Abstract
Purpose
The primary aim of this retrospective, single-centre analysis was to assess the performance of 68Ga-PSMA-11 PET/CT in prostate cancer (PCa) patients in early PSA failure after radical prostatectomy (RP). The secondary aim was to assess the potential impact of 68Ga-PSMA-11 PET/CT on treatment strategy.
Methods
68Ga-PSMA-11 PET/CT is performed in our institution within an investigational new drug (IND) trial in PCa patients with biochemical recurrence (BCR). The records of all patients enrolled between March 2016 and July 2017 were evaluated. These records were retrospectively analysed according to the following inclusion criteria: (a) RP as primary therapy, (b) proven BCR, ©) PSA levels in the range 0.2–0.5 ng/ml at the time of the 68Ga-PSMA-11 PET/CT investigation, and (d) no salvage radiotherapy (S-RT) performed after recurrence. The performance of 68Ga-PSMA-11 PET/CT was evaluated in terms of detection rate on a per-patient and a per-region basis (local vs. distant lesions). We further performed an intention-to-treat (ITT) analysis. The patient cohort was grouped into three subpopulations, blinded to the 68Ga-PSMA-11 PET/CT results, according to the patients’ characteristics and different patterns of treatment: (1) S-RT (with or without systemic treatment), (2) stereotactic body radiotherapy (SBRT) (with or without systemic treatment), and (3) systemic treatment. The treatment strategy was re-evaluated for each patient taking into consideration the 68Ga-PSMA-11 PET/CT images.
Results
We enrolled 119 PCa patients (mean age 66 years, range 44–78 years) with a mean PSA level at the time of 68Ga-PSMA-11 PET/CT of 0.34 ng/ml (median 0.32 ng/ml, SD ±0.09, range 0.20–0.50 ng/ml). 68Ga-PSMA-1 1 PET/CT was positive in 41 of the 119 patients, resulting in an overall detection rate of 34.4%. 68Ga-PSMA-11 uptake was observed in the prostate bed (3 patients, 2.5%), in the pelvic lymph nodes (21, 17.6%), in the retroperitoneal lymph nodes (4, 3.4%) and in the skeleton (21, 17.6%). Regarding ITT, 81 patients (68.1%) were considered possible candidates for S-RT only in the prostate bed and none of the patients (0%) for SBRT. According to the 68Ga-PSMA-11 PET/CT results, the intended treatment was changed in 36 patients (30.2%). According to the PET/CT results, S-RT was recommended in 70 patients (58.8%), only to the prostate bed in 58 (48.7%) and SBRT in 29 (24.4%). The intended RT planning was modified in 36 (87.8%) of 41 patients with a positive 68Ga-PSMA-11 PET/CT result.
Conclusion
In our patient series with PSA levels <0.5 ng/ml, 68Ga-PSMA-11 PET/CT had a detection rate of 34.4%. In the ITT analysis, 30.2% of patients had a change in the intended treatment. These data support the hypothesis that 68Ga-PSMA-11 PET/CT is a useful procedure in the management of PCa patients showing early recurrence after RP, and should be implemented in routine clinical practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the most frequent cancer in men in Western countries, and in men second only to lung cancer for mortality [1]. Localized or locally advanced PCa is mainly treated with radical prostatectomy (RP), external beam radiotherapy (EBRT), or brachytherapy. However, recurrence after radical treatment is frequent, occurring in up to approximately 50% of men [2,3,4]. Accordingly, after RP, increasing serum PSA levels greater than 0.2 ng/ml, confirmed by two consecutive measurements, can be reliably related to either residual or recurrent disease [5, 6]. Currently, salvage radiotherapy (S-RT) to the prostatic fossa, extended to the pelvic lymph nodes (LNs) in high-risk patients, is one of the most successful curative treatments in patients experiencing biochemical recurrence (BCR) after RP. Furthermore, the PSA level before S-RT is a highly significant predictor of disease progression, with more favourable outcomes observed at low PSA levels (0.50 ng/ml or lower) [7]. Some authors have suggested that S-RT at the earliest signs of recurrence after RP may result in long-term benefit in approximately 50% of patients, and the 2017 European Association of Urology (EAU) guidelines advise performing S-RT in patients experiencing BCR after RP as early as reasonably possible if PSA serum levels reach detectable levels (PSA <0.5 ng ml) [5]. On the other hand, up to 50% of patients may experience progression of the disease after S-RT. The most recent literature [8] confirms that a metastasis-directed therapy approach based on PET/CT imaging is safe and improves patient outcomes.
