Abstract
Purpose
Prostate-specific antigen (PSA) flare is a well-known phenomenon in patients with prostate cancer, but its impact during radium-223 dichloride (223RaCl2) therapy is still unclear. This radioisotope has shown to improve overall survival in metastatic castration-resistant prostate cancer (mCRPC). We sought to evaluate the impact of PSA flare on survival and its relation with metabolic parameters on 18F-labeled sodium fluoride PET/CT.
Methods
We conducted a retrospective study of 168 patients with mCRPC (median age 69; median PSA 29.7) receiving 223RaCl2. Overall survival (OS) and progression-free survival (PFS), estimated by the Kaplan–Meier method and compared using a log-rank test, were evaluated for patient groups corresponding to different definitions of PSA flare. Metabolic 18F-fluoride PET/CT data were analyzed as well.
Results
Immediate PSA decline was observed in 49 patients (29.2%), whereas no PSA response was observed in 59 patients (35.1%). PSA flare (defined as rise after the first cycle followed by decrease below the baseline) was observed in 20 patients (11.9%) and PSA flare followed by a decrease from peak but not below baseline was observed in 40 (23.8%). The first flare subgroup had a median PFS and OS of 20.8 and 23.9 months, respectively. These outcomes were not significantly different from patients with immediate PSA decrease, but were significantly better than in patients with persistent PSA elevation (3.1 months for PFS and 11.5 months for OS, p < 0.001). Moreover, the PSA flare group showed an alkaline phosphatase (ALP) decrease significantly greater than non-responders (p = 0.003). Metabolic 18F-fluoride PET/CT data were available in 35 patients at baseline and during 233RaCl2 therapy. The tumor burden reduction, expressed by ΔTLF10 and ΔFTV10, was more evident within PSA flare group below baseline than non-responders (p = 0.005 and 0.001, respectively).
Conclusions
This report suggests that a flare does not necessarily indicate lack of response to 223RaCl2 therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the second most common male cancer worldwide and the second cause of male cancer death in the USA. Bone is the main site of metastases, representing more than 90% of patients with metastatic disease. In addition, bone complications such as bone marrow failure, bone pain, pathological fracture, and spinal cord compression reduce significantly quality of life in these patients [1,2,3]. In the last decade, several advances in the treatment of metastatic castration-resistant prostate cancer (mCRPC) have been gained by the development of new drugs that have improved the overall survival (OS). 233Ra-dichloride (233RaCl2) therapy, one of such new agents, has been shown to prolong OS, delay time to symptomatic skeletal events, and improve quality of life in mCRPC patients, regardless of previous docetaxel treatment. Thus, the US Food and Drug Administration (USFDA) approved 233RaCl2 for treatment in mCRPC patients with symptomatic bone metastases [4,5,6].
Prostate-specific antigen (PSA) flare, which consists of an early and transient rise in the PSA level followed by a decline, is a well-known phenomenon observed in several studies during luteinizing hormone-releasing hormone (LHRH) agonist treatment and in almost 20% of patients in the course of systemic chemotherapy [7,8,9,10]. Nevertheless, at present, after an initial rise in PSA, there is no clear definition on the extent of the following PSA decline to be considered as a flare and its impact on treatment outcomes and patient management is still indeterminate. In literature, the phenomenon is defined as a decline of at least 50% from the baseline or from the PSA peak value, but also as an undefined PSA response [11, 12]. To our knowledge, there have been no studies investigating the PSA flare phenomenon during the course of 233RaCl2 therapy.
The aim of our study was to evaluate the prognostic impact of the PSA flare in mCRPC patients treated with 233RaCl2. We also investigated the possible correlation of this phenomenon with metabolic parameters by 18F-fluoride PET/CT.
Materials and methods
Waivers of informed consents and institutional board authorization were granted for this study. Clinical records of 168 patients with mCRPC and treated with 233RaCl2 at MD Anderson Cancer Center between August 2013 and February 2017 were retrospectively analyzed. All patients receiving at least one cycle of 223RaCl2 therapy were considered for the analyses. The following parameters were recorded for all patients: age, Gleason score (GS), treatment history, sites of metastases, Eastern Cooperative Oncology Group (ECOG) performance status, baseline serum PSA, PSA trend during treatment, and duration of 233RaCl2 treatment. Whole-body skeletal 18F-fluoride PET/CT was performed to determine eligibility for 233RaCl2 therapy and/or to restage the patients every three or after six cycles at the physician’s discretion. Nodal and visceral metastases were assessed visually on the images available (18F-fluorodeoxyglucose PET/CT, 18F-fluoride PET/CT, body CT scans, ultrasound, or MR imaging). Baseline characteristics of the patient population are displayed in Table 1.
