figure 2 provides an overview of the outcomes in the treatment cohort. Among the patients, 37 individuals (66%) died during the median follow-up period of 22 months. Specifically, there were 18 deaths (67%) in the [177Lu]Lu-PSMA-617-refractory group and 19 deaths (66%) in the [177Lu]Lu-PSMA-617-naïve group.
The median overall survival (OS) for the entire cohort was 15 months (95% CI, 10-19) as shown in Fig. 3A. Patients who had not previously received [177Lu]Lu-PSMA-617 therapy had a slightly higher median OS of 15 months compared to those who were refractory to prior [177Lu]Lu-PSMA-617 RLT, with a median OS of 10 months [HR: 1.124 (95% CI, 0.564-2.293); P=0.739] (Fig. 3B). Similarly, the estimated median radiological progression-free survival (rPFS) for the entire cohort was 9 months (95% CI, 7-15) as depicted in Fig. 4A. The median rPFS was 8 months in the [177Lu]Lu-PSMA-617-refractory group compared to 11 months in patients who received upfront [225Ac]Ac-PSMA-617 treatment (HR: 1.421; 95% CI: 0.733-2.758; P=0.297) (Fig. 4B).
In the univariate analysis, several factors were found to be predictive of poor overall survival (OS), including the absence of a ≥50% decline in prostate-specific antigen (PSA) levels (P=0.031), an Eastern Cooperative Oncology Group (ECOG) performance status ≥2 (P=0.048), and the presence of radiological progressive disease (rPD) (P < 0.001). However, in the multivariate analysis using the Cox-proportional hazards model, rPD emerged as the sole independent predictor of poor OS [HR: 8.264 (95% CI: 1.429-16.497); P=0.004]. This is further illustrated in Table 3 and Fig. 5A.
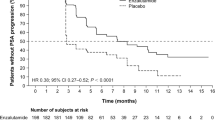
In terms of radiological progression-free survival (rPFS), the absence of any decline in prostate-specific antigen (PSA) levels was found to be a poor predictor [multivariate HR: 6.749, 95% CI: 1.949-23.370, P = 0.002]. Patients who demonstrated any PSA decline had an estimated median rPFS of 10 months, whereas those who did not experience any PSA decline had a median rPFS of only 3 months (P < 0.001) (Table 3, Fig. 5B).
Morphologic Response: PCWG-modified RECIST 1.1 criteria
Table 4 and Fig. 2 provide an overview of the morphologic response to [225Ac]Ac-PSMA-617 TAT. The objective response rate (ORR) was 14.2% (8 out of 56 patients), while the rDCR was 32.1% (18 out of 56 patients). Subgroup analysis revealed that 26% (7 out of 27) of patients in the [177Lu]Lu-PSMA-617-refractory group achieved an imaging-based rDCR, compared to 38% (11 out of 29) in the [177Lu]Lu-PSMA-617-naïve group (P=0.336). One patient (3.7%, 1 out of 27) who had previously received [177Lu]Lu-PSMA-RLT achieved a CR from [225Ac]Ac-PSMA-617 TAT. However, in the [177Lu]Lu-PSMA-617-naïve group, two patients initially achieved complete remission on interim evaluation but experienced disease relapse within a median duration of 13 months, resulting in the failure to sustain the CR. Disease progression was observed in 74% (20 out of 27) of patients in the [177Lu]Lu-PSMA-617-refractory group and 62% (18 out of 29) in the [177Lu]Lu-PSMA-617-naïve group. Among the 38 patients with disease progression, 33 experienced disease-specific deaths, while the remaining five patients were subjected to further treatments.
Clinical response
Table 5 demonstrates the significant reduction observed in the visual analog scale (VAS) score after treatment, with a median pre-therapy value of 8 compared to a post-therapy value of 5 (P < 0.001). Similarly, there was a notable decrease in the median analgesic score (AS) from 3 to 2 (P < 0.001). Furthermore, the median Karnofsky Performance Score (KPS) showed a significant improvement from 55 to 70 (P < 0.001), indicating enhanced functional status. Additionally, the Eastern Cooperative Oncology Group (ECOG) status exhibited a significant improvement, with the median score decreasing from 3 to 2 (P < 0.001).