In patients with recurrent and/or metastatic disease, computed tomography (CT), magnetic resonance (MRI) and bone scintigraphy can be used for detecting cancer site(s) of relapse [5], despite less than optimal accuracy. Many PET radiotracers, including choline, acetate, fluciclovine and fluorodeoxyglucose, have also been proposed for use in the investigation of recurrent PCa [9,10,11]. Interest in the use of prostate-specific membrane antigen (PSMA) for PET target imaging in PCa patients has recently increased [12, 13]. PSMA is a transmembrane glycoprotein with both intracellular and extracellular domains with increased expression in PCa cells [14]. Its transmembrane location and internalization after ligand binding make it a favourable target for imaging. The first agent released, labelled with 68Ga (PSMA-HBED-CC or PSMA-11), quickly became the most commonly used radiotracer for PSMA-based PET imaging. According to the most recent literature [13, 15,16,17], PSMA-11 PET/CT has a higher detection rate in the lower range of PSA levels during recurrence than direct competitors such as choline or fluciclovine PET/CT [15, 17,18,19,20,21]. Commercial interest in 18F-labelled PSMA has also increased because of the possibility of improving supply through large production volumes and long-distance delivery that would allow centres without an on-site cyclotron and/or a 68Ge/68Ga generator to perform PSMA imaging [22].
The primary aim of this retrospective, single-centre analysis was to assess the performance of 68Ga-PSMA-11 PET/CT in PCa patients with serum PSA levels ≤0.5 ng/ml during BCR after RP, assessed by per-patient and per-lesion analyses. The potential impact of 68Ga-PSMA-11 PET/CT on treatment strategy in this cohort of patients was also assessed.
Materials and methods
Inclusion criteria and intention-to-treat analysis
68Ga-PSMA-11 PET/CT is performed in our institution within an investigational new drug (IND) trial in PCa patients with BCR approved by the local ethics committee (EudraCT 2015-004589-27 OsSC). All the patients considered in the present retrospective analysis were patients enrolled in the prospective, single-centre trial. All patients provided signed informed consent prior to PET/CT scan. The records of all patients enrolled between March 2016 and July 2017 were retrospectively analysed. The inclusion criteria for the present analysis were: (a) RP as primary therapy, (b) proven BCR, ©) PSA levels in the range 0.2–0.5 ng/ml and (d) no S-RT performed after recurrence.
An intention-to-treat (ITT) analysis was also performed. The cohort of patients was grouped into three different subpopulations according to their characteristics and the different patterns of treatment. A radiation oncologist and a urologist, blinded to the PSMA results, defined the three subpopulations in accordance with the National Comprehensive Cancer Network (NCCN) guidelines (version 2.2017): (1) S-RT (with or without systemic treatment), (2) stereotactic body radiotherapy (SBRT, with or without systemic treatment), and ©) systemic treatment (androgen-deprivation therapy, ADT).
The radiation oncologist and the urologist adopted the NCCN guidelines (version 2.2017) with adjustments on the basis of the best clinical practice for patient treatment planning. Moreover, although SBRT in international guidelines such as the NCCN guidelines is not considered an option in oligometastatic patients, there is increasing evidence in the literature [23,24,25,26,27,28] suggesting its usefulness in this setting, and SBRT was included as a treatment option in our cohort of patients. According to the NCCN guidelines, S-RT to the prostate bed is suggested when conventional imaging is negative or positive only in the prostate bed. S-RT to the prostate bed and pelvic LNs was planned in pN1 patients with positive imaging in one or more pelvic LNs not previously treated with RT, whereas patients with imaging showing positive pelvic LNs, who had undergone previous radiation treatment were candidates for pelvic LN dissection (PLND). Simultaneous integrated boost intensity-modulated RT was considered together with S-RT when imaging was positive In the prostate bed or pelvic LNs. Moreover, in patients with positive conventional imaging showing five or fewer metastatic lesions (LNs and/or distant metastases) SBRT with or without ADT was suggested. Finally, patients with positive conventional imaging and more than five disease locations (LNs and/or distant metastases) were candidates for ADT with or without palliative RT to symptomatic lesions.