At present, as no official definition is available for PSA flare, we considered different definitions described in previous reports, although with chemotherapy drugs [10, 13]. A flare was defined as an increase of PSA after the first cycle of 233RaCl2 therapy followed by a decrease which could be: (1) a decrease less than baseline, (2) a ≥ 50% decrease from baseline, and (3) a decrease from the peak of PSA during treatment. Patients with persistent PSA rise during therapy with radium were considered non-responders, whereas immediate responders were those with an immediate and continue decrease of PSA after the first cycle.
The primary endpoint of the study was to evaluate OS, which was established from 233RaCl2 cycle start until date of death, and PFS, which was defined as the interval from beginning of 233RaCl2 therapy until date of death of any cause, or disease progression according the recommendations of the Prostate Cancer Working Group 3 (PCWG3) [14]. All deceased patients underwent complete follow-up until death. The secondary endpoint was to assess semi-quantitative parameters by 18F-fluoride PET/CT during 233RaCl2 therapy.
233RaCl2 treatment
233RaCl2 (Xofigo®, Bayer Healthcare Pharmaceuticals Inc.) treatment was administered per clinical standard of care to 168 mCRPC patients, all of them with bone metastases; additionally, 34% of these patients had visceral and/or nodal metastases. Eligibility criteria for treatment with 233RaCl2 consisted of being older than 18 years and signing the informed consent. Treatment was given if: hemoglobin (Hb) level >10 g/dL, absolute neutrophil count (ANC) ≥1.5 × 109/L, and platelet count ≥100 × 109/L. Patients with initially low levels of Hb (<10 g/dL) received a blood transfusion prior to 233RaCl2 therapy being started.
The used activity dose of 223RaCl2 was 50 kBq/kg body weight (1.4 μCi/kg) given at monthly intervals for 6 cycles [15]. The volume administered was calculated using the patient’s body weight (kg), the dosage level (50 kBq/kg body weight), the radioactivity concentration of the product at the reference date, and the decay correction factor provided with each vial. After each 233RaCl2 cycle, the patients were counseled regarding expected side effects and precautions of the radiopharmaceutical.
Semi-quantitative assessment of 18F-fluoride PET/CT
Images were acquired after 50–60 min of 18F-sodium fluoride injection (158–370 MBq), from the vertex of the skull to the feet. MIM Vista workstation (MIM Vista) was used for reconstruction and displaying images in the three different planes (transverse, coronal, and sagittal).
18F-Fluoride PET/CT images were analyzed by two board-certified nuclear medicine physicians. Whole-body skeletal tumor burden was determined by semi-quantitative analysis, with MIM Vista, on baseline and restaging fluoride images. The technique, already described by Etchebehere et al. [16], consists of drawing a rectangular semiautomatic volume of interest (VOI) in the whole-body image with caution to encompass all metastatic sites. When the whole-body VOI is drawn, the maximum standardized uptake value (SUVmax) threshold is set at 10, in order to exclude 99% of all normal bone uptake. Hence, only regions with a SUVmax of 10 or greater generated VOIs automatically by the computer and images were revised to manually exclude any sites of high uptake not related to bone metastases (i.e. urinary activity or degenerative disease). Subsequently, the following parameters were obtained: highest SUVmax among all the metastases, mean SUVmax of all metastases (mean10), and skeletal tumor burden, obtained by calculating the total fluoride skeletal metastatic uptake as a product of mean SUV x VOI10 (TLF10) and the total volume of fluoride bone metastases (FTV10).
Statistical analysis
Patient and clinical characteristics were summarized using descriptive statistics. Frequencies and percentages were provided for categorical variables; mean (SD) and median (range) were provided for continuous variables. For the statistical analysis, we divided our cohort in three categories: responders, which included patients who experienced flare (definitions 1, 2, and 3 mentioned above), non-responders, and immediate responders.
