Abstract
Rationale
Dopamine transporter (DAT) imaging is an important adjunct in the diagnostic workup of patients with Parkinsonism. 18F-FE-PE2I is a suitable PET radioligand for DAT quantification and imaging with good pharmacokinetics. The aim of this study was to determine a clinical optimal simplified reference tissue-based image acquisition protocol and to compare the discriminatory value and effect size for 18F-FE-PE2I to that for 123I-FP-CIT scan currently used in clinical practice.
Methods
Nine patients with early Parkinson’s disease (PD, 64.3 ± 6.8 years, 3M), who had previously undergone a 123I-FP-CIT scan as part of their diagnostic workup, and 34 healthy volunteers (HV, 47.7 ± 16.8 years, 13M) underwent a 60-min dynamic 18F-FE-PE2I PET-MR scan on a GE Signa 3T PET-MR. Based on dynamic data and MR-based VOI delineation, BPND, semi-quantitative uptake ratio and SUVR[t1–t2] images were calculated using either occipital cortex or cerebellum as reference region. For start-and-end time of the SUVR interval, three time frames [t1–t2] were investigated: [15–40] min, [40–60] min, and [50–60] min postinjection. Data for putamen (PUT) and caudate nucleus-putamen ratio (CPR) were compared in terms of quantification bias versus BPND and discriminative power.
Results
Using occipital cortex as reference region resulted in smaller bias of SUVR with respect to BPND + 1 and higher correlation between SUVR and BPND + 1 compared with using cerebellum, irrespective of SUVR [t1–t2] interval. Smallest bias was observed with the [15–40]-min time window, in accordance with previous literature. The correlation between BPND + 1 and SUVR was slightly better for the late time windows. Discriminant analysis between PD and HV using both PUT and CPR SUVRs showed an accuracy of ≥ 90%, for both reference regions and all studied time windows. Semi-quantitative 123I-FP-CIT and 18F-FE-PE2I values and relative decrease in the striatum for patients were highly correlated, with a higher effect size for 18F-FE-PE2I for PUT and CPR SUVR.
Conclusion
18F-FE-PE2I is a suitable radioligand for in vivo DAT imaging with high discriminative power between early PD and healthy controls. Whereas a [15–40]-min window has lowest bias with respect to BPND, a [50–60]-min time window at pseudoequilibrium can be advocated in terms of clinical feasibility with optimal discriminative power. The occipital cortex may be slightly preferable as reference region because of the higher time stability, stronger correlation of SUVR with BPND + 1, and lower bias. Moreover, the data suggest that the diagnostic accuracy of a 10-min static 18F-FE-PE2I scan is non-inferior compared with 123I-FP-CIT scan used in standard clinical practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The dopamine transporter (DAT), a membrane protein expressed on the presynaptic terminals of nigrostriatal dopaminergic neurons, is important for dopamine reuptake from the synaptic cleft. Degeneration of nigrostriatal dopaminergic neurons is a hallmark of Parkinson’s disease (PD) and is associated with progressive loss of dopamine transporters [1, 2]. PD affects 2–3% of population above the age of 65 years and is characterized by tremor, bradykinesia, rigidity, and a wide variety of non-motor symptoms [3]. DAT imaging plays a crucial role in the diagnostic workup of patients with clinically uncertain Parkinsonism as it allows in vivo assessment of dopaminergic system integrity [4].
Until now, DAT imaging in daily clinical practice is performed mainly using single photon emitting tracers such as 123I-FP-CIT-SPECT ([123I] N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)nortropane) [1, 5]. However, PET ligands allow better spatial resolution down to 3–4 mm [1, 5, 6]. Both 11C and 18F labeled PET radioligands bound to the nortropane analog PE2I have been investigated [7,8,9,10]. 18F-FE-PE2I ([18F]-(E)-N-(3-iodoprop-2-enyl)-2β-carbofluoroethoxy-3β- (4′-methyl-phenyl) nortropane) has faster kinetics compared with 11C-PE2I [7, 9] and can therefore be a very suitable radioligand for clinical imaging of the entire nigrostriatal pathway and DAT quantification in PD patients. Its affinity for DAT is comparable with 123I-FP-CIT (Ki = 12 nM) but selectivity is much better (PE2I affinity for serotonin transporter (SERT) and norepinephrine transporter (NET) is 29-fold and 58-fold lower, respectively, than for DAT) [7, 11].
