Abstract
Purpose
Our aim was to assess the prognostic value of [68 Ga]Ga-FAPI-04 positron emission tomography (PET) uptake in PDAC and to evaluate the correlation between in vivo lesional radioactivity with pathological characteristics of pancreatic ductal adenocarcinoma (PDAC).
Methods
We retrospectively analyzed treatment‐naïve PDAC patients who underwent preoperative [68 Ga]Ga-FAPI-04 PET/CT followed by pancreatectomy. The tracer uptake was determined as maximum tumor standardized uptake value (SUVmax), FAPI-avid tumor volume (FTV), total lesion FAP expression (TLF) as well total pancreatic uptake (TSUVmax), total FAPI-avid pancreatic volume (FPV), and total pancreatic FAP expression (TPF). Spearman’s correlation analysis was performed to evaluate the association between [68 Ga]Ga-FAPI-04 PET/CT imaging and ex vivo immunohistological FAP expression and pathological characteristics of surgical specimens (differentiation, size, vascularity, perineural invasion, and lymph node metastases). Kaplan–Meier and hazard ratio (HR, log-rank) methods were used to evaluate the prognostic value of [68 Ga]Ga-FAPI-04 PET/CT and clinicopathological factors.
Results
Thirty-seven surgical PDAC patients were included. The ex vivo expression of FAP was significantly associated with the tumor SUVmax and TLF. FAP expression was more abundant in poorly differentiated PDAC than in well- to moderately differentiated neoplasms. Tumor SUVmax or TLF and pancreatic TSUVmax or TPF were significantly correlated with tumor size, differentiation, and perineural invasion, respectively. SUVmax had a significant independent prognostic value for recurrence-free survival (HR = 2.46, P < 0.05), while [68 Ga]Ga-FAPI-04 TPF predicted overall survival (HR = 12.82, P < 0.05).
Conclusion
The in vivo [68 Ga]Ga-FAPI-04 uptake in localized PDAC showed a significant correlation with ex vivo FAP expression and aggressive pathological characteristics. [68 Ga]Ga-FAPI-04 PET/CT also presented a potential for postoperative prognostication of PDAC. Elevated fibroblast activity induced by obstructive pancreatitis might be associated with the patient's survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is one of the most aggressive solid malignant tumors. Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic malignancy, and its incidence is gradually increasing [1]. The prognosis for PDAC patients is poor, with a median survival of about 6 months and a 5-year survival rate of only 10% [1].
A majority of patients with resected PDAC remain susceptible to local recurrence or distant metastases, despite recent improvements in surgical approaches, systemic therapy, and follow-up strategies. Preoperative prediction methods are therefore urgently needed to enable better stratification of patients who will benefit from surgery [2].
PDAC is characterized by a complex desmoplastic stroma. Through direct or indirect effects on cancer cells and the tumor microenvironment, the stroma is considered to be a key factor in disease progression [3]. Targeting stromal components is a popular and promising strategy in PDAC treatment [4]. Cancer-associated fibroblasts (CAFs) are key components of the extracellular matrix of PDAC [3]. In PDAC, the function of CAFs remains controversial, as they exhibit both tumor-suppressive and tumor-promoting properties [5]. Fibroblast activation protein (FAP) was found to be highly and selectively expressed in CAFs [6]. Understanding the relationship between tumor FAP expression and tumor clinicopathological features will have important clinical significance. Besides, FAP was upregulated in acute/chronic pancreatitis [7]. PDAC patients with obstructive pancreatitis are more likely than non-pancreatitis individuals to experience early recurrences following curative pancreatectomy [8].
A popular imaging modality increasingly used in oncology research is [68 Ga]Ga-FAPI-04 PET/CT. However, the prognostic impact of [68 Ga]Ga-FAPI-04 PET/CT parameters on recurrence-free survival (RFS) and overall survival (OS) has been less frequently reported in PDAC. Therefore, this study aimed to investigate the in vivo [68 Ga]Ga-FAPI-04 uptake in primary PDAC lesions in relation to immunopathological characteristics including tumor grade, differentiation, invasion, and lymph node metastasis. In addition, we evaluated the prognostic value of preoperative [68 Ga]Ga-FAPI-04 PET/CT imaging parameters in PDAC lesions and pancreas tissue in patients with PDAC after curative surgery.
Methods and materials
Study population
We registered this study at ClinicalTrials.gov (NCT05275985) and received approval from the Institutional Ethics Committee of Peking Union Medical College Hospital (IRB protocol #ZS1810; Beijing, China). Informed written consent was provided by all participants before they participate in this study.