Kaplan–Meier survival curves demonstrated survival time distributions and log-rank tests for comparison between groups. The Wilcoxon signed rank test (paired, two-tailed) was applied for the comparison of the different response parameters for continuous variables and chi-square test or Fisher's exact test for categorical variables. All statistical tests used a significance level of 5%. Statistical analyses were performed using the Statistical Package for Social Sciences, version 22.0, for Windows (SPSS, Chicago, IL, USA).
Results
233RaCl2 doses were completed in 108 (64.2%) patients, 6 (3.7%) patients were still undergoing 233RaCl2 therapy, and 54 (32.1%) patients did not complete all 6 doses because of either progression, hematologic toxicity, or deteriorating ECOG status. Median age at the first cycle of 233RaCl2 therapy was 69 years. The mean number of doses performed was five (median, six doses). Only two patients performed one cycle, but due to persistent increase of PSA, were considered non-responders. After treatment, median follow-up was 8.4 months (range 0.5–36.7). The median OS time was 12.2 months (range 1.4–41.6). The median PFS was 6.2 months (range 0.6–41.6). Population characteristics and outcomes are shown in Tables 1 and 2.
The incidence of the PSA flare, according to different definitions, ranged from 9.5 to 35.7%. According to definition 1 (flare after the first cycle followed by a decrease below the baseline), 18 patients had a flare after the first cycle and 2 after the second cycle. On the other hand, among patients with flare until the peak of PSA (definition 3, which includes also patients from definition 1), 32 had a flare after the first cycle, 18 after the second, 6 after the third, and 4 after the fourth. The median initial PSA increase from the baseline was +20% in patients where flare persisted only for the first cycle and +39% in those who experienced a longer flare period. In this latter group, median interval to the PSA flare was 1.7 months, whereas it was 0.9 months in the first group with flare persisting only for the first cycle. Patients who experienced a flare only after the first dose showed a median PFS and OS of 20.8 and 23.9, respectively, while patients with a longer flare PFS and OS were shorter (9.8 and 18.3, respectively; Table 3). When we considered PSA flare with decrease below the baseline (definition 1), OS and PFS were considerably better than non-responders (PFS: 20.8 vs. 3.1 months, p < 0.001; OS: 23.9 vs. 11.5 months, p < 0.001; Fig. 1). Furthermore, patients with decline from the peak but not below baseline had a longer PFS and OS than non-responders (PFS: 7.9 vs. 3.1 months, p < 0.001; OS: 14.5 vs. 11.5 months, p = 0.04; Fig. 2). In contrast, no significant differences in PFS and OS were found among patients with flare, regardless of definition, and those with immediate decline in PSA level. Indeed, according to definition 1, PFS was 20.8 vs. 12.9 months [p = non-significant (ns)] and OS was 23.9 vs. 37.2 months (p = ns), respectively. According to definition 3, PFS was 9.8 vs. 12.9 months (p = ns) and OS 18.3 vs. 37.2 months (p = ns), respectively (Fig. 3). Finally, when stricter definition of PSA flare was used, such as PSA decline ≥50% less than baseline, similar OS and PFS results were obtained (data not shown). Additionally, we also evaluated alkaline phosphatase (ALP) trend during 223RaCl2 treatment and found that ALP reduction was significantly higher in the flare group compared to non-responders (median reduction: −25% and −7.6%, respectively, p = 0.003; Fig. 4), whereas we did not find a similar ALP flare phenomenon in our cohort. No significant differences between patients with visceral metastases and the other groups in terms of either survival or side effects to therapy were observed.
Semi-quantitative analysis by 18F-fluoride PET/CT
Semi-quantitative parameters on 18F-fluoride PET/CT were obtained at baseline and after 233RaCl2 therapy in 35 patients, 10 with PSA flare below baseline (definition 1), and 25 non-responders. Nine out of 10 patients completed all 6 cycles, and only 1 patient stopped after the fourth. Among non-responders, 16 patients completed all cycles, while 3 and 6 patients stopped after five and four cycles of radium, respectively.
The mean shrinkage of tumor lesions was significantly greater in the flare group, as demonstrated by ΔTLF10 (−44.4% for flare patients vs. +13.3% within non-responders, p = 0.005) and ΔFTV10 (−35.5% vs. +22.8% in non-responders, p = 0.001). On the other hand, no significant differences for ΔSUVmax and ΔSUVmean10 were found (Table 4).
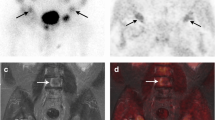
A typical example of flare during treatment with 233RaCl2 is illustrated in Fig. 5.