As most brain regions outside the nigrostriatal pathway express little or no DAT, several cortical or cerebellar reference regions can be considered. The use of a dynamic simplified reference tissue model (SRTM) approach has already been validated by several authors for 18F-FE-PE2I [7, 10, 12]. However, for clinical purposes, especially in an aged patient population, a shorter scan time and a simplified quantification protocol to improve patient tolerability are needed. Different approaches for static scanning of 18F-FE-PE2I have been investigated, but no general agreement has been reached so far [5, 12, 13]. Sonni et al. found that the 16.5–42-min time window was optimal as SUVR16.5–42 correlated best with BPND (r2 = 0.98), despite an underestimation of the BPND [13]. Ikoma et al. concluded that the SUVR estimates in the 70–90-min time frame were stable and correlated well with the BP2CM (r = 0.82–0.86), although the BPSUVR70–90 largely overestimated the BP2CM [12]. Jakobson et al. compared the diagnostic performance of 18F-FE-PE2I PET with 123I-FP-CIT SPECT and found that pseudoequilibrium was reached after 50 min for 18F-FE-PE2I, and SUVR50–75 was selected as the optimal time window [5]. In these three studies, the cerebellum (CBL) was used as reference region, although cerebellar time activity curves show slower kinetics than neocortical (e.g., occipital) regions [14]. For clinical DAT imaging using 123I-FP-CIT, the occipital cortex (OC) is widely used as a reference region because it can be delineated and quantified in the same midtransverse planes as the striatum [15].
In view of the existing debate, the aim of this study was to define an optimal static time window and reference region for analysis of 18F-FE-PE2I PET in a clinical setting, optimizing both correlation and bias with respect to SRTM-derived BPND as ground truth and propose a suitable time frame with optimal clinical applicability. In addition, we compared the discriminatory value and effect size for 18F-FE-PE2I with that for 123I-FP-CIT scan currently used in clinical practice, in a set of patients that underwent both investigations.
Materials and methods
Subject demographics (Tables 1 and 2)
For 18F-FE-PE2I, healthy volunteers (HV) were prospectively recruited through website advertisements and local newspapers. Main exclusion criteria were abnormal findings on clinical (neurological or general physical) examination, history of important neurological or psychiatric disorders, and major internal pathology (diabetes, cancer, etc.). For 123I-FP-CIT, 14 historical HV (58.1 ± 12.5 years, 5M) were used, which formed part of the European Normal Control Database (ENC-DAT) that were scanned in our center [16].
PD patients were prospectively recruited in the Movement Disorders Clinic of the University Hospitals Leuven. The diagnosis of PD was based on the UK Parkinson’s Disease Society Brain Bank criteria. Exclusion criteria were a history of neurological or psychiatric diseases other than PD and major internal pathology. All PD patients had previously undergone a 123I-FP-CIT scan as part of their diagnostic workup. Unified Parkinson’s Disease Rating Scale (UPDRS), Hoehn-Yahr (HY) scale, and Mini Mental State Examination (MMSE) scores were determined on medication. Levodopa equivalent daily dose (LEDD) was calculated as described [17].
Written informed consent was given by every participant. This study was approved by the local university hospital Ethics Committee for Research and the study was performed in accordance to the World Medical Association Declaration of Helsinki. All subjects signed an informed consent form.
Data acquisition
All subjects underwent a 60-min dynamic 18F-FE-PE2I PET-MR scan (GE Signa 3T PET-MR). For that, an activity of 144 ± 18 MBq 18F-FE-PE2I was administered. No arterial blood sampling or metabolite analysis was performed. All PD patients were on their standard dopaminergic medication.
PET data were collected in list mode and reconstructed using the following parameters (6 × 15 s, 3 × 30s, 3 × 60s, 3 × 180 s, 9 × 300 s) corrected for randoms, scatter, time-of-flight offset [18], and deadtime. Individual attenuation correction was performed using a validated in-house zero echo time (ZTE) approach [19]. Ordered Subsets Expectation Maximization (28 subsets with 4 iterations), including time-of-flight information, resolution modeling, and Gaussian postsmoothing with a Full-Width-at-Half-Maximum (FWHM) kernel of 4.5 mm (isotropic), was used for reconstruction.