All the participants with suspected pancreatic masses without previous treatment underwent a preliminary diagnostic work-up that usually included clinical (age, gender, symptoms, weight changes in recent half-year [weight loss was considered present if there is a 2 kg or greater loss]), biochemical [including serum carbohydrate antigen 199 (CA-199)], and radiologic imaging with multi-detector chest-abdominal-pelvis contrast-enhanced computed tomography (CE-CT). The protocol of CE-CT imaging was listed in the supplemental materials. Local resectability was classified as resectable, borderline resectable, or locally advanced on CE-CT by an experienced radiologist (> 5 years’ experience) according to NCCN criteria [9]. Then, sixty-four consecutive treatment‐naïve patients (37 males, 27 females; mean age: 60.1 ± 10.8 years) with clinical suspicion of primary PDAC were recruited from June 2020 to November 2021. The included patients underwent preoperative 68 Ga-FAPI-04 PET/CT imaging within 1 week prior to surgery. As for the further clinicopathological and prognostic analysis in this study, the exclusion criteria were (1) the absence of surgical therapy; (2) no histopathological results, (3) non-PDAC lesions on histology, and (4) no follow-up data. The patients diagnosed in clinical stages I–III (according to the 8th edition of the American Joint Committee on Cancer) and who underwent curative surgical resection at our institution were analyzed retrospectively. All patients included were either resectable or borderline resectable (the latter was assessed as feasible for directly curative surgery by surgeons experienced in pancreatic surgery) by CE-CT.
PET/CT imaging protocol and image processing
The interval between CE-CT and PET/CT was 3–15 days. The labeling method of our institution 68 Ga-FAPI-04 has been described previously [10]. The radiochemical purity determined by thin-layer chromatography was > 95%. Patients do not require special preparation prior to the [68 Ga]Ga-FAPI-04 PET/CT examination. After intravenous injection of 2.22–2.96 MBq (0.06–0.08 mCi)/kg of [68 Ga]Ga-FAPI-04, images were acquired on a PET/CT scanner (Polestar m660, SinoUnion, Beijing, China) for 2 min/bed. CT parameters were 120 kV, 160 mA, pitch 1.3, slice thickness 2.5 mm, and rotation time 0.5 s. The PET/CT scan was performed from the top of the head to the upper thigh. An ordered subset expectation maximization algorithm (two iterations, 10 subsets, Gaussian filter with full-width half-maximum of 4 mm, 192 × 192 matrix) was used for reconstruction of all PET images, corrected for CT-based attenuation, random events, and scattering.
Imaging analysis
All images were independently reviewed by two experienced (> 5 years) nuclear medicine physicians (D.J. and ZX.H.) who were masked to the medical history of the participants. Any disagreement would be discussed with another senior expert (experience > 15 years, L.H.) till reached an agreement. MIM software (MIM Software Inc., Cleveland, OH, USA) was used for 68 Ga-FAPI-04 PET/CT semi-quantitative analysis. Regions of interest (ROI) of the tumor were manually delineated on transaxial slices around intense radiopharmaceutical uptake foci. When a PDAC lesion could not be distinguished from distal obstructive pancreatitis on [68 Ga]Ga-FAPI-04 PET, the tumor extent on the corresponding low-dose CT and the previous CE-CT imaging was employed for delineation. The maximum standardized uptake value (SUVmax), FAPI-avid tumor volume (FTV, defined as the lesion volume of the ROI with the SUV threshold of 40%. The measurement method of metabolic tumor volume in 18F-FDG PET/CT is used as a reference [11]), and total lesion FAP expression (TLF, calculated by multiplying FTV by the corresponding mean SUV) were measured and calculated for each primary PDAC lesion. Besides, the ROI of the total pancreatic intense uptake foci had also been delineated, and total maximum standardized uptake value (TSUVmax), total FAPI-avid pancreatic volume (FPV), and total pancreatic FAP expression (TPF) were measured and calculated. Examples of tumor ROI and pancreas ROI outlines were shown in Supplemental Fig. 1.
Histology and immunohistochemistry of PDAC
A board-certified and experienced pathologist (H.Z.) specializing in digestive oncology who was blinded to patients’ clinical status performed all the macro- and microscopic examinations of all surgical specimens. The degree of differentiation in PDAC was divided into well, moderately, or poorly differentiated which were categorized according to the 2019 World Health Organization classification system [12]. Tumor size (long axis diameter), pathological TNM stage (I, II, or III), grade of differentiated (higher or lower differentiated), vascular invasion (present or absent), perineural invasion (present or absent), and lymph node (LN) metastasis (absent or present, and the number if present) were recorded for each PDAC specimen.