Example of flare: 18F–NaF PET/CT scans performed at baseline (a), after the third 223RaCl2 cycle (b), and after the sixth 223RaCl2 cycle (c). The baseline scan demonstrates osteoblastic metastases with TLF10 of 3.391 and the interim scan demonstrated an increase in uptake of some bone lesions, particularly the sternum, left humerus, lumbar spine, and pelvis with a TLF10 of 5.437. The scan after radium therapy demonstrated improvement of bone lesions (TLF10 of 2170) and reduction in ALP and PSA levels. The patient was still alive 20 months after 223RaCl2 therapy was started
Discussion
Our study highlights a better OS and PFS in mCRPC patients experiencing PSA flare in 233RaCl2 therapy than those with progressive PSA increase, most of whom were already treated with androgen deprivation therapy, cytotoxic chemotherapy, hormone therapy, and palliative radiotherapy. We also included patients with visceral metastases because they were previously treated and felt to be quiescent. Moreover, Etchebehere et al. [17] previously demonstrated a benefit to radium therapy in patients with more advanced disease, including visceral metastases.
Although the phenomenon has been described only in two case reports [18, 19], where patients had a significant pain response to treatment and stable bone disease after six cycles of 233RaCl2therapy, to our knowledge, this is the first large study which demonstrates a survival advantage in PSA flare patients during bone-targeted therapy. As the first study of PSA flare during 233RaCl2, comparison to other reports of PSA response is difficult because of differences in treatment dose, schedule, and concomitant therapies. Previously, PSA flare has been described during first-line docetaxel, cabacitaxel chemotherapy, and abiraterone treatment [11,12,13, 20, 21] with an incidence ranging from 8.3 to 30.6%. In our study, the incidence of PSA flare was comprised between 9.5 and 35.7%, depending upon the definition used for flare. When we defined flare with stricter criteria, i.e. a rise followed by a decrease of 50% from baseline, median PFS and OS were not significantly different from those in patients incurring an immediate PSA response. Similar results were observed in the abovementioned studies as well. Although our study has highlighted the relation between PSA flare and survival, a clear definition of PSA flare is still a matter of debate. In our opinion, for an accurate comparison, it is preferable to define a PSA flare only if the decrease is below the baseline (definition 1). On the other hand, a decrease from peak but not below the baseline could be mixed with phenomena of tumor progression. Additionally, a reduction of PSA flare ≥50% seems to be easier and faster to calculate in daily practice. The mechanism of a PSA flare induced by 233RaCl2, abiraterone, and other chemotherapeutic prostate drugs has not yet been understood. Our opinion, supported by data, is that the phenomenon is related to tumor response. 233RaCl2 is a targeted alpha emitter that selectively binds to areas of increased bone turnover in bone metastases, particularly into newly formed bone stroma, and emits high-energy alpha particles of short range. The high-energy alpha-particle radiation induces mainly DNA damage that result in a potent and highly localized cytotoxic effect in the target areas [22]. As such, we hypothesize that the observed PSA flare during 233RaCl2 treatment is due to PSA release from tumor cell lysis. Based on this mechanism of action, PSA flare may correspond to a high degree of tumor cell death and may be associated with more complete and more durable response. On the other hand, minor bone metastases or micro-metastases that are present before treatment could not be sufficiently irradiated and may progress during 233RaCl2 therapy. This might explain, together with any change in the tumor microenvironment, the persistent increase of PSA in a part of our patients and the different survival results.
We also found that serum ALP after 233RaCl2therapy was significantly lower in the flare groups compared to non-responders. Recently, Han and Hong [23] reported that ALP kinetics can identify PSA flare among men with mCRPC, although in patients treated with different chemotherapies, suggesting a potential role of ALP as a biomarker for treatment response. Indeed, ALP is an osteoblastic activity marker, so that variations in ALP levels might reflect changes in bone metastases more accurately than PSA levels in mCRPC, where PSA promoter/enhancer activity of metastatic cancer cells is not overexpressed [24, 25].
ALP flare is a well-established phenomenon during endocrine therapy in metastatic prostate cancer [26, 27]. However, we did not find any significant correlations between ALP flare and PSA flare, as most patients with PSA flare had decrease in ALP levels, suggesting a different mechanism of flare between these two markers.
