Abstract
Radium Ra-223 dichloride (radium-223, Xofigo®) is a targeted alpha therapy approved for the treatment of castration-resistant prostate cancer (CRPC) with symptomatic bone metastases and no known visceral metastatic disease. Radium-223 is the first targeted alpha therapy in this indication providing a new treatment option, with evidence of a significant survival benefit, both in overall survival and in the time to the first symptomatic skeletal-related event. The skeleton is the most common metastatic site in patients with advanced prostate cancer. Bone metastases are a clinically significant cause of morbidity and mortality, often resulting in bone pain, pathologic fracture, or spinal cord compression necessitating treatment. Radium-223 is selectively accumulated in the bone, specifically in areas of high bone turnover, by forming complexes with the mineral hydroxyapatite (the inorganic matrix of the bone). The alpha radiation generated during the radioactive decay of radium-223 produces a palliative anti-tumour effect on the bone metastases. The purpose of this guideline is to assist nuclear medicine specialists in evaluating patients who might be candidates for treatment using radium-223, planning and performing this treatment, understanding and evaluating its consequences, and improving patient management during therapy and follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radium Ra-223 dichloride (radium-223, Xofigo®) is a targeted alpha therapy approved for the treatment of castration-resistant prostate cancer (CRPC) with symptomatic bone metastases and no known visceral metastatic disease. Radium-223 is the first targeted alpha therapy in this indication providing a new treatment option, with evidence of a significant survival benefit, both in overall survival and in the time to the first symptomatic skeletal-related event.
The skeleton is the most common metastatic site in patients with advanced prostate cancer. Bone metastases are a clinically significant cause of morbidity and mortality, often resulting in bone pain, pathologic fracture, or spinal cord compression [1] necessitating treatment. The border zones of bone and bone metastases are often characterised by a high turnover promoting them as a target for bone seeking radiopharmaceuticals, such as radium-223. Radium-223 is selectively accumulated in the bone, specifically in areas of high bone turnover, by forming complexes with the mineral hydroxyapatite (the inorganic matrix of the bone). The alpha radiation generated during the radioactive decay of radium-223 produces a palliative anti-tumour effect on the bone metastases.
Purpose
The purpose of this guideline is to assist nuclear medicine specialists in:
-
Evaluating patients who might be candidates for treatment using radium-223, i.e. patients with castration-resistant prostate cancer (CRPC) with symptomatic bone metastases and no known visceral metastatic disease. For guidance on bone palliation with radionuclides in general see the upcoming separate EANM guideline for radionuclide therapy of bone metastases with beta emitting radionuclides.
-
Planning and performing this treatment.
-
Understanding and evaluating the consequences of this therapy.
-
Improving patient management during therapy and follow-up.
Systematic literature search
To provide the best evidence in the EANM guidelines on bone-seeking radiopharmaceuticals in metastatic bone disease (“Guideline on radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer” and “Guideline on bone palliation with bone-seeking radiopharmaceuticals”) an overall search strategy was designed to answer three PICO-questions (according to the PICO method, the acronym translates to Populations/People/Patient/Problem, Intervention(s), Comparison, Outcome) with regard to the clinically used bone-seeking radiopharmaceuticals in metastatic bone disease, including samarium-153 EDTMP, strontium-89 chloride/dichloride, rhenium-186 HEDP and radium-223 dichloride (designations shortened in the tables), as presented in Table 1. The Agency for Quality in Medicine conducted a systematic literature search in the data-bases Medline via Pubmed (http://www.ncbi.nlm.nih.gov/pubmed) and The Cochrane Library (http://onlinelibrary.wiley.com/cochranelibrary/search/) systematically to locate and obtain the articles relevant to the predefined PICO-questions. A total of 305 systematic reviews, meta-analyses and randomised controlled trials (RCT) and additional 1624 other clinical studies was identified to answer the PICO-question 3. Finally, the results of the included articles were summarised in evidence tables. Only studies focussing on radium-223 are reported in this document (see section on evidence tables).
