Abstract
Purpose
[18F]FAPI-42 is a new fibroblast activation protein (FAP)-specific tracer used for cancer imaging. Here, we describe the optimal acquisition time and in vivo evaluation of [18F]FAPI-42 and compared intra-individual biodistribution, tumor uptake, and detection ability to [68Ga]Ga-FAPI-04.
Methods
A total of 22 patients with various types of cancer received [18F]FAPI-42 whole-body positron emission tomography/computed tomography (PET/CT). Among them, 4 patients underwent PET/CT scans, including an early dynamic 20-min, static 1-h, and static 2-h scans. The in vivo biodistribution in normal organs and tumor uptake were semiquantitatively evaluated using the standardized uptake value (SUV) and tumor-to-background ratio (TBR). Furthermore, both [18F]FAPI-42 and [68Ga]Ga-FAPI-04 PET/CT were performed in 12 patients to compare biodistribution, tumor uptake, and tumor detection ability.
Results
[18F]FAPI-42 uptake in the tumors was rapid and reached a high level with an average SUVmax of 15.8 at 18 min, which stayed at a similarly high level to 2 h. The optimal image acquisition time for [18F]FAPI-42 was determined to be 1 h postinjection. For tumor detection, [18F]FAPI-42 had a high uptake and could be clearly visualized in the lesions. Compared to [68Ga]Ga-FAPI-04, [18F]FAPI-42 had the same detectability for 144 positive lesions. In addition, [18F]FAPI-42 showed a higher SUVmax in liver and bone lesions (P < 0.05) and higher TBRs in liver, bone, lymph node, pleura, and peritoneal lesions (all P < 0.05).
Conclusion
The present study demonstrates that the optimal image acquisition time of [18F]FAPI-42 is 1 h postinjection and that [18F]FAPI-42 exhibits comparable lesion detectability to [68Ga]Ga-FAPI-04.
Trial registration
Chinese Clinical Trial Registry (ChiCTR2100045757).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fibroblast activation protein (FAP) is a transmembrane serine protease that is expressed in stromal fibroblasts in more than 90% of epithelial cancers as well as in malignant cells in glioblastoma and pancreatic, breast, colorectal, cervical, and oral squamous cell carcinomas [1, 2]. Overexpression of FAP is associated with high local tumor motility and invasiveness, decreased survival, and poor prognosis in cancer patients [2,3,4]. It has been shown that FAP is also upregulated in lesions associated with wound healing, atherosclerotic plaques, rheumatoid arthritis, idiopathic pulmonary fibrosis, and hepatic fibrosis [5,6,7,8,9] but is expressed at a negligible or non-detectable level in most normal adult tissues under physiological conditions. Therefore, FAP is considered a good target for the diagnosis and treatment of tumors and nonmalignant diseases associated with FAP expression. Accordingly, FAP-targeted PET/CT has emerged as a new technique for imaging cancers. The most common FAP tracer is [68Ga]Ga-FAPI-04, which is a quinoline-based FAP ligand radiolabeled with the generator radionuclide 68Ga (T1/2 = 67.7 min, 88.9% β+). [68Ga]Ga-FAPI-04 PET/CT has been demonstrated to be a good imaging modality for 28 types of cancer and offers higher tumor to non-tumor (T/NT) contrast and higher tumor detectability compared to [18F]FDG PET/CT [10,11,12,13,14,15,16,17,18]. However, [68Ga]Ga-FAPI-04 is limited by the relatively short half-life of 68Ga and availability from the 68Ge/68Ga-generator, which allows only one or two elution per day and batch production of approximately 1‒3 patient doses per elution. In this context, 18F-labeled FAP tracers may represent a promising alternative for FAP imaging. 18F-fluorine, as is well known, is a positron emitter that is commonly available due to extensively equipped cyclotrons worldwide and can be produced in a large amount to meet the requirements of a large cohort of patients. 18F-labeled radiopharmaceuticals have a longer half-life (T1/2 = 109.8 min) and can thus be delivered over longer distances. Furthermore, the low positron energy of 18F (≤ 635 keV) enables a short positron linear range in tissue (2.3 mm), resulting in the highest resolution PET images of all available positron emitters [19].