Several simultaneous MR sequences were collected. For this study, we used the volumetric T1-weighted 3D BRAVO sequence (plane: sagittal; TE 3.2 ms; T4 8.5 ms; TI 450 ms; flip angle 12; receiver bandwidth 32; NEX 1).
For 123I-FP-CIT scan, thyroid protection (1 ml of Lugol) prior to injection was administered, and an activity of 170 ± 22 MBq 123I-FP-CIT was injected. 123I-FP-CIT scans of the 14 HV were performed on an E.cam system (Siemens, Erlangen, Germany). Thirty-minute 123I-FP-CIT scans of the 9 PD patients were performed on a GE Discovery 670 SPECT-CT (General Electric, Buc, France). As both groups were scanned on different dual-head cameras, reconstructions were performed offline with the same calculated attenuation correction (uniform attenuation 0.15/cm) to minimize scanner variance. Reconstructions were performed using iterative reconstruction with OSEM (5 iterations and 8 subsets, followed by 3 iterations with 4, 2, and 1 subsets, respectively), scatter correction (acquisition with dual energy window), and modeling of collimator blurring. Gaussian postsmoothing with a kernel of 7.5 mm FWHM was applied. The interval between 123I-FP-CIT scan and 18F-FE-PE2I scan in the 9 PD patients was 6.9 ± 3.8 months (range 1.6–13.0).
Data analysis
18F-FE-PE2I
Motion correction was implemented with a rigid co-registration of each frame to the average of the first 12 frames representing the first 6 min of the PET acquisition. Spatial normalization to the MNI template was performed in PMOD (v3.7, PMOD technologies, Zurich, Switzerland) using the 3D T1 BRAVO and probability maps transformation restricted to the subject-specific gray matter part of the whole brain with a fixed threshold of 0.3. Cerebellar, occipital, striatal, and substantia nigra (SN) time activity curves (TACs) were calculated using the PNEURO tool with the Hammers atlas (N30R83). For the caudate nucleus (CAU) and putamen (PUT), the PMOD parcellation was used as this module allows improved subcortical VOI delineation. BPND (SRTM model) and SUVR images were calculated using both OC and CBL as reference regions. SUVR[t1–t2] images at three time intervals [t1–t2] (minutes postinjection (pi)) were investigated: [15–40], [40–60], and [50–60].
123I-FP-CIT
Spatial normalization and VOI delineation (CAU left and right, PUT left and right, and OC) was performed in PMOD (v3.7) using the individual clinical T1-weighed MRI. The semi-quantitative uptake ratio (SUR) was calculated using the OC as reference region according to the following formula: (mean counts in each VOI)/(mean counts in OC).
General statistics
General statistics were calculated in SPSS (IBM Statistics v 25.0, IBM, NY, USA). For the Bland-Altman analysis, GraphPad Prism (GraphPad Software v5, San Diego, USA) was used.
The Spearman correlation coefficients for 18F-FE-PE2I were calculated between BPND + 1 and SUVR for CAU, PUT, and the ratio CAU/PUT (CPR). Similarly a Bland-Altman analysis was done for CAU, PUT, and CPR. The BPND + 1 was used as gold standard outcome measure. A discriminant analysis with leave-one-out approach was performed to discriminate between PD and HV using PUT and CPR. Additionally, Glass’ effect size (Δ) using the HV group’s standard deviation (SD), Δ = [(meanHV − meanPD)/SDHV], was determined to compare the ability of the different time windows, different regions, and both tracers to differentiate between PD patients and HV.
A Mann-Whitney U test was used for group differences between PD and HV for both 18F-FE-PE2I SUVR and 123I-FP-CIT SUR images with application of the exact significance option. Correlations between 18F-FE-PE2I SUVR50–60,occipital and 123I-FP-CIT SUR and percentage decrease for CAU and PUT compared with HV were determined using the Spearman correlation coefficients.
For all analyses, unilateral values for CAU, PUT, and CPR were used. For HV, we used the left-sided value for CAU, PUT, and CPR. For PD patients with lateralized symptoms (strictly unilateral or bilateral with asymmetry), the value contralateral to the clinically most affected side was used. If the patients had symmetrical symptoms, the left side was chosen.