All PDAC specimens were submitted for FAP immunohistochemistry (IHC). The FAP antibody (ab201178; Abcam) was diluted to 1:500. FAP expression was comprehensively assessed in cross-sectional areas throughout the tumor and adjacent non-malignant tissues. The semi-quantitative IHC score was determined by assigning the percentage of positive cells and their staining intensity under a light microscope at a magnification of × 100 by a pathologist who was blinded to all clinical data. The intensity score of FAP was graded as: 0 (none), 1 (weak), 2 (intermediate), or 3 (strong). The semi-quantitative percentages of positively stained cells were scored as follows: 0 (0–10%), 1 (10–25%), 2 (25–50%), 3 (51–75%), and 4 (75–100%). The final IHC score was the product of the intensity and percentage value and ranged from 0 to 12. Based on the IHC score, tumors were marked 0’ (negative; IHC score 0), 1’ (mild; IHC score 1–4), 2’ (moderate; IHC score 5–8), or 3’ (intense; IHC score 9–12). The representative examples of FAP IHC score were shown in Supplemental Fig. 2. Furthermore, the quantitative FAP staining proportion for IHC images was calculated by Aipathwell software (Wuhan Servicebio Co. Ltd).
Follow-up and outcome of PDAC patients
Laboratory and chest-abdominal-pelvis CE-CT/plain CT examinations were performed regularly after surgery (once every 3–6 months). Recurrence-free survival (RFS) was measured from the time of pre-operative [68 Ga]Ga-FAPI-04 PET/CT imaging to the first documented disease recurrence. Overall survival (OS) was measured from the time of pre-operative [68 Ga]Ga-FAPI-04 PET/CT imaging until death. Patients without an event were censored at the time of the last clinical assessment in June 2022. During a median follow-up of 12 months (7–24 months), 28 recurrences and 9 deaths occurred. The postoperative chemotherapy regimen included tegafur-albumin, paclitaxel/gemcitabine, leucovorin-fluorouracil-irinotecan-oxaliplatin (FOLFIRINOX), or concurrent chemoradiotherapy.
Statistical analysis
Data were analyzed using the PASW Statistics software (version 18.0; SPSS, Inc., Chicago, IL, USA). Quantitative values are expressed as mean ± standard deviation. Categorical variables are presented as numbers (percentages). The concordance between imaging and pathological findings was tested with the Spearman’s correlation coefficient. Medians were used as cutoffs for grouping the continuous variables. Differences between the two groups were compared using independent samples t test. For the receiver operating characteristic (ROC) analysis, the area under the curve (AUC) and the cut-off values for sensitivity and specificity of the different [68 Ga]Ga-FAPI-04 parameters were calculated to distinguish poorly differentiated PDAC from the well- or moderately differentiated tumors. Survival outcomes based on RFS and OS were analyzed by plotting Kaplan–Meier survival curves and calculating log-rank P values and hazard ratios (HR). P values less than 0.05 were regarded as statistically significant.
Results
Patient characteristics
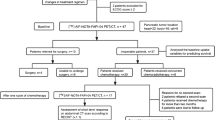
Of the 64 patients who were initially enrolled, 19 were excluded due to unresectable tumors, and 6 were excluded because of non-PDAC diseases. Thirty-seven patients with pathologically confirmed PDAC lesions remained. Figure 1 represents the flowchart of this study. Table 1 summarizes the characteristics of the patients with PDAC and the tumors themselves. Among the 37 patients, thirty-two patients were determined to have resectable lesions, while five patients had borderline resectable PDAC lesions by CE-CT.
The flowchart of the included patients. Abbreviations: SUVmax, tumor maximum standardized uptake value; FTV, FAP expression tumor volume; TLF, total lesion FAP expression; TSUVmax, total maximum standardized uptake value; FPV, FAP expression total pancreatic volume; TPF, total pancreatic FAP expression; RFS, recurrence-free survival; OS, overall survival
The correlation between 68 Ga-FAPI-04 uptake and histological FAP expression in PDAC lesions
Elevated ex vivo FAP expression was detected in all 37 PDAC IHC specimens (0’_none = 0, 1’_mild = 16, 2’_moderate = 11, and 3’_intense = 10). The quantitative FAP + proportion was 34.1% ± 17.5%. The primary tumor SUVmax, FTV, and TLF of [68 Ga]Ga-FAPI-04 were 14.1 ± 7.5, 23.8 ± 21.6 cm2, and 143.7 ± 127.6 cm3, respectively. The FAP IHC score was significantly associated with the SUVmax and TLF of [68 Ga]Ga-FAPI-04 PET/CT respectively (r = 0.78 and 0.44, P < 0.05). The SUVmax and TLF increased with a higher FAP IHC score. The SUVmax and TLF of FAP IHC 1’, 2’, and 3’ groups were 8.9 ± 2.6, 88.3 ± 54.1 cm3; 14.2 ± 3.2, 136.4 ± 89.7 cm3; and 24.3 ± 8.9, 221.0 ± 190.6 cm3, respectively (P < 0.05, Fig. 2). Moreover, significantly positive correlations were shown between FAP-positive proportion and the SUVmax and TLF (r = 0.80 and 0.64, P < 0.001, Table 2 and Fig. 2).