The 18F-labeled FAP ligand, [18F]FAPI-42, has the same pharmacophore as [68Ga]Ga-FAPI-04 with the exception of the replacement of the chelator DOTA by NOTA. In our previous work, the radiosynthesis and preclinical evaluation of [18F]FAPI-42 were reported [20]. Recently, [18F]FAPI-42 has been clinically introduced and exhibited good performance for depicting malignant tumors [21, 22]. However, it is not precisely clear whether [18F]FAPI-42 performs as well as [68Ga]Ga-FAPI-04 in the detection of tumors. Furthermore, it is also necessary to determine the optimal time for image acquisition. Here, we present data on the biodistribution of [18F]FAPI-42 and compare its biodistribution, tumor uptake, and detection ability to [68Ga]Ga-FAPI-04.
Materials and methods
Study design and patient population
This study was approved by the institutional review board of the Ethics Committee of Nanfang Hospital (No. NFEC-2020–205) and registered at the Chinese Clinical Trial Registry (ChiCTR2100045757). All patients signed an informed consent form before participation and all procedures were conducted in accordance with the Declaration of Helsinki.
Patients were consecutively recruited for enrollment in this study from April to July 2021. The inclusion criteria were as follows: (1) patients with newly diagnosed cancer or posttreatment cancer who had no antitumor therapy 3 months prior to the examination and (2) primary malignancies confirmed by pathology. The exclusion criteria were patients: (1) who had another primary malignancy at the time of examination; (2) who suffered from severe hepatic and renal insufficiency; and (3) who refused to undergo the scans.
The primary malignancy was diagnosed by biopsy and histopathological examination. Diagnosis of lymph node metastasis and distant metastasis was established by multiple imaging modalities, including magnetic resonance imaging (MRI) of the brain, chest CT, abdominal ultrasound, whole-body bone scan, and whole-body PET/CT.
Radiopharmaceuticals
The radiolabeling precursors were obtained from Nanchang TanzhenBio Co., Ltd. (Nanchang, China) with high chemical purity (> 95%). [18F]FAPI-42 was produced on an automated synthesis module (AllInOne module) as previously reported [23]. A detailed description of the radiosynthesis process and quality control of [18F]FAPI-42 have been published elsewhere [20]. [68Ga]Ga-FAPI-04 was manually synthesized as previously described [10, 12, 24]. Briefly, 68GaCl3 was achieved by adding 4.00 mL 68Ge/68Ga-generator eluate (0.05 M hydrochloric acid; 0.74–1.48 GBq) to a solution of DOTA-FAPI-04 (25 nmol) in 0.25 mol/L NaOAc aqueous (1 mL). The reaction mixture was heated at 100 °C for 10 min and purified by simple solid-phase extraction of the product by cartridge separation. Analysis and quality control of the prepared products were performed as previously reported [11, 12, 21].
Imaging procedures
PET/CT imaging was performed using a total-body PET/CT scanner (uEXPLORER, United Imaging Healthcare; Shanghai, China). All patients underwent [18F]FAPI-42 (209 ± 55 MBq) PET/CT scanning. Four patients underwent a 20-min dynamic PET scan before administration and additional PET scans 1 h and 2 h after administration of [18F]FAPI-42. Twelve patients also underwent [68Ga]Ga-FAPI-04 (110 ± 27 MBq) PET/CT scanning on 2 consecutive days.
Before administration of FAP tracers, patients were instructed to fast for at least 2 h in order to reduce hepatobiliary excretion. The PET/CT scan was performed approximately at 1 h after intravenous injection of [18F]FAPI-42/[68Ga]Ga-FAPI-04. The PET scan was performed with a 5-min single bed position 3D acquisition. Low-dose CT (80 mA, 120 kVp) was acquired for attenuation correction and all corrections applied to the reconstructed images and for anatomic localization of lesions. All data were reconstructed using list-mode OSEM-PSF-TOF [25, 26].
Any drug-related side effects were recorded and the vital parameters of the patients were observed for 1 week.