Toxicity and adverse events
Table 6 provides information on treatment-emergent adverse events (AEs) observed during [225Ac]Ac-PSMA-617 targeted alpha therapy (TAT). The most commonly reported symptomatic side effect was fatigue, with grade 1/2 fatigue experienced by 70% of patients (39/56) and grade 3 fatigue observed in two patients (3.5%). Other frequent grade 1/2 symptomatic side effects included loss of appetite (43%), myalgia (38%), and xerostomia (32%). However, these side effects resolved within 5 weeks after treatment. Grade 1/2 xerostomia was observed only after a median of 3 cycles of [225Ac]Ac-PSMA-617 therapy and lasted for a median duration of 1 month. One patient experienced grade 3 ascites followed by pleural effusion and unfortunately died. Two patients discontinued treatment after the second cycle of [225Ac]Ac-PSMA-617 due to grade 2 fatigue and anorexia. Additionally, grade 2 constipation occurred in 13 patients, which was successfully managed with the use of laxatives.
Before initiating treatment with [225Ac]Ac-PSMA-617, grade 1/2 anemia was present in 52 patients (92.8%), but this percentage decreased to 7.1% (4/56) at the end of follow-up. Two patients (3.5%) had persistent grade 3 anemia throughout the follow-up period. Grade 1 leukopenia was observed in 12 patients (21.4%) at baseline and remained at a similar percentage (19.6%, 11/56) at the end of follow-up. Grade 3 leukopenia occurred in three patients (5.3%), but it was normalized in two patients (3.5%). Seven patients (12.5%) experienced grade 3 elevation in alkaline phosphatase (ALP), and all of these patients had extensive skeletal involvement. No grade 3/4 toxicities related to alanine aminotransferase or aspartate aminotransferase were reported. Only one patient (1.7%) experienced grade 3 nephrotoxicity. There was a significant improvement in mean baseline ALP levels after treatment [656.4 ± 900 IU/mL vs. 326 ± 530 IU/mL, P=0.032]. Statistically significant changes in other pre- and post-treatment parameters were observed, but these values remained within normal limits (Table 7). In patients with grade 3 hematological adverse events, treatment was temporarily halted and then reinitiated after the parameters improved.
Discussion
Currently, the FDA-approved radionuclide therapies for the treatment of metastatic castration-resistant prostate cancer (mCRPC) are [223Ra]RaCl2 (limited to skeletal metastasis) and [177Lu]Lu-PSMA-617. Among the different alpha emitters, bismuth-213 (with a half-life of 46 minutes) and actinium-225 have shown promising potential. However, when comparing the two, [225Ac]Ac-PSMA-617, with its longer half-life of 10 days, demonstrates a significantly higher therapeutic index, making it more suitable for clinical application.
Following the promising initial outcomes of targeted alpha therapy using [225Ac]Ac-PSMA-617 for metastatic castration-resistant prostate cancer (mCRPC) in a limited number of research centers worldwide [7,8,9,10, 13, 14], several clinical trials are currently in progress. In our short-term analysis, we investigated the preliminary clinical experience of [225Ac]Ac-PSMA-617 targeted alpha therapy in a cohort of 28 patients with mCRPC who had either exhausted or showed resistance to beta-emitting [177Lu]Lu-PSMA-617 radioligand therapy. Our findings revealed favorable treatment responses with minimal incidences of adverse effects [10].
The present study represents an extended cohort (N=56) investigation with an extended duration of follow-up (median follow-up duration of 22 months). Although it does not conform to the ideal design of a prospective, two-armed randomized controlled trial, this study provides a comprehensive and thorough comparison of the long-term survival outcomes associated with [225Ac]Ac-PSMA-617 targeted alpha therapy in two distinct groups of patients: those who were refractory to [177Lu]Lu-PSMA-617 and those who were treatment-naïve. The study ensures a complete matching of demographics, clinical parameters, and disease burden between both groups, thus strengthening the reliability of the findings.