Radiopharmaceuticals and PET/CT acquisition
68Ga-PSMA was synthesized in the radiopharmacy of the Service of Nuclear Medicine of the S.Orsola Malpighi University Hospital of Bologna. 68Ga-PSMA-HBED-CC(Glu-NH-CO-NH-Lys-(Ahx)-[[68Ga]Ga(N,N′-bis-[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N,N′-diacetic acid]) (68Ga-PSMA-11) was prepared using a procedure similar to that described by Eder et al. [29], transferred to a cassette-based automated synthesis module (Modular-Lab, PharmTracer; Eckert & Ziegler, Berlin) based on acetone-free cation exchange postprocessing.
68Ga-PSMA-11 PET/CT imaging was performed on either a Discovery STE or a Discovery 710 PET/CT hybrid system (GE Healthcare) after intravenous injection of 68Ga-PSMA–ligand complex at a mean dose of 2 MBq/kg of 68Ga-PSMA-11 (dose range 150 ± 50 MBq). The tomographs were validated for proper quantification and quality of the images recorded. Daily quality control procedures were performed. Patients did not need any preparation before the procedure. An attenuation-corrected whole-body scan (skull base to mid-thighs) in 3D (emission time 2 min per bed position with an axial field of view of 15.6 cm per bed position) starting 60 min after tracer injection was acquired. A low-dose CT scan was performed for attenuation correction of the PET emission data and contrast medium was not used. Emission data were also corrected for scatter and random coincidence events by dedicated software. All 68Ga-PSMA-11 PET/CT images were analysed with dedicated software (eNTEGRA; GE Healthcare).
Image interpretation
Reading and interpretation of PET/CT images was done independently by two nuclear medicine physicians with oncology experience. Disagreements were resolved by consensus. The criteria used to define PSMA-positive lesions suspicious for PCa location were in accordance with the current literature [30,31,32]. Visual interpretation was the main criterion to reach the final diagnosis. Semiquantitative analysis of all suspected lesions was performed by calculating the maximum standardized uptake value (SUVmax). The dimensions of pathological findings were assessed using the low-dose CT scan. The uptake of 68Ga-PSMA-11, i.e. the tracer concentration of the region detected in the image, was quantified in terms of SUVmax. Each suspicious PET lesion was categorized as either positive or negative. Any area of focal uptake of PSMA higher than the surrounding background without correlation with physiological tracer uptake was the main criterion of positivity, aside from the presence of lesions on the low-dose CT scan. PET-positive lesions were classified as suspected local relapse (prostate/prostate bed relapse and/or iliac LNs and/or pararectal LNs) or suspected distant relapse (retroperitoneal LNs and/or LNs above the iliac bifurcation and/or bone lesions and/or visceral lesions). An interobserver analysis was not performed in this study. 68Ga-PSMA-11 PET/CT findings were validated when feasible by histology or correlated with lesion-targeted imaging, including 11C-choline PET/CT, pelvic MRI, contrast-enhanced CT and/or bone scan.
Statistical analysis
All the data reported are expressed as means, medians, standard deviations (SD) and ranges. PSA kinetics were calculated according to the method described by Khan et al. [33] and at least two PSA measurements were performed during the 6 months prior to the PET/CT scan. Demographic and clinical variables were assessed by a descriptive analysis. Continuous variables were compared between the two groups using the nonparametric Mann-Whitney test in view of the asymmetric distribution of the variables. All tests were two-sided. Statistical significance was assumed for p values less than 0.05. All data were assessed using the SPSS Statistics software package (version 21.0.0, IBM Corp., Armonk, NY).