Moreover, our data suggest a possible correlation between PSA flare and a lower tumor burden as shown by ΔTLF10 ΔFTV10 on 18F-fluoride PET/CT, which was recently identified as a prognostic factor of outcome in 233RaCl2 therapy [28]. Our observations are in line with those reported by Modi et al. [29], where a significant correlation with number of bone metastases was found.
In a clinical setting, our findings have useful implications because an initial PSA rise is not necessarily signal disease progression, but may be either a non-response or may represent a prognostic favorable flare. It is essential for oncologists to recognize flare phenomenon in order to avoid a premature interruption of 233RaCl2 therapy. Then, as the PSA trend is only a part of the anticancer therapy response, new monitoring means are being developed (circulating tumor cells and circulating DNA) and new drugs appear to be effective without determining PSA variance [30,31,32,33].
The main limitation of this study was the retrospective design. Second, approximately one third of patients discontinued 233RaCl2 therapy and were switched to chemotherapy (or secondary hormone therapy) according to the oncologist’s decision, although it is well known that switching to another therapy has worse outcomes compared to patients who continued with 233RaCl2 therapy despite progression [28]. Finally, the timing of PSA measurements and scanning intervals during the alpha emitter therapy varied among the patients, although this aspect is similar to the real-world clinical practice.
Conclusion
In summary, for the first time, our study confirms the PSA flare phenomenon in a large series of mCRPC patients treated with 233RaCl2. Patients who experienced PSA flare had significantly better survival than those with persistent PSA increase (“non-responders”) and similar to those with immediate PSA decline. Thus, we strongly recommend that 233RaCl2 therapy should not be discontinued after an early and transient PSA rise. In particular, physicians should be aware of the possibility of PSA flare induced by 233RaCl2therapy during, at least, the first 2 months of treatment, which does not represent a sign of disease progression. However, our study highlights the need to find a standard and validate PSA flare definition and thresholds to ensure homogeneity among studies.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–64.
Lipton A. Implications of bone metastases and the benefits of bone-targeted therapy. Semin Oncol. 2010;37(Supplement 2):S15-S29.
Joung JY, Ha YS, Kim IY. Radium Ra 223 dichloride in castration-resistant prostate cancer. Drugs Today (Barc). 2013;49(8):483–90.
Kluetz PG, Pierce W, Maher VE, et al. Radium Ra 223 dichloride injection: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res. 2014;20(1):9–14.
Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–23.
Sugiono M, Winkler MH, Okeke AA, Benney M, Gillatt DA. Bicalutamide vs cyproterone acetate in preventing flare with LHRH analogue therapy for prostate cancer--a pilot study. Prostate Cancer Prostatic Dis. 2005;8(1):91–4.
Noguchi K, Uemura H, Harada M, et al. Inhibition of PSA flare in prostate cancer patients by administration of flutamide for 2 weeks before initiation of treatment with slow-releasing LH-RH agonist. Int J Clin Oncol. 2001;6(1):29–33.
Nelius T, Filleur S. PSA surge/flare-up in patients with castration-refractory prostate cancer during the initial phase of chemotherapy. Prostate. 2009;69(16):1802–7.
Angelergues A, Maillet D, Flechon A, et al. Prostate-specific antigen flare induced by cabazitaxel-based chemotherapy in patients with metastatic castration-resistant prostate cancer. Eur J Cancer. 2014;50(9):1602–9.
Sella A, Sternberg CN, Skoneczna I, Kovel S. Prostate-specific antigen flare phenomenon with docetaxel-based chemotherapy in patients with androgen-independent prostate cancer. BJU Int. 2008;102(11):1607–9.
Nelius T, Klatte T, de Riese W, Filleur S. Impact of PSA flare-up in patients with hormone-refractory prostate cancer undergoing chemotherapy. Int Urol Nephrol. 2008;40(1):97–104.
Ueda Y, Matsubara N, Tabata KI, et al. Prostate-specific antigen flare phenomenon induced by Abiraterone acetate in chemotherapy-naive patients with metastatic castration-resistant prostate Cancer. Clin Genitourin Cancer. 2017;15(2):320–5.
Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate Cancer: updated recommendations from the prostate Cancer clinical trials working group 3. J Clin Oncol. 2016;34(12):1402–18.
Kluetz PG, Pierce W, Maher VE, et al. Radium Ra 223 dichloride injection: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res. 2014;20:9–14.