Presentation in evidence tables
For the identified publications the SIGN checklists and SIGN grading for assignment of levels of evidence was used (Table 2). The category 1(+) was added in order to classify studies, which have a level of evidence between 1+ and 1−. The assigned reference ID within the literature search and the level of evidence of studies related to radium-223 are also reported in the references section.
Background information and definitions
Definitions
Therapy
Radionuclide therapy with radium-223 refers to the single or repeated intravenous administration (treatment cycles) of Ra-223. Radium-223 is currently only commercially available as radium-223 dichloride in aqueous solution (Xofigo®, formerly Alpharadin). We refer at this point in principle to the most recent summary of product characteristics (SmPC) [2].
Radionuclide/radioactive compound
Radium-223 is produced from an actinium-227 (Ac-227) generator. In this generator, actinium-227 decays via its daughter radionuclide thorium-227 (Th-227) (half-life 18.7 d) to radium-223. Samples obtained from the generator are purified by a chromatographic process in order to minimise the residual presence of actinium-227 (Ac-227) and thorium-227 (Th-227). The six-stage-decay of radium-223 to stable lead-207 (Pb-207) occurs via a chain of short-lived daughter nuclides, and is accompanied predominantly by alpha emissions (Table 3). There are also beta and gamma emissions with different energies and emission probabilities. Radium-223 and its daughter nuclides radon-219 (Rn-219), polonium-215 (Po-215), lead-211 (Pb-211), bismuth-211 (Bi-211), and thallium-207 (Tl-207), reach equilibrium after a couple of hours. To facilitate reading, this mixture is not stated in detail, but simply referred to as radium-223. The fraction of energy emitted from radium-223 and its daughters as alpha-particles is 95.3% (energy range of 5–7.5 MeV). The fraction emitted as beta-particles is 3.6% (average energies are 0.445 MeV and 0.492 MeV), and the fraction emitted as gamma-radiation is 1.1% (energy range of 0.01–1.27 MeV). The total decay energy is 28 MeV.
Castration-resistant prostate cancer (CRPC)
CRPC is characterised by testosterone serum levels <50 ng/dL or <1.7 nmol/L, three consecutive rises of prostate-specific antigen (PSA), resulting in two 50% increases over the nadir, PSA progression despite consecutive hormonal manipulations, progression of known osseous metastases or appearance of new lesions on imaging, e.g. bone scintigraphy [3].
Symptomatic bone metastases
Two or more bone metastases in conjunction with one or more of the following symptoms: pain, impairment of function or mobility, pathological fracture, tumour-induced hypercalcaemia. This definition also covers remaining bone metastases after surgical or orthopaedic interventions or external beam radiotherapy.
Background
The skeleton is the most common metastatic site in patients with advanced prostate cancer. Bone metastases are present in more than 90% of patients who die from prostate carcinoma [4]. They are a clinically significant cause of morbidity and mortality, often resulting in bone pain, pathologic fracture, or spinal cord compression [1] necessitating treatment.
Radium-223 is an isotope of radium, which is a chemical element belonging to the second main group. Members of that group possess physiological similarities with calcium promoting them as “bone seekers”. Radium-223 is thus selectively accumulated in the bone, specifically in areas of high bone turnover [5], e.g. the border zones of bone and bone metastases. Radium-223 accumulates in these areas by forming complexes with the mineral hydroxyapatite (the inorganic matrix of the bone). The high linear energy transfer of the alpha radiation generated during its radioactive decay results in a high probability of DNA double-strand breaks in the adjacent cells. In combination with noxious effects on the microenvironment/matrix of metastases (including osteoblasts and osteoclasts) an anti-tumour effect on the metastases is produced. The penetration depth of alpha particles from radium-223 in soft tissue is less than 0.1 mm (corresponding to approximately 10 cell diameters) leading to a highly localised effect. Thus, detrimental effects on healthy tissues near to the metastases are minimised.