Imaging interpretation
The acquired CT and PET images were sent to a MedEx (MedEX Technology Ltd. Co., Beijing) workstation for registration, fusion, and measurement. Two experienced nuclear physicians with more than 10 years of certificated experience for each scan independently performed image interpretation and any disagreements were resolved by consensus. Any focal accumulations of [18F]FAPI-42 or [68Ga]Ga-FAPI-04 that were higher than the background were interpreted as a positive lesion. Tumor tracer uptake was quantified by the maximal standardized uptake value (SUVmax) and target-to-background ratio (TBR). The region of interest (ROI) was drawn along the margins of the lesion on the axial PET image and automatically adapted to a three-dimensional volume of interest at a 60% isocontour, which was used to measure the SUVmax. TBR was calculated by dividing the SUVmax of the lesion by the SUVmean of the organs (brain background for brain lesions, lung background for lung and pleura lesions, aortic lumen background for lymph nodes, liver background for liver lesions, adrenal glands background for adrenal gland lesions, L5 background for bone lesions, gluteal muscle background for all other lesions). Tumors with a diameter larger than 1 cm were defined as large lesions, while tumors with a diameter smaller than 1 cm were defined as small lesions.
Statistical analysis
Normally distributed variables are reported as the means ± standard deviations while skewed variables are reported as the medians (range). The differences in SUVmax and TBR between [18F]FAPI-42 and [68Ga]Ga-FAPI-04 were evaluated using the paired t-test (normally distributed variables) or Wilcoxon signed-rank test (skewed variables). Analyses were performed using (SPSS, version 22.0; IBM, Armonk, NY). Two-tailed P values less than 0.05 were considered indicative of a statistically significant difference.
Results
Patient characteristics
A total of 22 patients (12 males, 10 females; median age, 55.5 years; range, 23–74 years) with various malignant tumors were prospectively enrolled, including 6 patients with lung cancer, 6 patients with liver cancer, 5 patients with colorectal cancer, and 1 patient each with stomach cancer, esophageal cancer, carcinosarcoma of the cheek, celiac neuroendocrine carcinoma, and clear cell carcinoma of the bladder (Table 1). Of the 22 patients enrolled, 18 patients had increased levels of tumor markers in the serum, while 4 patients had a normal level of tumor markers. Detailed information is presented in Supplementary Table 1. Among them, 12 patients were newly diagnosed and the other 10 patients had relapse tumors after single or multiple modality treatments. Twelve patients had IV staged diseases, 17 patients had local advanced staged diseases, and 3 patients had early staged diseases (Table 1).
Safety
The mean injection activity of [18F]FAPI-42 was 209 ± 55 MBq (n = 22) with molar activity of 52–186 GBq/µmol (n = 8); for the [68Ga]Ga-FAPI-04 examination, the mean injection activity was 110 ± 27 MBq (n = 12) with molar activity of 29–44 GBq/µmol (n = 8). No drug-related side effects were reported during or after [18F]FAPI-42 or [68Ga]Ga-FAPI-04 PET/CT. PET imaging was well tolerated by all patients. Vital parameters remained stable and no patient reported any new symptoms during the observation period.
Dynamic scans of [18F]FAPI-42
The maximum intensity projection (MIP) and biodistribution data assessed by SUV kinetics for one patient (male) are shown in Fig. 1. The images and SUV kinetics for the other three patients are available in Supplementary Figs. 1, 2, and 3. The pooled SUVmax and tumor SUVmax/organ SUVmean for all four patients that underwent this scan are summarized in Fig. 2a and b. The highest average normal organ SUVmax at all time points was observed in the kidneys, decreasing from an average SUVmax of 238 at 10 min to 27.1 by 2 h (decline of 89%). Tracer uptake in the tumor was rapid and showed excellent retention with an average SUVmax of 15.8 at 18 min, and 15.3 at 2 h (decrease of 3%). The SUVmax of the organs decreased in all patients 5 min postinjection (p.i.) to the last time point, while TBRs increased with time (with exception of the gallbladder TBR). The highest TBR among all time points was observed in the lungs, small intestine, and bone, with a ratio of 54.1, 40.3, and 26.9.