Rohren EM, Etchebehere EC, Araujo JC, et al. Determination of skeletal tumor burden on 18F-fluoride PET/CT. J Nucl Med. 2015;56(10):1507–12.
Etchebehere EC, Milton DR, Araujo JC, et al. Factors affecting (223)Ra therapy: clinical experience after 532 cycles from a single institution. Eur J Nucl Med Mol Imaging. 2016 Jan;43(1):8–20.
McNamara MA, George DJ. Pain, PSA flare, and bone scan response in a patient with metastatic castration-resistant prostate cancer treated with radium-223, a case report. BMC Cancer. 2015;15:371.
De Vincentis G, Follacchio GA, Frantellizzi V, Liberatore M, Monteleone F, Cortesi E. Prostate-specific antigen flare phenomenon during 223Ra-dichloride treatment for bone metastatic castration-resistant prostate Cancer: a case report. Clin Genitourin Cancer. 2016;14(5):e529–33.
Olbert PJ, Hegele A, Kraeuter P, Heidenreich A, Hofmann R, Schrader AJ. Clinical significance of a prostate-specific antigen flare phenomenon in patients with hormone-refractory prostate cancer receiving docetaxel. Anti-Cancer Drugs. 2006;17(8):993–6.
Thuret R, Massard C, Gross-Goupil M, et al. The postchemotherapy PSA surge syndrome. Ann Oncol. 2008;19(7):1308–11.
Humm JL, Sartor O, Parker C, Bruland OS, Macklis R. Radium-223 in the treatment of osteoblastic metastases: a critical clinical review. Int J Radiat Oncol Biol Phys. 2015;91(5):898–906.
Han KS, Hong SJ. Serum alkaline phosphatase differentiates prostate-specific antigen flare from early disease progression after docetaxel chemotherapy in castration-resistant prostate cancer with bone metastasis. J Cancer Res Clin Oncol. 2014;140:1769–76.
Tsui KH, Wu L, Chang PL, et al. Identifying the combination of the transcriptional regulatory sequences on prostate specific antigen and human glandular kallikrein genes. J Urol. 172:2029–34.
Tsui KH, Feng TH, Chung LC, et al. Prostate specific antigen gene expression in androgen insensitive prostate carcinoma subculture cell line. Anticancer Res. 28:1969–76.
Pelger RC, Lycklama A, Nijeholt GA, et al. The flare in alkaline phosphatase activity post-orchidectomy predicts which patient may benefit from early chemotherapy in metastatic prostate cancer. Prostate. 50:119–24.
Nakashima J, Ozu C, Nishiyama T, et al. Prognostic value of alkaline phosphatase flare in patients with metastatic prostate cancer treated with endocrine therapy. Urology. 56:843–7.
Etchebehere EC, Araujo JC, Fox PS, Swanston NM, Macapinlac HA, Rohren EM. Prognostic factors in patients treated with 223Ra: the role of skeletal tumor burden on baseline 18F-fluoride PET/CT in predicting overall survival. J Nucl Med. 2015;56(8):1177–84.
Modi D, Hwang C, Mamdani H, et al. Radium-223 in heavily pretreated metastatic castrate-resistant prostate Cancer. Clin Genitourin Cancer. 2016;14(5):373–380.e372.
Smith DC, Smith MR, Sweeney C, et al. Cabozantinib in patients with advanced prostate Cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31(4):412–9.
Kwee S, Song M-A, Cheng I, Loo L, Tiirikainen M. Measurement of circulating cell-free DNA in relation to 18F-Fluorocholine PET/CT imaging in chemotherapy-treated advanced prostate Cancer. Clin Transl Sci. 2012;5(1):65–70.
Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10(3):233–9.
de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate Cancer. Clin Cancer Res. 2008;14(19):6302–9.
Acknowledgements
Prof. Elba C. Etchebehere and Dr. Dong Dai are acknowledged for their valuable contribution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study herein presented was approved by the local review board and performed in accordance with the principles of good clinical practice, with the Declaration of Helsinki, and with the national regulations regarding clinical trials. Waivers of informed consent and authorization were granted for the retrospective analysis.
Conflicts of interest
The authors have declared no conflicts of interest.
Rights and permissions
About this article
Cite this article
Castello, A., Macapinlac, H.A., Lopci, E. et al. Prostate-specific antigen flare induced by 223RaCl2 in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 45, 2256–2263 (2018). https://doi.org/10.1007/s00259-018-4051-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4051-y