Results
Population characteristics
We enrolled 119 consecutive patients with BCR after RP. Their median age was 66 years (range 44–78 years) and all patients underwent 68Ga-PSMA-11 PET/CT with a serum PSA level in the range 0.2–0.5 ng/ml (mean PSA value 0.34 ng/ml, median 0.32 ng/ml; SD ±0.09). Of the 119 patients, 9 (7.6%) were receiving ADT at the time of the scan and 28 (23.5%) received ADT during recurrence. None of the patients enrolled had had previous S-RT. Five patients (4.2%) had had previous S-PLND. Complete clinical data are presented in Table 1.
PET/CT performance
In the patient-based analysis, 68Ga-PSMA-11 PET/CT was positive in 41 patients (34.4%). Pathological PSMA uptake was observed in the prostatic fossa in 3 patients (2.5%), in pelvic LNs in 21 (17.6%), in retroperitoneal LNs in 4 (3.4%) and in bone in 21 (17.6%). No visceral metastases (e.g. lung or liver) were observed. Additionally, 25 patients (21.0%) had a single PSMA-positive lesion, 13 patients (10.9%) had two to five PSMA-positive lesions (oligometastatic patients), and 3 patients (2.5%) had more than five PSMA-positive lesions (multimetastatic patients). Regarding bone metastasis, 8 patients (6.7%) had two or more bone lesions and 13 patients (10.9%) had a single bone lesion. Table 2 shows PSA levels, PSA doubling time (PSAdt) and PSA velocity (PSAvel) in both PSMA-positive and PSMA-negative patients.
The lesion-based analysis showed a total of 86 lesions detected with 68Ga-PSMA-11 PET/CT: 3 in the prostatic fossa, 32 pelvic LNs, 10 extrapelvic LNs (including retroperitoneal nodes) and 41 bone lesions. These results are presented in Table 3.
The 68Ga-PSMA-11 PET/CT results were validated mostly by clinical follow-up (correlative imaging and/or decreasing or increasing PSA levels after S-RT). Among the 41 positive patients, 23 had clinical follow-up. To the best of our knowledge, none of these was considered false-positive. Negative scans were considered negative by definition. The Mann-Whitney test showed a significant difference between 68Ga-PSMA-11 PET/CT-positive and 68Ga-PSMA-11 PET/CT-negative patients in PSA levels (higher values in positive patients, p = 0.035), PSAdt and PSAvel (faster kinetics in positive patients; p < 0.001 for PSAdt, p = 0.007 for PSAvel). The analysis comparing PSA levels and PSA kinetics in PET-positive and PET-negative patients is presented in Table 2.
ITT analysis
The radiation oncologist and urologist identified 74 patients (62.2%) as possible candidates for S-RT in the prostatic fossa, 7 (5.9%) for S-RT in the prostatic fossa and pelvic LNs, 0 (0%) for SBRT, and 38 (31.9%) for ADT. After reviewing the 68Ga-PSMA-11 PET/CT results, the radiation oncologist and urologist changed their treatment strategy in 36 patients (30.2%). The ITT analysis performed according to 68Ga-PSMA-11 PET/CT findings showed that 70 patients (58.8%) became candidates for S-RT (to the prostatic fossa in 58, 48.7%; to both the prostatic bed and the pelvic LNs in 12, 10.1%), 29 patients (24.4%) became candidates for SBRT (to local LNs in 11 patients; to distant LNs in 1; to bone in 18), 1 patient (0.8%) became a candidate for S-PLND due to previous LN RT, and 21 patients (17.6%) became candidates for ADT. The results of the ITT analysis are presented in detail in Table 4 and Fig. 1. Therefore, of 41 patients positive on 68Ga-PSMA-11 PET/CT, radiotherapy planning was modified in 36 |87.8%). The clinical management of the remaining five PSMA-positive patients was not significantly modified in comparison with the PSMA-blinded ITT analysis, due to: (1) previous pelvic RT in high-risk patients with PSMA-positive pelvic LNs (two patients), (2) PSMA scan suggesting distant multimetastatic spread (three patients) (Figs. 2, 3 and 4).