a Maximum intensity projection (MIP) of [18F]FAPI-42 at 5, 10, 15, 20, 65, and 120 min p.i. in patient 21 (female). b Time-activity curves of [18F]FAPI-42 at different time points following tracer injection. c The ratios of tumor SUVmax/organ SUVmean at different time points following [18F]FAPI-42 injection. The ratio of tumor SUVmax/brain SUVmean was high and is excluded from the plot
Pooled tumor and organ SUVmax (a) and ratios of tumor SUVmax/organ SUVmean at different time points following [18F]FAPI-42 injection (b). Pooled small and large tumor lesions SUVmax (c) and the ratios of tumor SUVmax/aortic lumen SUVmean (d) at 15, 65, and 120 min following [18F]FAPI-42 injection. Results are shown as the mean and standard deviation for 4 patients
The SUVmax and TBR (blood) for large and small lesions are summarized in Fig. 2c and d. Although some small lesions could be visualized at the early 10-min scan, other lesions were still indistinct due to low TBR (blood) (2.6 ± 0.64). At the 1-h scan, although the increase in SUVmax was not significantly different than the 10-min scan (11.3 ± 4.1 vs. 7.7 ± 1.5, P = 0.05), the TBR (blood) increased significantly to a much higher level (8.1 ± 2.7 vs. 2.6 ± 0.64, P = 0.0076), and the small lesions were all clearly visualized (Fig. 1). The TBR further increased from 1 to 2 h; however, this did not result in more lesions being detected. For detection of large tumors, the lesions could be clearly visualized at the early 10-min scan, although they were clearer at the 1-h and 2-h scans.
Biodistribution of [18F]FAPI-42 and comparison with [68Ga]Ga-FAPI-04 PET/CT
In the total 22 patients, [18F]FAPI-42 PET/CT demonstrated intense radioactivity in the urinary tract, indicating that the kidneys were the main excretory organs. Intense uptake of the radioactivity was also observed in the gallbladder and common bile duct, implying that some [18F]FAPI-42 was also eliminated via the hepatobiliary system. Moderate uptake of radioactivity was seen in other organs in some patients, including the submandibular gland (14/22), thyroid (12/22), and pancreas (19/22), similar to the findings of the dynamic scan. Only minimal or mild physiological uptake was observed in other organs and tissue, including the brain, parotid, oral mucosa, lung, myocardium, liver, intestine, fat, spine, and muscle. Radioactivity in bones was low, suggesting that no defluorination occurred in vivo (Fig. 3).
Compared with [18F]FAPI-42, [68Ga]Ga-FAPI-04 showed similar uptake of radioactivity in the urinary tract (9.6 ± 7.3 vs. 9.5 ± 7.4, t = 0.022, P = 0.983), but lower accumulation of radioactivity in the gallbladder and common bile duct (10.5 ± 6.2 vs. 0.9 ± 1.1, t = 4.461, P = 0.001 and 4.2 ± 2.3 vs.1.1 ± 0.4, t = 3.753, P = 0.003, respectively). Accumulation of [68Ga]Ga-FAPI-04 in the parotid, salivary gland, thyroid, and pancreas was also lower than that of 18F-FAPI-42 (P < 0.05). There were no significant differences in uptake between the two tracers in other organs or tissues (all P > 0.05; Fig. 3).
Comparison of tumor detection and tumor uptake between [18F]FAPI-42 and [68Ga]Ga-FAPI-04 PET/CT
On 18F-FAPI -42 PET/CT images, the uptake of [18F]FAPI-42 in tumors was intense, and the lesions could be clearly visualized (Fig. 4). [18F]FAPI-42 PET/CT was positive in all 22 enrolled patients. [18F]FAPI-42 PET/CT depicted primary and relapse tumors in 16 patients, suspected lymph node metastases in 14 patients, and suspected distant metastases in 14 patients, including lung lesions in 4 patients, pleural lesions in 5 patients, liver lesions in 6 patients, bone lesions in 4 patients, peritoneal lesions in 2 patients, and other suspected metastases in 3 patients. In the 12 patients who received dual-tracer scans, both PET/CT demonstrated the same positive detection for 144 lesions (Table 2). In the visual analysis, [18F]FAPI-42 and [68Ga]Ga-FAPI-04 PET/CT showed comparable results for primary tumors, lymph node lesions, pleural lesions, and peritoneal lesions in terms of both patient-based and lesion-based comparison (Table 2). However, liver and bone lesions were more clearly visualized by [18F]FAPI-42 PET/CT than [68Ga]Ga-FAPI-04 PET/CT (P < 0.05; Fig. 5).