This real-world study encompasses a diverse range of patients with varying ECOG performance statuses, and the administration of [225Ac]Ac-PSMA-617 targeted alpha therapy was based on compassionate grounds. In a clinical trial setting, many of these patients, who had a poor prognosis, may have been excluded from receiving this therapy. Unlike previous reports that primarily included patients with ECOG ≤2 [7,8,9, 13, 14], our study population consisted of 67.8% (38/56) of patients with ECOG 3/4. This study thus, reflected practical scenarios where patients often experience a decline in ECOG status to 3/4 after undergoing extensive prior treatments and exhibit unfavorable laboratory profiles. Despite the inclusion of a challenging patient cohort with mCRPC, our results are comparable to or even superior to studies involving more selectively chosen patients. We believe that this could be attributed to the enhanced radiobiological characteristics of Actinium-225. Furthermore, we propose that the findings of this real-world clinical study can be safely and effectively extrapolated to support individualized patient-centered management in future trial designs for patients with mCRPC.
In our study, a significant PSA decline of ≥50% was observed in 68% (38/56) of patients, and 91% (51/56) of patients experienced any PSA decline. The median time of disease progression was 9 months. Recent meta-analyses by Ballal et al. [20], Lee et al. [12], and Maa et al [11]. have reported greater than 50% PSA decline rates of approximately 59%, 61%, and 65.8%, respectively, in patients treated with [225Ac]Ac-PSMA targeted alpha therapy. These meta-analyses also indicated that over 80% of patients showed any PSA decline after receiving [225Ac]Ac-PSMA targeted alpha therapy. Notably, the response rates and PSA decline results observed with [225Ac]Ac-PSMA-617 treatment were higher compared to a prior meta-analysis for [177Lu]Lu-PSMA radioligand therapy by Yadav et al. [5] (46%) and a phase two clinical trial of [177Lu]Lu-PSMA-617 by Hofman et al. [21] (57%). Our data further demonstrated a positive association between any PSA decline and a PSA decline of ≥50% with improved survival outcomes. These findings are consistent with previous studies that consistently showed better progression-free survival with any PSA decline [7,8,9,10, 13, 14].
Disease progression is inevitable after a certain point for all anti-cancer treatment options including approximately 30% of patients with prostate cancer who develop resistance to [177Lu]Lu-PSMA-617 radioligand therapy [5]. A crucial question to consider is whether [225Ac]Ac-PSMA-617 therapy can improve disease control following progression with [177Lu]Lu-PSMA-617. In our study, the observed objective response rate (ORR) and disease control rate (DCR) with [225Ac]Ac-PSMA-617 were 14.2% and 32%, respectively. Interestingly, we found that the ORRs and DCRs were higher in patients who had not received prior [177Lu]Lu-PSMA-617 treatment (17% and 38%) compared to those who were refractory to [177Lu]Lu-PSMA-617 (11% and 26%). This trend aligns with our earlier short-term results, where patients who were naïve to [177Lu]Lu-PSMA-617 RLT exhibited superior molecular responses compared to those with previous exposure (78% vs. 38.5%) [10]. It is noteworthy that even among patients who had already experienced progression on [177Lu]Lu-PSMA-617 treatment, disease control was observed in 26% of cases. Moreover, these patients demonstrated an additional extended median overall survival of 10 months and a median progression-free survival of 8 months. This observation suggests that Actinium-225-based alpha particle treatment may overcome resistance in tumor cells that are resistant to beta particles, leading to therapeutic response.
According to a meta-analysis, the short-term evidence of [225Ac]Ac-PSMA-617 shows a molecular response rate of approximately 54% across seven studies involving 124 patients [20]. However, in the current study, only one patient achieved a sustained complete image-based response to treatment. Two patients without prior exposure to [177Lu]Lu-PSMA-617 RLT and with extensive bone metastases initially showed promising complete molecular responses but experienced disease progression within a median of 13 months after treatment. It is important to note that despite the favorable response (69% PSA-RR) and improved radiological progression-free survival (rPFS) (median 11 months) in the upfront [225Ac]Ac-PSMA-617 treatment group, it does not appear to have a significant impact on overall survival (OS) (median 15 months). Sathekge et al.[14] have also reported estimated median PFS and OS durations of 15.2 months (95% CI, 13.1-17.4) and 18 months (95% CI, 16.2-19.9), respectively in their cohort. Our findings, along with the results reported by Sathekge et al. [14], suggest that achieving sustained disease-free survival in these patients remains challenging. Despite a prolonged rPFS, it does not necessarily correspond to a prolonged OS. This emphasizes the need for further investigation into optimizing treatment strategies, considering factors such as treatment sequence, combination therapies, and individual patient characteristics. Alternatively, it could also imply that an upfront treatment with [225Ac]Ac-PSMA-617 treatment is not necessary except in circumstances where diffuse bone marrow involvement is present.