The correlation between [68 Ga]Ga-FAPI-04 uptake and histological FAP expression and the ROC curves of [68 Ga]Ga-FAPI-04 to diagnose poorly differentiated PDAC. a The bar plot indicated the significant difference of [68 Ga]Ga-FAPI-04 SUVmax between FAP-IHC score 1’, 2’, and 3’ groups. b The scatter plot showed a strong correlation between [68 Ga]Ga-FAPI-04 SUVmax and the FAP positive proportion. c The ROC curve represented the diagnosis efficiency of [68 Ga]Ga-FAPI-04 SUVmax to distinguish poorly from well + moderately differentiated PDAC. d The difference of [68 Ga]Ga-FAPI-04 TLF between FAP-IHC score 1’, 2’, and 3’ groups. e The correlation between [68 Ga]Ga-FAPI-04 TLF and the FAP positive proportion. f The ROC curve of TLF to diagnose poorly from well + moderately differentiated PDAC
Besides, FAP is overwhelmingly expressed in the tumor stroma of PDAC (Fig. 3), but occasionally expression is observed in tumor cells in 5/37 (13.5%) of PDAC specimens (Supplemental Fig. 3). The detailed performance of [68 Ga]Ga-FAPI-04 PET/CT at different FAP expression levels was shown in Supplemental Figs. 4-6.
This representative case represented the performance of [68 Ga]Ga-FAPI-04 PET/CT and FAP- immunohistochemistry (IHC) in different differentiation PDAC lesions. a–e A 65-year-old male patient with well-differentiated PDAC in the pancreatic tail (2.1 × 1.7 cm). The images of [68 Ga]Ga-FAPI-04 PET, CT, and axial fusion (a–c, arrows) showed focal uptake (SUVmax = 7.2, FTV = 13.1 cm2, and TLF = 42.0 cm3). d–e The HE staining and FAP-IHC of the lesion showed a mild expression of FAP in the tumor stroma (IHC score = 1’, × 10). f–j A 60-year-old male patient had increased [68 Ga]Ga-FAPI-04 uptake in moderately differentiated PDAC in the pancreatic tail (2.5 × 2.4 cm, SUVmax = 13.5, FTV = 16.5 cm2, and TLF = 87.6 cm3, f–h, arrows). i–j The images of HE and FAP staining showed moderate FAP expression in the tumor stroma (IHC score = 2’, × 10). k–o A 72-year-old female with poorly differentiated PDAC (4.6 × 4.4 cm) underwent [68 Ga]Ga-FAPI-04 PET/CT. The image of [68 Ga]Ga-FAPI-04 PET, CT, and axial fusion (k-m, arrows) showed increased uptake in the pancreatic lesions (SUVmax = 31.5, FTV = 38.1 cm2, and TLF = 555.9 cm3). n–o The HE and FAP IHC staining showed intense FAP overexpression in the tumor stroma (IHC score = 3’, × 10)
The relationship between [68 Ga]Ga-FAPI-04 uptake and tumor clinicopathological features
The correlations between [68 Ga]Ga-FAPI-04 uptake, FAP expression, and tumor clinicopathological features were shown in Fig. 4. The difference in [68 Ga]Ga-FAPI-04 PET/CT parameters between different groups were listed in Table 2 and Supplemental Table 1. The difference in FAP IHC between different clinicopathological groups was listed in Supplemental Table 2. Notably, the histological FAP expression and the SUVmax, TSUVmax, TLF, and TPF of [68 Ga]Ga-FAPI-04 PET/CT were significantly correlated with the histological differentiation of PDAC. Poorly differentiated PDAC exhibited significantly increased FAP IHC score (2.6 ± 0.7), more FAP positive proportion (48.4% ± 15.1%), higher SUVmax (20.0 ± 8.4), higher TSUVmax (21.3 ± 8.5), higher TLF (227.1 ± 169.5 cm3), and TPF (528.9 ± 169.5 cm3) values than did well- and moderately differentiated PDAC (Figs. 3, 4 and 5, Table 2, Supplemental Table 1). The optimized threshold of the [68 Ga]Ga-FAPI-04 SUVmax for distinguishing poorly differentiated PDAC lesions from well- and moderately differentiated PDAC was 12.3. The corresponding sensitivity, specificity, and area under the curve (AUC) of the ROC curve were 92.2%, 73.9%, and 0.90, respectively. The [68 Ga]Ga-FAPI-04 TLF cut-off value for distinguishing the poorly differentiated PDAC from the well- and moderately differentiated PDAC was 128.4; sensitivity, specificity, and AUC were 71.4%, 73.9%, and 0.80, respectively (Fig. 2).