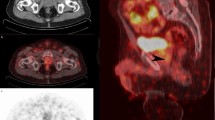
A 68-year-old patient treated with RP and PLND for PCa (GS 4 + 4, pT2cN0(0/12)Mx, PSA nadir 0.01 ng/ml). No adjuvant therapies were performed and BCR occurred 3 years later with a PSA level of 0.20 ng/ml. The PSA level increased to 0.26 ng/ml and the patient was selected for 11C-choline PET/CT imaging which was negative. A subsequent 68Ga-PSMA-11 PET/CT scan revealed pathological PSMA uptake in the right prostate bed (arrows, SUVmax 24.9) close to a surgical clip. The patient was later treated with S-RT to a planned target volume which included the PET-positive finding. A PSA level of 0.03 ng/ml 12 months after treatment indicated a complete response
A 65-year-old patient treated with RP and PLND for PCa (GS 4 + 3, pT3bN0Mx). Adjuvant RT and S-PLND were performed. BCR occurred 5 years later with a PSA level of 0.30 ng/ml. No ADT was administered and 68Ga-PSMA-11 PET/CT imaging (PSA level 0.45 ng/ml at the time of the scan) revealed significant uptake in a single left internal iliac lymph node (arrows, SUVmax 20, maximum diameter 6 mm)
A 60-year-old patient treated with RP and PLND for PCa (GS = 5 + 4, pT3bpN1Mx). MRI was performed 5 months after BCR and showed metastatic lesions in two lumbar vertebrae. 68Ga-PSMA-11 PET/CT imaging (PSA level 0.40 ng/ml at the time of the scan) revealed metastatic bone disease in multiple areas of PSMA uptake without significant alterations on CT images
Discussion
Despite the retrospective analysis, the patient series was enrolled prospectively within an IND trial, and none of the included patients received S-RT during recurrence. Only a few studies are currently evaluating the clinical role of PSMA PET/CT in a homogeneous cohort of relapsing patients showing very low levels of PSA (<0.5 ng/ml) after RP. Nevertheless, our results show a slightly lower detection rate (34.4%) to that found previously in patients with this range of PSA levels [34,35,36,37] and lower than that of 68Ga-PSMA-PET/MRI [38]. In a retrospective multicentre study in 270 patients, Calais et al. [36] found a detection rate of 40.5% in a subpopulation with PSA levels <0.5 ng/ml. A meta-analysis by Perera et al. [20] showed that the detection rate in patients with PSA levels in the range 0.2–0.99 ng/ml is in the range 44–69%. Emmett et al. [39] investigated 164 patients with recurrence after RP with PSA levels in the range 0.05–1.0 ng/ml. PSMA PET/CT was positive in 61% of the patients: 38 in the prostatic fossa, 41 in pelvic LNs, and 23 with distant lesions. Finally, in a recent study by Rauscher et al. [40], PSMA PET/CT was positive in 74 of 134 patients (55%) with PSA levels in the range 0.2–0.5 ng/ml after RP.
The lower detection rate observed in our study in comparison with that found in other studies can be explained by the fact that our patient series comprised a very selected and homogeneous cohort of consecutive patients with proven BCR after RP and without previously administered S-RT. Furthermore, only 7.6% of patients had received ADT at the time of the scan. The concomitant administration of ADT in patients with recurrence with increasing PSA levels has recently been reported as the most significant predictor of a positive 68Ga-PSMA-11 PET/CT scan [40].
A single PSMA-positive lesion was observed in 21.0% of our patients, while oligometastatic disease was observed in 10.9% and multimetastatic disease in 2.5%. These results are similar to those found by Ceci et al. [13] in 70 patients with BCR or persistently elevated PSA levels after radical therapy (RP or EBRT). In their study, 11.4% of patients had oligometastatic disease. In a subpopulation of patients with PSA levels <0.5 ng/ml, Mamede et al. [41] found that 11C-choline PET/CT identified the site of PCa relapse in 21.1% of patients. Therefore our results are consistent with those of Morigi et al. [21] showing a higher detection rate with 68Ga-PSMA-11 than with 11C-choline in PCa patients with very low PSA levels previously treated with radical intent and with increasing PSA levels.