While there is increasing evidence supporting the superiority of [225Ac]Ac-PSMA-617 over [177Lu]Lu-PSMA-617 RLT, concerns regarding its toxicity persist. Xerostomia has been consistently reported as the most common treatment-related adverse event, accounting for approximately 77.1% of cases according to a recent meta-analysis of 201 patients [11]. However, all studies agree that dry mouth symptoms are transient, with only a limited number of cases (3.0%) reaching grade 3 xerostomia [11, 12]. In our previously published short-term data [10], we observed grade 1/2 xerostomia in 29% (8/28) of patients, and our long-term follow-up results showed a similar prevalence (32%). Importantly, none of our patients discontinued treatment due to xerostomia, unlike 10% of participants in other groups. Various techniques, such as saline irrigation and steroid injection, have been shown to improve salivary gland function and enhance treatment tolerability, but high-level evidence on different techniques is currently lacking in the literature [22].
The hematologic toxicities associated with [225Ac]Ac-PSMA-TAT in our study were predominantly of grade 1/2, with minimal occurrences of grade 3/4 toxicities as reported in other studies (ranging from 1.8% to 7%) [9, 10]. In contrast, Feuerecker et al. [23] reported higher rates of grade 3/4 hematological toxicities, including anemia, thrombocytopenia, and leukopenia (35%, 19%, and 27%, respectively). However, a pooled analysis of 6 studies by Ma et al. [11] reported relatively lower rates of grade 3 anemia, leukopenia, and thrombocytopenia (7.5%, 4.5%, and 5.5% respectively, out of 201 patients). Nephrotoxicity, specifically grade 3 impairment of kidney function, was observed in only 1.7% of our patients compared to rates reported by Sathekge et al. (5.6-6.8%) [9, 13, 14]. A meta-analysis by Ma et al. [11] also indicated that only 3.0% of 201 patients experienced grade 3 nephrotoxicity. Fatigue, dysgeusia, nausea, and constipation were additional toxicities observed at varying rates in different studies, but these were transient and did not disrupt the treatment regimen.
Limitations
Although the treatment protocol, follow-up, and patient groups based on prior exposure to [177Lu]Lu-PSMA-617 RLT were well-matched, the retrospective design and the low patient number of this study are its main limitations.
Conclusion
In conclusion, the use of [225Ac]Ac-PSMA-617 TAT in this cohort of heavily pre-treated mCRPC patients was seen to be safe with promising benefits in median OS and rPFS. Moreover, reasonable disease control and survival benefits were also observed even in patients refractory to [177Lu]Lu-PSMA-RLT. Further research is warranted to optimize treatment strategies, taking into account factors such as treatment sequence, combination therapies, and individual patient characteristics so as to contribute to improved overall survival.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Alam MR, Singh SB, Thapaliya S, et al. A Review of 177Lutetium-PSMA and 225Actinium-PSMA as emerging theranostic agents in prostate cancer. Cureus 2022;14(9):e29369. https://doi.org/10.7759/cureus.29369.
Labriola MK, Atiq S, Hirshman N, Bitting RL. Management of men with metastatic castration-resistant prostate cancer following potent androgen receptor inhibition: a review of novel investigational therapies. Prostate Cancer Prostatic Dis. 2021;24:301–9.
Lawal IO, Bruchertseifer F, Vorster M, Morgenstern A, Sathekge MM. Prostate-specific membrane antigen-targeted endoradiotherapy in metastatic prostate cancer. Curr Opin Urol. 2020;30:98–105.
Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1091–1103.
Yadav MP, Ballal S, Sahoo RK, Dwivedi SN, Bal C. Radioligand Therapy With 177Lu-PSMA for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Am J Roentgenol. 2019;213:275–85.