MIP images of [18F]FAPI-42 (a) and [68Ga]Ga-FAPI-04 (b) PET/CT in 6 representative patients (patients 3, 1, 2, 5, 8, and 7, from left to right) with different histologically proven tumor entities (c; sorted by tumor entity). Scale bar: 100 μm. Ca, cancer; HCC, hepatocellular carcinoma; IT, interventional therapy
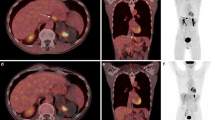
PET/CT images of [18F]FAPI-42 and [68Ga]Ga-FAPI-04 in patient 9 (male) with newly diagnosed lung adenocarcinoma. Both PET/CT detected the primary lung cancer (size 3.5 × 2.3 cm) in the upper lobe of the right lung with intense uptake of [18F]FAPI-42 (SUVmax 21.0; a and e) and [68Ga]Ga-FAPI-04 (SUVmax 17.3; d and f). Some bone metastases were more clearly visualized by [18F]FAPI-42 (a and i) compared with [68Ga]Ga-FAPI-04 PET/CT (d and j). The diagnosis of right lung cancer and bone metastasis was confirmed by pathology (k, l). Scale bar: 200 μm. a, d: MIP images; b, c and e, f: axial CT and fused PET/CT of the lung; g, h and i, j: axial CT and fused PET/CT of the mediastinum; k, l: pathological images of the right lung and sternal lesions. The primary lung cancer is indicated by red arrows; bone metastasis is indicated by white arrows
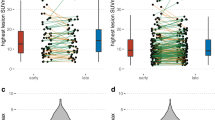
No significant differences in SUVmax between [18F]FAPI-42 and [68Ga]Ga-FAPI-04 were found in the primary tumors, lymph node lesions, pleural lesions, or peritoneal lesions (all P > 0.05; Fig. 6). However, uptake of [18F]FAPI-42 was observed to be higher in liver and bone lesions (P < 0.05; Fig. 6). Similarly, no significant difference in TBR was found between [18F]FAPI-42 and [68Ga]Ga-FAPI-04 in the primary tumors and lung lesions (P > 0.05). However, the TBR of [18F]FAPI-42 was significantly higher compared to [68Ga]Ga-FAPI-04 not only in liver and bone lesions (P < 0.05) but also in the lymph node, pleura, and peritoneum lesions (P < 0.05; Fig. 7).
Discussion
68Ga-labeled FAP probes have recently been proven useful for the detection of tumors with high expression of FAP and can be used for imaging various cancers [12, 27,28,29]. To take advantage of the favorable properties of fluorine‐18, a few 18F-labeled FAPI probes have been introduced for PET imaging [21, 22, 28, 30]. However, clinical data comparing 68Ga-labeled and 18F-labeled FAP tracers are scarce and the suitable time for the detection of tumors also needs to be identified for 18F-labeled FAP probes. In the present study, we compared biodistribution and lesion detectability between the FAP tracers [18F]FAPI-42 and [68Ga]Ga-FAPI-04 in cancer patients. In addition, the optimal image acquisition time for [18F]FAPI-42 was established.
The biodistribution of [18F]FAPI-42 in four cancer patients from 0 to 2 h after tracer administration demonstrated rapid and high tumor uptake and satisfactory retention, while normal organs showed rapid radioactivity decreases and excretion through the kidneys. Combined with the biodistribution results in another 12 cancer patients, [18F]FAPI-42 demonstrated that the excretion pathway of [18F]FAPI-42 is predominantly from urinary and biliary systems, which is similar to reports for the tracers [18F]FAPI-74, [68Ga]Ga-FAPI-04, and [68Ga]Ga-FAPI-46 [12, 28, 31].