Results
Demographics and parametric images
Nine PD patients for 18F-FE-PE2I and 123I-FP-CIT, 34 HV for 18F-FE-PE2I, and 14 HV for 123I-FP-CIT were included. Tables 1 and 2 show the demographic variables and clinical parameters for the three groups. Kinetic optimization was performed on all 18F-FE-PE2I scans. For comparison of 18F-FE-PE2I between PD and HV, an age- and gender-matched subgroup of 18F-FE-PE2I HV was used (Table 2).
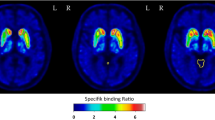
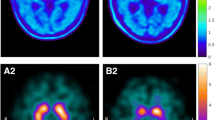
All 9 18F-FE-PE2I and 123I-FP-CIT scans of the PD patients were visually abnormal. Figure 1 shows a typical 18F-FE-PE2I example of midtransverse slices for BPND and the various SUVR[t1–t2] parametric images for both a PD patient and a HV. An example of 18F-FE-PE2I and 123I-FP-CIT for a PD patient and HV is illustrated in Fig. 2.
Midtransverse 18F-FE-PE2I BPND and SUVR parametric images, for all 3 time windows and both reference regions. a Images of a Parkinson’s disease (PD) patient (M, age 67 years, UPDRS mot = 23, HY = 2 ). b A healthy volunteer (HV) (F, age 52 years, UPDRS mot = 3). M, male; y, years; UPDRS III, UPDRS part III; HY, Hoehn and Yahr stage
123I-FP-CIT (upper row) and 18F-FE-PE2I (lower row) images of a 67-year-old Parkinson’s disease (PD) patient (Hoehn and Yahr 2) and healthy volunteers (HV) (45 years old for 123I-FP-CIT, 52 years old for 18F-FE-PE2I). Images in radiological orientation. The red arrow points to the uptake in the substantia nigra
Optimal time window and reference region selection for 18F-FE-PE2I
The Bland-Altman plots of CAU, PUT, and CPR separately for the different time windows and reference regions are shown in Fig. 3. Biases for CAU, PUT, and CPR are reported in Table 3. For CAU and PUT, the absolute value of the bias was generally smaller for PD patients compared with HV. In contrast, CPR biases were smaller for HV than for PD patients. The bias was overall smaller when OC was used as reference region compared with CBL.
Result of the Bland-Altman analysis 18F-FE-PE2I: bias or mean difference between BPND + 1 and SUVR in different time windows for patients with Parkinson’s disease (PD) (n = 9) and healthy volunteers (HV) (n = 34). Dotted lines show the upper and lower 95% limit of agreement; values are shown between brackets. a Cerebellum as reference region. b Occipital cortex as reference region
Looking at the different time intervals, the smallest absolute values of the bias was found for the [15–40] interval, with the exception of CPR in HV where most late time windows had a smaller bias. Bias for CPR was overall small (0.193 or smaller). The bias for CAU, PUT, and CPR was mainly negative for the two late time intervals due to an average overestimation of BPND + 1.
Correlations between BPND + 1 and SUVR for the different time windows are shown per reference region in Fig. 4. For CAU, PUT, and CPR, we found highly significant correlations (ρ > 0.71, p < 0.001) between BPND + 1 and SUVR values for all three time windows and both reference regions. However, the correlation for the [15–40] (range ρ [0.71–0.90]) was lower than for the [40–60] (range ρ [0.88–0.94]) and the [50–60]-min (range ρ [0.91–0.96]) time interval. For all regions and all time windows, the correlation was equal or higher with the OC as reference region compared with the CBL.
Correlation (Spearman) between 18F-FE-PE2I BPND + 1 and SUVR, for each time window and reference region for patients with Parkinson’s disease (PD) (n = 9) and healthy volunteers (HV) (n = 34). Dotted line shows line of identity. a Cerebellum as reference region. b Occipital cortex as reference region
Group differences and discriminant analysis between PD patients and age-matched HV using 18F-FE-PE2I and 123I-FP-CIT
The boxplot of the SUVR[t1–t2] values for the PD patients and HV for every time window is shown in Fig. 5. The effect sizes were 4.1 for the early time window, 4.1 for the intermediate time window, and 3.9 for the late time window for OC, and 3.8, 3.7, and 3.9 for CBL, respectively.