The correlation between imaging and pathological features in PDAC. a–b A 58-year-old female patient with poorly differentiated PDAC. [68 Ga]Ga-FAPI-04 PET/CT cross-sectional fusion image showed the pancreatic head occupancy (a, arrow) with SUVmax of 23.4 and TLF of 170.2 cm3. The image of immunohistochemistry shows significant FAP overexpression, and the score of FAP-IHC was 3’ (b, × 10). c–d A 51-year-old male patient with well-differentiated PDAC. The SUVmax of [68 Ga]Ga-FAPI-04 PET/CT was 8.5 (c, arrow, TLF = 42.2 cm3) and FAP IHC score was 1’ (d, × 10). e–f A 50-year-old male patient with a 4.5-cm long diameter PDAC lesion in the tail of the pancreas, [68 Ga]Ga-FAPI-04 with SUVmax of 23.9 and TLF of 138.4 cm3 (e, arrow), FAP staining suggesting significant high expression of FAP (FAP IHC score = 3’, f, × 10). g–h [68 Ga]Ga-FAPI-04 PET/CT fusion image of a 58-year-old female patient with PDAC of the pancreatic neck (long diameter = 2.1 cm, SUVmax = 6.2, TLF = 80.9 cm3, g, arrow) and FAP staining showed mild FAP expression (FAP-IHC score = 1’, h, × 10). i–j A 59-year-old male PDAC patient (SUVmax = 12.7, TLF = 205.2 cm3, i, arrow) with perineural invasion. FAP-IHC score was 3’ (j, × 10). k–l A 67-year-old female PDAC patient without perineural invasion, the SUVmax and TLF were 9.2 and 46.4 cm3, respectively (k, arrow), and FAP IHC score was 1’ (l)
Furthermore, both tumor size and perineural invasion were significantly correlated to [68Ga]Ga-FAPI-04 PET/CT values. Correspondingly, elevated SUVmax, TSUVmax, FTV, TLF, and TPF of [68Ga]Ga-FAPI-04 PET/CT were observed in larger tumor sizes (length ≥ 3.4 cm) than in smaller size groups (Fig. 5, Table 2). Moreover, perineural invasions were observed in 21 (56.8%) patients. The SUVmax, TSUVmax, and TLF of [68Ga]Ga-FAPI-04 and FAP-IHC scores in the perineural invasion (+) group were significantly higher than those in the perineural invasion (−) group. There were no significant correlations between resectability on CE-CT, tumor stage, the status of vascular invasion, or the existence and number of LN metastases and PET/CT imaging parameters or FAP expression (Supplemental Table 2).
Survival analysis
The results of Kaplan–Meier plots of the RFS and OS analyses are listed in Table 3. The median RFS and OS of the entire sample were 11 and 23 months, respectively. Age, sex, stage (I–II vs. III), tumor size, tumor site (head/neck vs. body/tail), the level of CA-199, or weight change (weight losing vs. weight stable) were not associated with RFS and OS (Table 3). For the IHC of FAP, the median RFS for patients with FAP IHC score 1’–2’ and score 3’ was 13 months vs 5 months, respectively (HR = 4.14, P < 0.01). The median OS for patients with score 3’ was less than that in the lower FAP expression group (12.5 vs. 23 months, respectively). Survival was significantly shorter in poorly differentiated PDAC than in well- or moderately differentiated PDAC, whether in terms of OS (13 vs. 23 months) or RFS (6 vs. 16 months) (Table 3). The Kaplan–Meier plots of RFS and OS for different groups of differentiation, FAP IHC score, and FAP positive proportion were shown in Supplemental Fig. 7.
For the [68 Ga]Ga-FAPI-04 imaging parameters, the median RFS for patients with high (> 12.4) and low (≤ 12.4) SUVmax was 8 vs 13 months, respectively (P < 0.01), while the other PET/CT parameters showed no statistically significant predictive value for RFS. The median OS for patients with high and low SUVmax were 20 and 23 months, respectively (P = 0.02). Furthermore, FTV (12 vs. 23 months, P = 0.02), TLF (12 vs. 13 months, P = 0.04), FPV (13 vs. 23 months, P = 0.006), and TPF (12 vs. 23 months, P = 0.003) showed similar OS trends after stratification based on their respective median value (Table 3 and Fig. 6). Furthermore, for multivariate analyses including clinical and imaging features, [68 Ga]Ga-FAPI-04 SUVmax had a significant independent prognostic value for RFS (HR = 2.46, P < 0.05), while [68 Ga]Ga-FAPI-04 TPF was a significant independent prognostic predictor for predicting OS (HR = 12.8, P < 0.05).