Indications
Intravenous administration of radium-223 dichloride (Xofigo®) is approved by the European Medicines Agency (EMA) under the EMEA procedure number EU/1/13/873/001. The approved indication is the treatment of adult patients with castration-resistant prostate cancer, symptomatic bone metastases and no known visceral metastases. Safety and efficacy of radium-223 in this setting were demonstrated in several phase two and three studies [6,7,8,9,10,11]. Radium-223 should be administered only by persons authorised to handle radiopharmaceuticals in designated clinical settings and facilities and after evaluation of the patient by a qualified nuclear medicine specialist.
Contraindications
The safety and efficacy of radium-223 in children and adolescents below 18 years of age have not been studied. There is no relevant use of this medicinal product in the paediatric population in the approved indication.
Radium-223 is not indicated if visceral metastases are known.
Absolute
-
There are no absolute contraindications to the use of radium-223 specified in the summary of product characteristics (SmPC) [2].
Relative
-
Karnofsky score < 50% or ECOG performance status >2 (at least in an out-patient setting, hospitalisation should be considered in these cases)
-
Limited bone marrow reserve:
-
Before the first administration/cycle:
-
Absolute neutrophil count <1.5 ∙ 109/L
-
Platelet count <100 ∙ 109/L
-
Haemoglobin <10.0 g/dL
-
-
Before subsequent administrations/cycles:
-
Absolute neutrophil count <1.0 ∙ 109/L
-
Platelet count <50 ∙ 109/L
-
-
-
Faecal incontinence (hospitalisation is strongly advised in these cases)
Risk factors and precautions for use
-
There is an increased risk of haematological adverse reactions such as neutropenia and thrombocytopenia in patients with evidence of compromised bone marrow reserve e.g. following prior cytotoxic chemotherapy [6] and/or radiation treatment (external beam radiation therapy, EBRT) or in patients with advanced diffuse metastatic infiltration of the bone (“superscan” in a bone scan). These patients should be treated only after a careful benefit-risk assessment. Close monitoring is necessary.
-
Safety and efficacy of radium-223 in patients with inflammatory bowel disease have not been studied. Because radium-223 is excreted in the faeces, radiation exposure of the intestine may have detrimental effects in the course of these diseases. Radium-223 should only be administered after a careful benefit/risk assessment in patients with acute inflammatory bowel disease.
-
Because radium-223 is excreted in the faeces, a relatively higher radiation exposure of the intestine can be expected in patients with serious constipation (e.g. due to opioid medication). However, constipation is not a contraindication for the use of radium-223.
-
In patients with untreated imminent or established spinal cord compression, treatment with standard of care, as clinically indicated, should be completed before starting or resuming treatment with radium-223.
-
In patients with bone fractures that require orthopaedic stabilisation the intervention should be performed before starting or resuming treatment with radium-223. A period of six to 10 weeks seems sufficient for adequate consolidation after orthopaedic intervention, but the effective point in time depends on the radiographic assessment.
-
There is a low risk of developing osteonecrosis of the jaw (ONJ) in patients treated with bisphosphonates and radium-223. In all observed cases of ONJ the patients were exposed to prior or concomitant bisphosphonates (e.g. zoledronic acid) and prior chemotherapy (e.g. docetaxel) [2]. It is unclear whether it is the sole risk of bisphosphonate and chemotherapy, or whether the risk for ONJ is further increased by radium-223.
-
Radium-223 contributes to a patient’s cumulative radiation exposure, which may be associated with an increased risk of cancer. In particular, the risk for osteosarcoma, myelodysplastic syndrome and leukaemias may be increased. However, in view of the patients to be treated these risks are low. Moreover, there is much less if any concern in elderly patients (aged >75 years) regarding these risks. No cases of radium-223-induced cancer have been reported in clinical trials during follow-up of up to 3 years [2]. Of the 600 patients treated with radium-223 in the randomised trial, 33% were 75 years of age and over.