The secondary aim of the current study was to evaluate the impact of 68Ga-PSMA-11 PET/CT on treatment strategy through an ITT analysis. The ITT analysis showed that the theoretical treatment strategy was changed in 36 patients (30.2%). Especially, when the 68Ga-PSMA-11 PET/CT result was positive, the radiation oncologist and urologist opted for a change in the intended treatment in 87.8% of patients. In particular, SBRT was reconsidered as a viable option while S-RT was often tailored to include the PSMA-positive lesions within the irradiation field. Therefore, in this selected group of patients, PSMA imaging led to a significant change in management in approximately one third of the overall patient population. These findings are consistent with those of Afaq et al. [42] and Calais et al. [36] in subgroups with PSA levels in the range 0.2–0.5 ng/ml. Nevertheless, these results are not consistent with those of other studies performed in less selected and more heterogeneous populations in which a change in management was reported in 76% of patients [43].
According to our results, 68Ga-PSMA-11 PET/CT should be suggested in patients in the early phase of BCR after RP, namely those showing very low PSA levels. As reported in the literature, PSA levels ≤0.50 ng/ml are significantly associated with more favourable outcomes after S-RT [7]. Accordingly, the inclusion of the positive 68Ga-PSMA-11 PET/CT findings in the field of irradiation may allow a tailored approach, thus reducing the incidence of failure of S-RT [8]. Recently Emmett et al. [39] observed a treatment response (defined as PSA decrease of >50%) in 71 of 99 patients (71.7%) in whom S-RT was performed according to the 68Ga-PSMA-11 PET/CT results.
The present study had some limitations. The analysis was performed retrospectively and only one centre was involved. Another limitation is the lack of an interobserver analysis for PET/CT reading. Moreover, to determine the impact of 68Ga-PSMA-11 PET/CT on therapeutic management, an ITT analysis was performed. The design of this study precluded analysis of the impact of 68Ga-PSMA-11 PET/CT on clinical outcomes. However, in order to reproduce the most reliable scenario, the definition of the possible treatment, as assessed by the radiation oncology and urologist was obtained by consensus and blinded to the 68Ga-PSMA-11 PET/CT images. Finally, the lack of validation by histology is a common limitation in imaging studies, especially in recurrent PCa, since the biopsy of PET-positive lesions is generally not feasible.
Conclusion
In this cohort of 119 consecutive patients with recurrent PCa with PSA levels <0.5 ng/ml 68Ga-PSMA-11 PET/CT showed a detection rate of 34.4%. In particular, in 19.3% of patients the site of disease was found to be outside the pelvis, namely extrapelvic LNs or bone metastases. The ITT analysis showed that 30.2% of patients had a change in the intended treatment blinded to 68Ga-PSMA-11 PET/CT results. These results support the hypothesis that 68Ga-PSMA-11 PET/CT is a valid procedure in the management of patients with recurrent PCa with low PSA levels after radical surgery, and support the implementation of this imaging procedure in routine clinical practice.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. https://doi.org/10.3322/caac.20107.
Rosenbaum E, Partin A, Eisenberger MA. Biochemical relapse after primary treatment for prostate cancer: studies on natural history and therapeutic considerations. J Natl Compr Cancer Netw. 2004;2:249–56.
Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol. 2007;51:1175–84. https://doi.org/10.1016/j.eururo.2007.01.015.
Punnen S, Cooperberg MR, D'Amico AV, Karakiewicz PI, Moul JW, Scher HI, et al. Management of biochemical recurrence after primary treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2013;64:905–15. https://doi.org/10.1016/j.eururo.2013.05.025.
Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. https://doi.org/10.1016/j.eururo.2016.08.002.
Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H. Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol. 2001;165:1146–51.
Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41. https://doi.org/10.1200/jco.2006.08.9607.
Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, Bruycker AD, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–53. https://doi.org/10.1200/jco.2017.75.4853.