Representative the Kaplan Meier plots of OS in the patients. a–c The Kaplan–Meier plots of OS for 37 patients with PDAC, which stratified by the median of SUVmax, FTV, and TLF of [68 Ga]Ga-FAPI-04 PET/CT. d–f The Kaplan–Meier plots of OS for 37 patients with PDAC, which stratified by the median of TSUVmax, FPV, and TPF of 68 Ga-FAPI-04 PET/CT
Discussion
In the present study, we correlated [68 Ga]Ga-FAPI-04 uptake with IHC FAP expression level and histopathological features in PDAC specimens. A significant correlation between [68 Ga]Ga-FAPI-04 PET uptake and FAP expression was found in the PDAC specimen. We also found that elevated [68 Ga]Ga-FAPI-04 uptake was associated with poor pathological differentiation and advanced tumor stage in PDAC. Notably, our results provide the first evidence for survival analysis and prognostic stratification of PDAC patients after surgery based on [68 Ga]Ga-FAPI-04 PET/CT.
CAFs interact with cancer cells and other stromal cells through a network of signaling pathways and mediators and are major regulators of the tumor microenvironment (TME) [13]. However, CAFs have paradoxical roles in cancer inhibition and promotion [14]. Distinct CAF populations express different fibroblast markers, and FAP is the one of more classical and most recently studied [15]. Several studies have demonstrated that FAP-overexpressing fibroblasts have been associated with epithelial cancer growth, invasion, metastasis, and therapeutic resistance [14, 16, 17]. Due to the overexpression of FAP in cancer-promoting CAFs and its limited expression in normal tissues, FAP has been considered a prime diagnostic and therapeutic target with potential for clinical application [18].
As previously reported, FAP is almost solely expressed in stromal fibroblasts of epithelial cancers [19]; however, this is still controversial [20]. Other reports have shown that apart from a paracrine mechanism in CAFs, PDAC cells might also directly contribute to stromal desmoplasia and progression through an autocrine mechanism involving the FAP protein [21]. Min Shi et al. reported that FAP was substantially expressed in both the fibroblasts and carcinoma cells of PDAC tissues (73.1% and 76.1%, respectively). Our study showed that FAP expression is predominant in the tumor stroma of PDAC, 5/37 (13.5%) of PDAC specimens also observed FAP expression in tumor cells. Further research is needed to investigate the interaction between tumor cells and CAFs through FAP protein in the tumor microenvironment.
PET/CT, as functional imaging technology, provides rapid, reproducible, and non-invasive in vivo assessment of receptor expression [22]. Our results furthermore demonstrated that the uptake of [68 Ga]Ga-FAPI-04 correlated significantly with the expression level of FAP in PDAC lesions, which is an excellent imaging modality for visualizing FAP expression ex vivo. Moreover, the parameters of [68 Ga]Ga-FAPI-04 were positively correlated with tumor differentiation, size, and perineural invasion in PDAC. A similar but statistically insignificant trend was evident for the tumor stage and vascular invasion. The results of our study indicate that preoperative non-invasive [68 Ga]Ga-FAPI-04 PET/CT has great potential for predicting the tumor histological invasiveness and outcomes of PDAC[23]. The underlying mechanism may include the activation of invasiveness, cell cycle progression (shift from G0/G1 to S/G2/M), immune suppression, and angiogenesis by FAP-expressing fibroblasts in PDAC [21].
Radical surgical resection of PDAC tumors can theoretically prevent tumor dissemination and metastatic tumor development. However, the recurrence rate after surgery for PDAC remains remarkably high and is correlated with an unfavorable survival prognosis [24]. The prognostic value of the clinical features, imaging, and biochemical markers of PDAC was evaluated previously [25, 26]. However, the significant heterogeneity of these studies in terms of treatment strategies and imaging protocols makes it difficult to form generalized conclusions. Prognostic implications of [18F]F-FDG have been outlined in predicting early recurrences and survival [27, 28] in PDAC, [18F]F-FDG, whereas the low numbers of patients cohort in these studies limited the strength for clinical significance and potential. Integrated dual tracer PET-CT ([68 Ga]Ga-FAPI and [18F]F-FDG) imaging providing tumor metabolism and TME activity may help in disease characterization, predicting and optimizing therapy, and delivering independent prognostic information.