-
Depending on the volume administered, this medicinal product can contain up to 2.35 mmol (54 mg) sodium per dose. This has to be taken into consideration by patients on a sodium-controlled diet.
Interaction with other medicinal products and other forms of interaction
No clinical interaction studies have been performed.
-
Interactions with calcium, phosphate, or vitamin D cannot be excluded due to physiological relationships.
-
Concomitant chemotherapy or external radiotherapy with radium-223 may have additive effects on bone marrow suppression (see section on risk factors and precautions for use) [2]. Safety and efficacy of concomitant therapy with radium-223 have not been established in dedicated studies. If chemotherapy, other systemic radioisotopes, or hemibody external radiotherapy is necessary during the treatment period, radium-223 should only be continued after a careful benefit-risk assessment. Close monitoring is then necessary.
-
The efficacy and safety of other concomitant anticancer therapies (e.g. abiraterone, enzalutamide) have only been established in an international early access programme done after the ALSYMPCA study and before regulatory approval of radium-223 as a prospective, interventional, open-label, single-arm, phase 3b study [12].
-
The efficacy and safety of cytotoxic chemotherapy performed after treatment with radium-223 has been examined in an exploratory analysis of the ALSYMPCA trial [9] data [13]. The available data indicates that chemotherapy following radium-223, regardless of prior docetaxel, is feasible and appears to be well tolerated [13]. Patients receiving chemotherapy after treatment with radium-223 had a similar haematological profile compared to patients receiving chemotherapy after placebo [13].
-
There are no data from dedicated studies regarding the optimal therapeutic sequence of radium-223 and other life prolonging drugs (e.g. abiraterone, enzalutamide, chemotherapy). However, in the absence of large-scale clinical trials, there are recommendations based on expert opinions from the Prostate Cancer Radiographic Assessments for Detection of Advanced Recurrence II Working Group [14].
Procedure
In addition to the following general recommendations for treatment with radium-223 there are detailed remarks on practical implementation of radium-223 treatment that were developed after a research consultancy agency observed radium-223 procedures and conducted interviews with key staff members involved in treatment delivery in eleven nuclear medicine centres across six European countries. Key consensus recommendations were then developed in a meeting of experts from these centres [15].
Facility and personnel requirements
-
Radium-223 may be received, used and administered only by authorised persons in designated settings. Its receipt, storage, use, transfer and disposal are subject to national regulations. The facilities required will depend on national legislation and regulations for the handling of alpha emitters. The facility needs to be authorised for performing this treatment. Adequate handling of radium-223 is of high importance due to the high relative biological effectiveness of incorporated radium-223. The facility in which treatment is administered must have appropriate personnel, radiation safety equipment, procedures available for handling and disposal of waste (see section on waste disposal), handling of contamination, monitoring personnel for accidental contamination and controlling contamination spread.
-
Clinicians involved in the therapy with radium-223 must be knowledgeable about and compliant with all applicable national and local legislation and regulations.
-
Physicians responsible for treating patients with radium-223 should have an understanding of the clinical pathophysiology and natural history of the disease, should be familiar with other forms of therapy and should be able to liaise closely with other physicians involved in managing the patient.
-
The nuclear medicine specialist is responsible for the radium-223 treatment, aftercare and follow-up and their coordination in close liaison with the referring physicians and other physicians involved in managing the patient (depending on national legislation and regulations). The nuclear medicine specialist is responsible for the validity of the indication for treatment with radium-223. The nuclear medicine specialist is liable to discuss the technical and clinical aspects of treatment with the patient prior to therapy. A long-term follow-up has to be ensured, among other things, to allow an oncologic quality control and to detect possible long-term adverse effects of the therapy. Involved physicians are encouraged to report any suspected adverse reaction via national reporting systems.
-
Given that it is a standardised procedure there is no medical necessity to consult a medical physics expert. However, depending on national legislation and regulations a medical physics expert can be consulted.
Therapy prerequisites, data required
-
Patient data (age, weight).