The results of the discriminant analysis are shown in Table 4. All time frame windows and both the PUT and CPR resulted in high discriminative power of more than 90%. Accuracy was identical or higher using the OC as reference region.
The Mann-Whitney U test showed a significant difference between PD and HV using both tracers for the CAU (p = 0.002 for 18F-FE-PE2I and p = 0.002 for 123I-FP-CIT) and PUT (p < 0.001 for 18F-FE-PE2I and p = 0.001 for 123I-FP-CIT). CPR was only significantly different for 18F-FE-PE2I (p < 0.001). Effect sizes were 1.5, 3.9, and − 13.2 for the CAU, PUT, and CPR with 18F-FE-PE2I, and 1.5, 1.7, and − 0.4 for the CAU, PUT, and CPR with 123I-FP-CIT (Table 5). The percentage DAT decrease in PD patients was 22.0 ± 16.6% for CAU and 59.4 ± 6.7% for PUT with 18F-FE-PE2I SUVR50–60,occipital, and 22.7 ± 12.4% for CAU and 33.4 ± 10.2% for PUT with 123I-FP-CIT SUR (Fig. 6). In addition, 18F-FE-PE2I, unlike 123I-FP-CIT, allowed measurement of DAT levels in the SN. 18F-FE-PE2I showed a decrease for SN of 17.3 ± 8.6% in PD compared with HV, with an effect size of 1.7 (Fig. 6c).
Percentage decrease of 123I-FP-CIT semi-quantitative uptake ratio and 18F-FE-PE2I standardized uptake value (50-60 min post-injection, occipital cortex as pseudoreference region) in Parkinson’s disease patients compared with healthy volunteers in the caudate nucleus (a), putamen (b), and substantia nigra (c)
18F-FE-PE2I SUVR50–60,occipital and 123I-FP-CIT SUR for CAU and PUT were significantly correlated with ρ = 0.87 (p = 0.002), 0.88 (p = 0.002), and 0.92 (p < 0.001). The percentage decrease in PD compared with HV with 18F-FE-PE2I and 123I-FP-CIT for the CAU and PUT were also significantly correlated, with ρ = 0.87 (p = 0.002) and 0.88 (p = 0.002), respectively, as shown in Fig. 7. In the PD group, no correlation was observed between motor UPDRS scores and either 18F-FE-PE2I or 123I-FP-CIT uptake.
Discussion
18F-FE-PE2I-PET has been shown to be an excellent imaging tool for in vivo DAT quantification in the entire nigrostriatal tract [1, 7, 10]. However, some discussion exists about the optimal reference region and time window. Therefore, in the present study, we aimed to define the optimal static scanning protocol for clinical applications.
Until now, only the CBL has been used as a reference region for 18F-FE-PE2I-PET scans. However, Odano et al. showed that the CBL has slower kinetics compared with the OC, suggesting that a cortical region (e.g., OC) may be better suited as a reference region. Additionally, the OC is situated in the same transversal plane as the striatum, which facilitates delineation. Moreover, the OC is also utilized as reference region for clinical 123I-FP-CIT-SPECT DAT scans. Therefore, we performed a head-to-head comparison between CBL and OC as reference regions. Our results demonstrated smaller bias, higher correlations, and identical or higher discriminative power using OC for all time windows. Therefore, in future studies, the OC should be considered as the reference region for 18F-FE-PE2I-PET scans instead of the CBL.
In contrast to some research purposes where closeness to BPND as gold standard is the key, discriminative power and clinical feasibility are most important for clinical practice. Therefore, we compared the three time windows in terms of quantification, discriminative power, and clinical feasibility.
First, we looked at the quantification. Overall, the smallest bias was observed with the [15–40] time window compared with the late time windows. However, the correlation between BPND + 1 and SUVR was slightly better for the late time windows compared with [15–40]. Therefore, for absolute quantification, the early time window seems more optimal as bias was substantially smaller with only a slight decrease in correlation.