Interestingly, we discovered that tumor-extrinsic inflammation might be related to patients’ survival. Although the relationship between obstructive pancreatitis and PDAC patients’ prognoses is not entirely clear, we believed that chronic obstructive pancreatitis was significantly involved in subsequent tumorigenesis, disease progression, and surgical complications [8]. Furthermore, the SUVmax of [68 Ga]Ga-FAPI-04 PET/CT served as an independent prognostic value for RFS of PDAC, while the TPF of [68 Ga]Ga-FAPI-04 PET/CT was an independent predictor for OS of PDAC.
The major limitation of our study was the small sample size. However, we believe that in this exploratory study, we have adequately demonstrated the prognostic trend using [68 Ga]Ga-FAPI-04 PET/CT in PDAC patients after surgery. Secondly, the lack of uniformity in the protocols of chemo/radiotherapy after surgery may have effects on the accuracy of prognostic results. Further studies are needed on 68 Ga-FAPI PET/CT for the prediction of systemic treatment outcomes and survival assessment.
Conclusion
In vivo [68 Ga]Ga-FAPI-04 PET uptake correlated strongly with ex vivo FAP expression in PDAC lesions. Ex vivo FAP overexpression and elevated uptake of [68 Ga]Ga-FAPI-04 were associated with increased histopathological and immunohistochemical characteristics of tumor risk in PDAC. Notably, in vivo quantification of CAF activation by [68 Ga]Ga-FAPI-04 PET/CT may be valuable in the preoperative prognostication of advanced PDAC. We found overall elevated FAPI uptake engaged intra- and extra-tumor regions in pancreas tissue could help preoperative prognostication.
Data availability
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request.
References
Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008–20. https://doi.org/10.1016/S0140-6736(20)30974-0.
Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267(5):936–45. https://doi.org/10.1097/SLA.0000000000002234.
Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17(8):487–505. https://doi.org/10.1038/s41575-020-0300-1.
Nicolle R, Blum Y, Marisa L, Loncle C, Gayet O, et al. Pancreatic adenocarcinoma therapeutic targets revealed by tumor-stroma cross-talk analyses in patient-derived xenografts. Cell Rep. 2017;21(9):2458–70. https://doi.org/10.1016/j.celrep.2017.11.003.
Madsen CD. Pancreatic cancer is suppressed by fibroblast-derived collagen I. Cancer Cell. 2021;39(4):451–3. https://doi.org/10.1016/j.ccell.2021.02.017.
Niedermeyer J, Kriz M, Hilberg F, Garin-Chesa P, Bamberger U, et al. Targeted disruption of mouse fibroblast activation protein. Mol Cell Biol. 2000;20(3):1089–94. https://doi.org/10.1128/MCB.20.3.1089-1094.2000.
Luo Y, Pan Q, Yang H, Peng L, Zhang W, et al. Fibroblast activation protein-targeted PET/CT with 68Ga-FAPI for imaging IgG4-related disease: comparison to 18F-FDG PET/CT. J Nucl Med. 2021;62(2):266–71. https://doi.org/10.2967/jnumed.120.244723.
Feng Q, Li C, Zhang S, Tan CL, Mai G, et al. Recurrence and survival after surgery for pancreatic cancer with or without acute pancreatitis. World J Gastroenterol. 2019;25(39):6006–15. https://doi.org/10.3748/wjg.v25.i39.6006.
Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw. 2019;17(5.5):603–5. https://doi.org/10.6004/jnccn.2019.5007.
Shi X, Xing H, Yang X, Li F, Yao S, Zhang H, et al. Fibroblast imaging of hepatic carcinoma with (68)Ga-FAPI-04 PET/CT: a pilot study in patients with suspected hepatic nodules. Eur J Nucl Med Mol Imaging. 2021;48(1):196–203. https://doi.org/10.1007/s00259-020-04882-z.
Mokoala KMG, Lawal IO, Lengana T, Popoola GO, Boshomane TMG, et al. The association of tumor burden by 18F-FDG PET/CT and survival in vulvar carcinoma. Clin Nucl Med. 2021;46(5):375–81. https://doi.org/10.1097/RLU.0000000000003549.
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, WHO Classification of Tumours Editorial Board, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–8. https://doi.org/10.1111/his.13975.
Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144(6):1210–9. https://doi.org/10.1053/j.gastro.2012.11.037.
Neuzillet C, Tijeras-Raballand A, Ragulan C, Cros J, Patil Y, et al. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol. 2019;248(1):51–65. https://doi.org/10.1002/path.5224.