Umbehr MH, Müntener M, Hany T, Sulser T, Bachmann LM. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013;64:106–17. https://doi.org/10.1016/j.eururo.2013.04.019.
Yu CY, Desai B, Ji L, Groshen S, Jadvar H. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: a critical analysis of literature. Am J Nucl Med Mol Imaging. 2014;4:580–601.
Castellucci P, Ceci F, Graziani T, Schiavina R, Brunocilla E, Mazzarotto R, et al. Early biochemical relapse after radical prostatectomy: which prostate cancer patients may benefit from a restaging 11C-choline PET/CT scan before salvage radiation therapy? J Nucl Med. 2014;55:1424–9. https://doi.org/10.2967/jnumed.114.138313.
Afshar-Oromieh A, Haberkorn U, Eder M, Eisenhut M, Zechmann CM. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imaging. 2012;39:1085–6. https://doi.org/10.1007/s00259-012-2069-0.
Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. 68Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42:1284–94. https://doi.org/10.1007/s00259-015-3078-6.
Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–40.
Rauscher I, Maurer T, Beer AJ, Graner FP, Haller B, Weirich G, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016;57:1713–9. https://doi.org/10.2967/jnumed.116.173492.
von Eyben FE, Picchio M, von Eyben R, Rhee H, Bauman G. 68Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2016. https://doi.org/10.1016/j.euf.2016.11.002.
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–74. https://doi.org/10.2967/jnumed.115.154153.
Calais J, Fendler WP, Herrmann K, Eiber M, Ceci F. Head-to-head comparison of 68Ga-PSMA-11 PET/CT and 18F-Fluciclovine PET/CT in a case series of 10 patients with prostate cancer recurrence. J Nucl Med. 2018;59:789–94. https://doi.org/10.2967/jnumed.117.203257.
Gasch C, Düwel C, Kopka K, Kratochwil C, Vinsensia M, Eiber M, et al. Significance of PSMA imaging in prostate cancer. Urologe A. 2017;56:3–12. https://doi.org/10.1007/s00120-016-0293-0.
Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive (68)Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–37. https://doi.org/10.1016/j.eururo.2016.06.021.
Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–90. https://doi.org/10.2967/jnumed.115.160382.
Giesel FL, Will L, Kesch C, Freitag M, Kremer C, Merkle J, et al. Biochemical recurrence of prostate cancer: initial results with [18F]PSMA-1007 PET/CT. J Nucl Med. 2018;59:632–5. https://doi.org/10.2967/jnumed.117.196329.
Sridharan S, Steigler A, Spry NA, Joseph D, Lamb DS, Matthews JH, et al. Oligometastatic bone disease in prostate cancer patients treated on the TROG 03.04 RADAR trial. Radiother Oncol. 2016;121:98–102. https://doi.org/10.1016/j.radonc.2016.07.021.
Ingrosso G, Trippa F, Maranzano E, Carosi A, Ponti E, Arcidiacono F, et al. Stereotactic body radiotherapy in oligometastatic prostate cancer patients with isolated lymph nodes involvement: a two-institution experience. World J Urol. 2017;35:45–9. https://doi.org/10.1007/s00345-016-1860-0.
Ost P, Jereczek-Fossa BA, Van As N, Zilli T, Tree A, Henderson D, et al. Pattern of progression after stereotactic body radiotherapy for oligometastatic prostate cancer nodal recurrences. Clin Oncol (R Coll Radiol). 2016;28:e115–20. https://doi.org/10.1016/j.clon.2016.04.040.
Saluja R, Cheung P, Zukotynski K, Emmenegger U. Disease volume and distribution as drivers of treatment decisions in metastatic prostate cancer: from chemohormonal therapy to stereotactic ablative radiotherapy of oligometastases. Urol Oncol. 2016;34:225–32. https://doi.org/10.1016/j.urolonc.2016.02.016.
Ost P, Jereczek-Fossa BA, As NV, Zilli T, Muacevic A, Olivier K, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2016;69:9–12. https://doi.org/10.1016/j.eururo.2015.07.004.