The optimal image acquisition time for [18F]FAPI-42 was determined to be 1 h p.i. by comparing the ratios of small/large lesions to the aortic lumen at different time points, which is consistent with the optimal image acquisition time of [18F]FAPI-74 reported previously [28]. Although the uptake of [18F]FAPI-42 in the tumors was rapid and high, the low TBR (blood) at the early 10-min scan made it unfavorable to detect some small lesions. It may not be necessary to postpone the [18F]FAPI-42 scan to 2 h because it did not show more tumor detection. It was observed that the radioactivity in the pancreas was obviously reduced in three patients after 2 h, which may be useful for the detection of pancreatic carcinoma. A similar phenomenon was reported such that [68Ga]Ga-FAPI-04 detected pancreatic carcinoma using a 3-h delay scan to reduce the physiologic radioactivity in the pancreas [32].
The biodistribution of [18F]FAPI-42 in normal organs and tumor lesions was head-to-head compared with [68Ga]Ga-FAPI-04 in the same twelve patients. The SUVmax analysis of the biodistribution of [68Ga]Ga-FAPI-04 in our cohort was comparable with previously described data for [68Ga]Ga-FAPI-04 [12, 31]. Comparison of the biodistribution of [18F]FAPI-42 and [68Ga]Ga-FAPI-04 in normal organs showed somewhat higher tracer uptake for [18F]FAPI-42; however, this did not influence diagnostic accuracy (Fig. 4). This result might be due to the different lipophilicity of the NOTA-chelator and DOTA-chelator groups. Compared to [68Ga]Ga-FAPI-04, [18F]FAPI-42 showed higher uptake in the parotid gland, submandibular gland, thyroid, myocardium, gallbladder, biliary tract, and pancreas, which might be caused by the different lipophilicity of the NOTA-chelator and DOTA-chelator groups. This shortcoming might influence the detection of lesions in these regions, especially for pancreatic, gallbladder, and biliary tract tumors. [18F]FAPI-42 accumulation in bone was low on PET/CT images in all patients, which indicated that no defluorination occurred in vivo. Of note, [18F]FAPI-42 showed high bone uptake in preclinical mice models, as reported in our previous work and in the literature [21].
The present study revealed that [18F]FAPI-42 may be a good alternative tracer to [68Ga]Ga-FAPI-04. Through head-to-head comparison in 12 patients, our results showed that [18F]FAPI-42 had high uptake in the lesions and the same detectability for the primary tumors and suspected metastases compared to [68Ga]Ga-FAPI-04, which indicated that replacing DOTA with NOTA and chelation with 18F-AlF did not affect the binding capacity of [18F]FAPI-42 to FAP. The visual analysis showed [18F]FAPI-42 has superior potential for detection of lesions in the liver and bones with higher TBRs in the liver, bone, lymph node, pleura, and peritoneal lesions compared to [68Ga]Ga-FAPI-04. In the study, we selected various tumors for [18F]FAPI-42 scanning, including [18F]FDG avid tumors, such as lung cancer, colorectal cancer, and sarcoma, and some tumors prone to be [18F]FDG non-avid, such as gastric cancer and hepatocellular cancer [33]. Our data indicated that all lesions were positive on [18F]FAPI-42 scan, which implied that [18F]FAPI-42 may play a supplementary role to [18F]FDG, similar to recently reported of [68Ga]Ga-FAPI-04 [21].
There are some limitations of the present study. Firstly, the dynamic scan was performed at early 20 min, but not 60 min, which could not provide more information concerning dynamic biodistribution of [18F]FAPI-42 in vivo. In addition, our study is limited by the small sample size of the enrolled patients and limited tumor types. Further research is needed to investigate the real clinical value of [18F]FAPI-42 in diagnosing and staging various tumors.
Conclusion
The biodistribution of [18F]FAPI-42 showed high TBRs that increased over time and confirmed that 1 h was a suitable time for image acquisition. [18F]FAPI-42 and [68Ga]Ga-FAPI-04 exhibited comparably high lesion detectability in patients with various cancers. Due to its more convenient availability and easy production, [18F]FAPI-42 can serve as a practical alternative to [68Ga]Ga-FAPI-04 in routine applications.
References
Bušek P, Mal R, Šedo A. Dipeptidyl peptidase IV activity and / or structure homologues (DASH) and their substrates in cancer. Int J Biochem Cell Biol. 2004;36:408–21.