Menezes S, Okail MH, Jalil SMA, Kocher HM, Cameron AJM. Cancer-associated fibroblasts in pancreatic cancer: new subtypes, new markers, new targets. J Pathol. 2022;257(4):526–44. https://doi.org/10.1002/path.5926.
Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020;39(3):783–803. https://doi.org/10.1007/s10555-020-09909-3.
Henry LR, Lee HO, Lee JS, Klein-Szanto A, Watts P, Ross EA, et al. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13(6):1736–41. https://doi.org/10.1158/1078-0432.CCR-06-1746.
Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: cancer-associated fibroblasts and their markers. Int J Cancer. 2020;146(4):895–905. https://doi.org/10.1002/ijc.32193.
Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, et al. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37(2):154–8. https://doi.org/10.1097/MPA.0b013e31816618ce.
Kawase T, Yasui Y, Nishina S, Hara Y, Yanatori I, Tomiyama Y, et al. Fibroblast activation protein-α-expressing fibroblasts promote the progression of pancreatic ductal adenocarcinoma. BMC Gastroenterol. 2015;15:109. https://doi.org/10.1186/s12876-015-0340-0.
Shi M, Yu DH, Chen Y, Zhao CY, Zhang J, Liu QH, et al. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J Gastroenterol. 2012;18(8):840–6. https://doi.org/10.3748/wjg.v18.i8.840.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60(6):801–5. https://doi.org/10.2967/jnumed.119.227967.
Herreros-Villanueva M, Gironella M, Castells A, Bujanda L. Molecular markers in pancreatic cancer diagnosis. Clin Chim Acta. 2013;418:22–9. https://doi.org/10.1016/j.cca.2012.12.025.
Chikamoto A, Inoue R, Komohara Y, Sakamaki K, Hashimoto D, Shiraishi S, et al. Preoperative high maximum standardized uptake value in association with glucose transporter 1 predicts poor prognosis in pancreatic cancer. Ann Surg Oncol. 2017;24(7):2040–6. https://doi.org/10.1245/s10434-017-5799-1.
Dunet V, Halkic N, Sempoux C, Demartines N, Montemurro M, Prior JO, et al. Prediction of tumour grade and survival outcome using pre-treatment PET- and MRI-derived imaging features in patients with resectable pancreatic ductal adenocarcinoma. Eur Radiol. 2021;31(2):992–1001. https://doi.org/10.1007/s00330-020-07191-z.
Smith RA, Bosonnet L, Ghaneh P, Raraty M, Sutton R, Campbell F, et al. Preoperative CA19–9 levels and lymph node ratio are independent predictors of survival in patients with resected pancreatic ductal adenocarcinoma. Dig Surg. 2008;25(3):226–32. https://doi.org/10.1159/000140961.
Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med. 2014;55(6):898–904. https://doi.org/10.2967/jnumed.113.131847.
Yamamoto T, Sugiura T, Mizuno T, Okamura Y, Aramaki T, et al. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2015;22(2):677–84. https://doi.org/10.1245/s10434-014-4046-2.
Funding
This work was sponsored in part by the National Natural Science Foundation of China (Grant No. 82071967), the National Key Research and Development Program of China (Grant No. 2020YFC2002702), CAMS Initiative for Innovative Medicine (No. CAMS-2018-I2M-3–001), CAMS initiative for innovative medicine (2016ZX310174-4), Tsinghua University-Peking Union Medical College Hospital Initiative Scientific Research Program (Grant No. 52300300519), and Capital’s Funds for Health Improvement and Research (CFH-2018–2-4014).
Author information
Authors and Affiliations
Contributions
All authors were involved in the study conception and design. Jie Ding, Zhixin Hao, Hua Huang, Qiaofei Liu Wenjing Liu, and Chao Ren were involved in acquisition of data. Jie Ding and Jiangdong Qiu were involved in analysis and interpretation of the data. Jie Ding, Xiang Li, and Jiangdong Qiu were involved in drafting of the manuscript. All authors were involved with critical revisions of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Institutional Ethics Committee of the Peking Union Medical College Hospital (Beijing, China, IRB protocol #ZS1810).
Consent to participate
Informed consent was obtained from all individual participants included in the study for publication.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in this article.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ding J and Li X shared the authorship.
Jie Ding is the first author.
This article is part of the Topical Collection on Oncology - Digestive tract
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, J., Qiu, J., Hao, Z. et al. Prognostic value of preoperative [68 Ga]Ga-FAPI-04 PET/CT in patients with resectable pancreatic ductal adenocarcinoma in correlation with immunohistological characteristics. Eur J Nucl Med Mol Imaging 50, 1780–1791 (2023). https://doi.org/10.1007/s00259-022-06100-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-06100-4