Yao HH, Hong MK, Corcoran NM, Siva S, Foroudi F. Advances in local and ablative treatment of oligometastasis in prostate cancer. Asia Pac J Clin Oncol. 2014;10:308–21. https://doi.org/10.1111/ajco.12256.
Eder M, Neels O, Müller M, Bauder-Wüst U, Remde Y, Schäfer M, et al. Novel preclinical and radiopharmaceutical aspects of [(68)Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals. 2014;7:779–96. https://doi.org/10.3390/ph7070779.
Rauscher I, Maurer T, Fendler WP, Sommer WH, Schwaiger M, Eiber M. (68)Ga-PSMA ligand PET/CT in patients with prostate cancer: how we review and report. Cancer Imaging. 2016;16:14. https://doi.org/10.1186/s40644-016-0072-6.
Fanti S, Minozzi S, Morigi JJ, Giesel F, Ceci F, Uprimny C, et al. Development of standardized image interpretation for 68Ga-PSMA PET/CT to detect prostate cancer recurrent lesions. Eur J Nucl Med Mol Imaging. 2017;44:1622–35. https://doi.org/10.1007/s00259-017-3725-1.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–24. https://doi.org/10.1007/s00259-017-3670-z.
Khan MA, Carter HB, Epstein JI, Miller MC, Landis P, Walsh PW, et al. Can prostate specific antigen derivatives and pathological parameters predict significant change in expectant management criteria for prostate cancer? J Urol. 2003;170:2274–8. https://doi.org/10.1097/01.ju.0000097124.21878.6b.
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. https://doi.org/10.1007/s00259-014-2949-6.
Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:92–101. https://doi.org/10.1007/s00259-016-3490-6.
Calais J, Czernin J, Cao M, Kishan AU, Hegde JV, Shaverdian N, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/ml: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–7. https://doi.org/10.2967/jnumed.117.201749.
Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–68. https://doi.org/10.1007/s00259-017-3711-7.
Kranzbühler B, Nagel H, Becker AS, Müller J, Huellner M, Stolzmann P, et al. Clinical performance of (68)Ga-PSMA-11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur J Nucl Med Mol Imaging. 2018;45:20–30. https://doi.org/10.1007/s00259-017-3850-x.
Emmett L, van Leeuwen PJ, Nandurkar R, Scheltema MJ, Cusick T, Hruby G, et al. Treatment outcomes from 68Ga-PSMA PET/CT-informed salvage radiation treatment in men with rising PSA after radical prostatectomy: prognostic value of a negative PSMA PET. J Nucl Med. 2017;58:1972–6. https://doi.org/10.2967/jnumed.117.196683.
Rauscher I, Düwel C, Haller B, Rischpler C, Heck MM, Gschwend JE, et al. Efficacy, predictive factors, and prediction nomograms for 68Ga-labeled prostate-specific membrane antigen–ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol. 2018;73:656–61 https://doi.org/10.1016/j.eururo.2018.01.006.
Mamede M, Ceci F, Castellucci P, Schiavina R, Fuccio C, Nanni C, et al. The role of 11C-choline PET imaging in the early detection of recurrence in surgically treated prostate cancer patients with very low PSA level <0.5 ng/mL. Clin Nucl Med. 2013;38:e342–e5. https://doi.org/10.1097/RLU.0b013e31829af913.
Afaq A, Alahmed S, Chen SH, Lengana T, Haroon A, Payne H, et al. Impact of 68Ga-prostate-specific membrane antigen PET/CT on prostate cancer management. J Nucl Med. 2018;59:89–92. https://doi.org/10.2967/jnumed.117.192625.
Albisinni S, Artigas C, Aoun F, Biaou I, Grosman J, Gil T, et al. Clinical impact of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis of a multidisciplinary approach. BJU Int. 2017;120:197–203. https://doi.org/10.1111/bju.13739.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Farolfi, A., Ceci, F., Castellucci, P. et al. 68Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA <0.5 ng/ml. Efficacy and impact on treatment strategy. Eur J Nucl Med Mol Imaging 46, 11–19 (2019). https://doi.org/10.1007/s00259-018-4066-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4066-4