Kind F, Michalski K, Yousefzadeh-Nowshahr E, Meyer PT, Mix M, Ruf J. Bone marrow impairment during early [177Lu]PSMA-617 radioligand therapy: Haematotoxicity or tumour progression? EJNMMI Res. 2022;12:20.
Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225 Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J Nucl Med. 2016;57:1941–44.
Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225 Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J Nucl Med. 2017;58:1624–31.
Sathekge M, Bruchertseifer F, Knoesen O, et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46:129–38.
Yadav MP, Ballal S, Sahoo RK, Tripathi M, Seth A, Bal C. Efficacy and safety of 225 Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients. Theranostics. 2020;10:9364–77.
Ma J, Li L, Liao T, Gong W, Zhang C. Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2022;12:796657.
Lee DY, Kim Y il. Effects of 225Ac-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer: A Meta-Analysis. J Nucl Med. 2022;63:840–846.
Sathekge M, Bruchertseifer F, Vorster M, et al. mCRPC Patients Receiving 225 Ac-PSMA-617 Therapy in the Post–Androgen Deprivation Therapy Setting: Response to Treatment and Survival Analysis. J Nucl Med. 2022;63:1496–1502.
Sathekge M, Bruchertseifer F, Vorster M, et al. Predictors of Overall and Disease-Free Survival in Metastatic Castration-Resistant Prostate Cancer Patients Receiving 225Ac-PSMA-617 Radioligand Therapy. J Nucl Med. 2020;61:62–69.
Scher HI, Morris MJ, Stadler WM, et al. Trial Design and Objectives for Castration -Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18.
Goldmacher G. (n.d.) Imaging Response Criteria Training for PCWG-Modified RECIST 1.1. Published by Articulate® Storyline. https://www.articulate.com. Accessed 24 July 2022.
McCaffery M &Pasero C. Pain: Clinical Manual. 2nd ed. St Louis, MO: Mosby; 1999.
Crooks V, Waller S, Smith T, Hahn TJ. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol. 1991;46:139 –44.
Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Publish Date: November 27, 2017.
Ballal S, Yadav MP, Sahoo RK, Tripathi M, Dwivedi SN, Bal C. 225Ac-PSMA-617-targeted alpha therapy for the treatment of metastatic castration-resistant prostate cancer: A systematic review and meta-analysis. Prostate. 2021;81:580–91.
Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33.
Rathke, H., Kratochwil, C., Hohenberger, R. et al. Initial clinical experience performing sialendoscopy for salivary gland protection in patients undergoing 225Ac-PSMA-617 RLT. Eur J Nucl Med Mol Imaging. 2019;46:139–47.
Feuerecker B, Tauber R, Knorr K, et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur Urol. 2021;79:343–50.
Author information
Authors and Affiliations
Contributions
The study's conception and design involved contributions from all authors. Sanjana Ballal, Madhav P Yadav, Swayamjeet Satapathy, and Shobhana Raju were responsible for patient enrollment, treatment, follow-up, material preparation, data collection, and analysis. The initial draft of the manuscript was written by Sanjana Ballal and Madhav Prasad Yadav. Chandrasekhar Bal, Madhavi Tripathi, and Nishikant A Damle processed, reported the images, and evaluated responses to treatment, Dr. Ranjit Kumar Sahoo was the Medical Oncologist and referred patients for treatment.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose
Disclosures
The authors have nothing to disclose.
Conflict of Interest
The authors have no conflict of interest
Ethical Clearance
Ethical clearance received Ref. No IEC-518/2018, RP-18/2018.
Informed Consent
Written informed consent was obtained from all patients to participate in the study and for the use of clinical information to analyze data.
Support
The Indian Council of Medical Research, under project No. 3/2/3/96/2019/NCD-III, provided funding support for a portion of the manpower involved in this work.
Disclaimer
This work has not been submitted elsewhere as a full article and is not under consideration by any other journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ballal, S., Yadav, M.P., Satapathy, S. et al. Long-term survival outcomes of salvage [225Ac]Ac-PSMA-617 targeted alpha therapy in patients with PSMA-expressing end-stage metastatic castration-resistant prostate cancer: a real-world study. Eur J Nucl Med Mol Imaging 50, 3777–3789 (2023). https://doi.org/10.1007/s00259-023-06340-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06340-y