Second, we performed a discriminant analysis to determine the respective discriminative power. The discriminant analysis for the PUT and CPR between the PD patients and HV resulted in high sensitivity, high specificity, and high accuracy using all time windows and both reference regions. Effect sizes were similar for all three time windows. In conclusion, all time windows are able to distinguish between PD and HV.
Finally, we addressed the clinical feasibility. Scan length is of critical importance especially in patients with neurodegenerative diseases such as PD. Long acquisition may result in substantial discomfort for this vulnerable patient population. Also, unexpected practical issues may disturb the suggested time schedule. Therefore, the shortest possible acquisition time window during pseudoequilibrium is preferred for clinical use. Likewise, for the current clinical standard, 123I-FP-CIT SPECT, scanning is performed 3 to 6 h pi when pseudoequilibrium is reached. For 18F-FE-PE2I-PET, pseudoequilibrium is reached at 50 min pi, reducing the waiting time significantly compared with 123 I-FP-CIT SPECT. Moreover, our data showed that a short 10-min static scan appeared to be sufficient for clinical practice.
The correlation in binding potential and binding ratios between the two tracers was high, and the discriminatory value between healthy volunteers and patients was similar which is in line with the findings by Jakobson et al. [5]. Furthermore, effect sizes of PUT and CPR values between patients and controls for 18F-FE-PE2I were substantially higher, which is due to the combination of a higher dynamic range for 18F-FE-PE2I in striatal abnormalities in patients (the percentage DAT decrease measured by 18F-FE-PE2I was approximately 60% compared with 30% for 123I-FP-CIT) as well as lower intersubject variability. Both can be due to better imaging statistics per VOI and better spatial resolution with lower partial volume effects. It can therefore be reasonably assumed that, also in a clinical population of uncertain Parkinsonism, 18F-FE-PE2I will be able to discriminate larger differences between patients with neurodegenerative Parkinsonism versus conditions without nigrostriatal DAT involvement such as essential tremor, psychogenic, or drug induced Parkinsonism.
In conclusion, our results suggest to scan early, 15 to 40 min pi, in case absolute quantification is the main aim. For clinical practice the [50–60] time frame seems most optimal both in terms of accuracy and clinical feasibility.
There are some limitations to our study. First, we had a relatively small patient group (9 PD patients). Nevertheless, even with 9 patients, good effect sizes were obtained. Second, the dynamic scan was acquired during 60 min; therefore, our late time frame during pseudoequilibrium lasted only 10 min which may have caused lower signal to noise. However, even with a 10-min time frame, we obtained similar discriminative power compared with the other time windows. Finally, our study only enrolled patients with an established clinical diagnosis of PD. Patients with unclear Parkinsonism were not included. Therefore, conclusions about clinical usefulness of 18F-FE-PE2I needs to be verified in a clinically representative sample of patients with uncertain Parkinsonism.
Conclusion
OC as reference region seems preferable over CBL because of the higher time stability, higher correlation of SUVR with BPND + 1, and lower bias. Of the three time frames, [15–40] shows the lowest bias and may therefore be the optimal time window for quantification studies. For clinical use, [50–60] seems more optimal as correlations of SUVR with BPND + 1 are higher and pseudoequilibrium is reached. Direct comparison in patients with PD shows higher differences between patients and controls as well as larger effect sizes, thereby suggesting at least non-inferiority of 18F-FE-PE2I compared with 123I-FP-CIT.
References
Fazio P, Svenningsson P, Cselenyi Z, Halldin C, Farde L, Varrone A. Nigrostriatal dopamine transporter availability in early Parkinson’s disease. Mov Disord. 2018;33(4):592–9.
Uhl GR. Dopamine transporter: basic science and human variation of a key molecule for dopaminergic function, locomotion, and Parkinsonism. Mov Disord. 2003;18(Suppl 7):S71–80.
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013.
Brooks DJ. Molecular imaging of dopamine transporters. Ageing Res Rev. 2016;30:114–21.
Jakobson Mo S, Axelsson J, Jonasson L, Larsson A, Ogren MJ, Ogren M, et al. Dopamine transporter imaging with [(18)F]FE-PE2I PET and [(123)I]FP-CIT SPECT-a clinical comparison. EJNMMI Res. 2018;8(1):100.
Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun. 2008;29(3):193–207.
Sasaki T, Ito H, Kimura Y, Arakawa R, Takano H, Seki C, et al. Quantification of dopamine transporter in human brain using PET with 18F-FE-PE2I. J Nucl Med. 2012;53(7):1065–73.
Varrone A, Halldin C. New developments of dopaminergic imaging in Parkinson’s disease. Q J Nucl Med Mol Imaging. 2012;56(1):68–82.
Varrone A, Toth M, Steiger C, Takano A, Guilloteau D, Ichise M, et al. Kinetic analysis and quantification of the dopamine transporter in the nonhuman primate brain with 11C-PE2I and 18F-FE-PE2I. J Nucl Med. 2010;52(1):132–9.
Fazio P, Svenningsson P, Forsberg A, Jonsson EG, Amini N, Nakao R, et al. Quantitative analysis of (1)(8)F-(E)-N-(3-iodoprop-2-enyl)-2beta-carbofluoroethoxy-3beta-(4′-methyl-phenyl) nortropane binding to the dopamine transporter in Parkinson disease. J Nucl Med. 2015;56(5):714–20.
Pinborg LH, Ziebell M, Frokjaer VG, de Nijs R, Svarer C, Haugbol S, et al. Quantification of 123I-PE2I binding to dopamine transporter with SPECT after bolus and bolus/infusion. J Nucl Med. 2005;46(7):1119–27.
Ikoma Y, Sasaki T, Kimura Y, Seki C, Okubo Y, Suhara T, et al. Evaluation of semi-quantitative method for quantification of dopamine transporter in human PET study with (1)(8)F-FE-PE2I. Ann Nucl Med. 2015;29(8):697–708.
Sonni I, Fazio P, Schain M, Halldin C, Svenningsson P, Farde L, et al. Optimal acquisition time window and simplified quantification of dopamine transporter availability using 18F-FE-PE2I in healthy controls and Parkinson disease patients. J Nucl Med. 2016;57(10):1529–34.
Odano I, Varrone A, Hosoya T, Sakaguchi K, Gulyas B, Padmanabhan P, et al. Simplified estimation of binding parameters based on image-derived reference tissue models for dopamine transporter bindings in nonhuman primates using [(18)F]FE-PE2I and PET. Am J Nucl Med Mol Imaging. 2017;7(6):246–54.
Vermeulen RJ, Wolters EC, Tissingh G, Booij J, Janssen AG, Habraken J, et al. Evaluation of [123I] beta-CIT binding with SPECT in controls, early and late Parkinson’s disease. Nucl Med Biol. 1995;22(8):985–91.
Varrone A, Dickson JC, Tossici-Bolt L, Sera T, Asenbaum S, Booij J, et al. European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging. 2013;40(2):213–27.
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–53.
Rezaei A, Schramm G, Van Laere K, Nuyts J. Estimation of crystal timing properties and efficiencies for the improvement of (joint) maximum-likelihood reconstructions in TOF-PET. IEEE Trans Med Imaging. 2019.
Schramm G, Koole M, Willekens S, Ahmadreza R, Van Weehaeghe D, Delso G et al. Regional accuracy of ZTE-based attenuation correction in static and dynamic brain PET/MR. Medical Physics. 2018.
Acknowledgments
The authors explicitly want to thank Mr. Kwinten Porters and Mr. Jef Van Loock for their contributions to the scanning and data handling, and the PET radiopharmacy and medical physics teams of UZ Leuven for their skilled contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Koen Van Laere and Wim Vandenberghe are Senior Clinical Investigators of the Fund for Scientific Research, Flanders, Belgium (FWO). Donatienne Van Weehaeghe and Aline Delva are PhD fellows of the FWO. Jenny Ceccarini is a postdoctoral fellow of the FWO. There are no other conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (UZ/KU Leuven Ethical Committee Belgium) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Rights and permissions
About this article
Cite this article
Delva, A., Van Weehaeghe, D., van Aalst, J. et al. Quantification and discriminative power of 18F-FE-PE2I PET in patients with Parkinson’s disease. Eur J Nucl Med Mol Imaging 47, 1913–1926 (2020). https://doi.org/10.1007/s00259-019-04587-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04587-y