Liu F, Qi L, Liu B, Liu J, Zhang H, Che DH, et al. Fibroblast activation protein overexpression and clinical implications in solid tumors: a meta-analysis. PLoS ONE. 2015;10:1–18.
Yang L, Ma L, Lai D. Over-expression of fibroblast activation protein alpha increases tumor growth in xenografts of ovarian cancer cells. Acta Biochim Biophys Sin. 2013 928–37.
Busek P, Mateu R, Zubal M, Kotackova L, Sedo A. Targeting fibroblast activation protein in cancer–prospects and caveats. Front Biosci. 2018 1933–68.
van der Geest T, Roeleveld DM, Walgreen B, Helsen MM, Nayak TK, Klein C, et al. Imaging fibroblast activation protein to monitor therapeutic effects of neutralizing interleukin-22 in collagen-induced arthritis. Rheumatol. 2018;57:737–47.
Brokopp CE, Schoenauer R, Richards P, Bauer S, Lohmann C, Emmert MY, et al. Fibroblast activation protein is induced by inflammation and degrades type i collagen in thin-cap fibroatheromata. Eur Heart J. 2011;32:2713–22.
Fan MH, Zhu Q, Li HH, Ra HJ, Majumdar S, Gulick DL, et al. Fibroblast activation protein (FAP) accelerates collagen degradation and clearance from lungs in mice. J Biol Chem. 2016;291:8070–89.
Lay AJ, Zhang HE, Mccaughan GW, Gorrell MD, Fap S, Fap C, et al. Fibroblast activation protein in liver fibrosis. Front Biosci. 2019 1–17.
Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci U S A. 1988;85:3110–4.
Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59:1415–22.
Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F]FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04769-z.
Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jäger D, et al. A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med. 2018;59:1423–9.
Kuten J, Levine C, Shamni O, Pelles S, Wolf I, Lahat G, et al. Head-to-head comparison of [68Ga]Ga-FAPI-04 and [18F]FDG PET/CT in evaluating the extent of disease in gastric adenocarcinoma. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05494-x.
Shi X, Xing H, Yang X, Li F, Yao S, Zhang H, et al. Fibroblast imaging of hepatic carcinoma with 68Ga-FAPI-04 PET/CT : a pilot study in patients with suspected hepatic nodules. Eur J Nucl Med Mol Imaging. 2021;48(1):196–203.
Kesch C, Yirga L, Dendl K, Handke A, Darr C, Krafft U, et al. High fibroblast‑activation‑protein expression in castration‑resistant prostate cancer supports the use of FAPI‑molecular theranostics. Eur J Nucl Med Mol Imaging. 2021 1–5.
Windisch P, Röhrich M, Regnery S, Tonndorf-martini E, Held T, Lang K, et al. Fibroblast activation protein (FAP) specific PET for advanced target volume delineation in glioblastoma. Radiother Oncol. 2020;150:159–63.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–5.
Wang H, Zhu W, Ren S, Kong Y, Huang Q, Zhao J. Ga-FAPI-04 versus 18F-FDG PET/CT in the detection of hepatocellular carcinoma. Front Oncol. 2021;11:693640.
Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chemie-Int Ed. 2008;47:8998–9033.
Hu K, Li J, Wang L, Huang Y, Li L, Ye S, et al. Preclinical evaluation and pilot clinical study of [18F]AlF-labeled FAPI-tracer for PET imaging of cancer associated fibroblasts. Acta Pharm. Sin. B. 2021 In press. https://doi.org/10.1016/j.apsb.2021.09.032
Wang S, Zhou X, Xu X, Ding J, Liu S, Hou X, et al. Clinical translational evaluation of Al18F-NOTA-FAPI for fibroblast activation protein-targeted tumour imaging. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05470-5.
Jiang X, Wang X, Shen T, Yao Y, Chen M, Li Z, et al. FAPI-04 PET/CT Using [18F]AlF labeling strategy: automatic synthesis, quality control, and in vivo assessment in patient. Front Oncol. 2021;11:1–9.
Huang Y, Li H, Ye S, Tang G, Liang Y, Hu K. Synthesis and preclinical evaluation of an Al18F radiofluorinated bivalent PSMA ligand. Eur J Med Chem. 2021;221:113502.
Loktev A, Lindner T, Burger EM, Altmann A, Giesel F, Kratochwil C, et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J Nucl Med. 2019;60:1421–9.
Tan H, Sui X, Yin H, Yu H, Gu Y, Chen S, et al. Total-body PET/CT using half-dose FDG and compared with conventional PET/CT using full-dose FDG in lung cancer. Eur J Nucl Med Mol Imaging. 2021;48:1966–75.
Spencer BA, Berg E, Schmall JP, Omidvari N, Leung EK, Abdelhafez YG, et al. Performance evaluation of the uEXPLORER total-body PET/CT scanner based on NEMA NU 2–2018 with additional tests to characterize PET scanners with a long axial field of view. J Nucl Med. 2021;62:861–70.
Altmann A, Haberkorn U, Siveke J. The latest developments in imaging of fibroblast activation protein. J Nucl Med. 2021;62:160–7.
Giesel FL, Adeberg S, Syed M, Lindner T, Jiménez-Franco LD, Mavriopoulou E, et al. FAPI-74 PET/CT using either 18F-AlF or cold-kit 68Ga labeling: biodistribution, radiation dosimetry, and tumor delineation in lung cancer patients. J Nucl Med. 2021;62:201–7.
Meyer C, Dahlbom M, Lindner T, Vauclin S, Mona C, Slavik R, et al. Radiation dosimetry and biodistribution of 68Ga-FAPI-46 PET imaging in cancer patients. J Nucl Med. 2020;61:1171–7.
Toms J, Kogler J, Maschauer S, Daniel C, Schmidkonz C, Kuwert T, et al. Targeting fibroblast activation protein: radiosynthesis and preclinical evaluation of an 18F-labeled FAP inhibitor. J Nucl Med. 2020;61:1806–13.
Wang S, Zhou X, Xu X, Ding J, Liu T, Jiang J, et al. Dynamic PET/CT imaging of 68Ga-FAPI-04 in chinese subjects. Front Oncol. 2021;11:1–11.
Rohrich M, Naumann P, Giesel FL, Choyke PL, Staudinger F, Wefers A, et al. Impact of 68Ga-FAPI PET/CT imaging on the therapeutic management of primary and recurrent pancreatic ductal adenocarcinomas. J Nucl Med. 2021;62:779–86.
Mankoff DA, Eary JF, Link JM, Muzi M, Rajendran JG, Spence AM, et al. Tumor-specific positron emission tomography imaging in patients: [18F]fluorodeoxyglucose and beyond. Clin Cancer Res. 2007;13:3460–9.
Acknowledgements
We would like to thank all the patients who participated in this study. We also thank the staff at the Department of Nuclear Medicine for their contribution to this work.
Funding
This work was supported in part by the National Natural Science Foundation of China (91949121), Guangdong Basic and Applied Basic Research Foundation (2021A1515011099), Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2017J010), and Nanfang Hospital Talent Introduction Foundation of Southern Medical University (123456).
Author information
Authors and Affiliations
Contributions
Conception and design, K. Hu and G. Tang; acquiring data, S. Huang, Y. Tian, Q. Wang, C. Xiao, L. Wang, and Y. Han; analyzing data, L. Wang, Y. Han, Y. Tian, K. Hu, and H. Wu; drafting the manuscript: K. Hu, L. Wang, and H. Wu; revising the manuscript, G. Tang and Y. Han; and enhancing the manuscript’s intellectual content, H. Wu. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All procedures involving human participants were carried out in accordance with the Ethics Committee of Nanfang Hospital (No. NFEC-2020–205) and registered in the Chinese Clinical Trial Registry (ChiCTR2100045757).
Consent to participate
Written informed consent was obtained from all participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kongzhen Hu and Lijuan Wang contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, K., Wang, L., Wu, H. et al. [18F]FAPI-42 PET imaging in cancer patients: optimal acquisition time, biodistribution, and comparison with [68Ga]Ga-FAPI-04. Eur J Nucl Med Mol Imaging 49, 2833–2843 (2022). https://doi.org/10.1007/s00259-021-05646-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05646-z