-
Inclusion criteria for treatment with radium-223: Therapy is indicated in castration-resistant prostate cancer, symptomatic bone metastases, and no known visceral metastases. The presence of lymph node metastases is not an explicit exclusion criterion. However, the presence of lymphadenopathies exceeding 3 cm, bulky disease or distant lymph node metastases should be a reason for a careful benefit/risk evaluation when choosing radium-223 as the only systemic treatment, as there is no evidence of benefit in this particular context. A documentation of the symptoms and their intensity before the start of the treatment is recommended. Eligibility for the treatment should be assessed in close liaison with an urooncologist or oncologist. Preferably, patients eligible for radium-223 therapy should be referred from a prostate cancer centre or urooncology or oncology department with expertise in the management of advanced prostate cancer after an interdisciplinary discussion in a tumour board (depending on national legislation and regulations).
-
Exclusion of visceral metastases: Visceral metastases usually occur late in the course of the disease. The liver is the organ most often affected [16]. A recent abdominal CT (not older than 3 months) is reasonable as a standard examination. Rapidly rising PSA values (doubling time below 1 month) or values exceeding 100 ng/mL require more extensive cross sectional imaging (e.g. PET/CT, CT, MRI) of the thorax and abdomen, e.g. with a hybrid imaging technique. [18F]-Fluorethylcholine, [11C]-Choline, and [68Ga]-PSMA are possible tracers for PET/CT or PET/MRI imaging.
-
Karnofsky score or ECOG performance status.
-
Prior therapies: Chemotherapies (date of last application), external beam radiotherapy (treatment areas, date of last treatment), radionuclide therapies (isotope, activity, date of last treatment).
-
Current medication, in particular chemotherapy, bisphosphonates/denosumab, calcium, phosphates, vitamin D (s. section on interaction with other medicinal products and other forms of interaction).
-
Recent bone scan or [18F]-Fluoride-PET/CT [17] (not older than 3 months) with evidence of bone metastases. In the case of a short PSA doubling time the most recent imaging should not be older than 4 weeks.
-
Recent blood count (not older than 10 days before application of radium-223) before each therapy cycle.
Patient information and instructions
-
Patients should receive both written and verbal information about the procedure prior to therapy. Depending on national legislation and regulations, a patient information sheet/consent form must be signed by the patient and the physician responsible of the patient (the nuclear medicine physician or the referring physician) in the same session at least 24 h before the first scheduled administration of radium-223. This consent refers to the whole treatment (i.e. all applications within the regular schedule). Thus, a new consent must only be obtained in cases of relevant unexpected changes in the course of the therapy or disease.
-
Patients should be informed that this therapy is not likely to cure their disease, but is a disease controlling treatment. Aims of the treatment include prolonging overall survival, maintaining disease control, preventing skeletal complications [11], reducing bone pain and improving symptoms and quality of life should be discussed.
-
In the case that a patient does not meet the inclusion criteria or exhibits contraindications to radium-223, a radionuclide therapy with a beta-emitter (i.e. samarium-153-EDTM) may be taken into consideration (see corresponding EANM guideline for radionuclide therapy of bone metastases with beta emitting radionuclides). However, these radiopharmaceuticals are effective in pain palliation but have not proven survival benefit.
-
Patients must be informed of the potential side effects of therapy.
-
Patients must be advised to reduce unnecessary radiation exposure and contamination to household members, caregivers and the public. Written instructions should be provided.
-
Patients could receive a contact card to be carried at all times after each injection. If issued, the card should provide details on radium-223 treatment and (24 h) contact numbers for use if travelling through airport security or in the event of a medical emergency [18].
Side effects
-
The adverse reactions observed with radium-223 are represented in the Table 4 [2]. They are classified according to System Organ Class. The most appropriate MedDRA term is used to describe a certain reaction and its synonyms and related conditions. Adverse reactions from clinical trials are classified according to their frequencies. Frequencies are defined as: very common (≥ 1/10), common (≥ 1/100 to <1/10), uncommon (≥ 1/1000 to <1/100). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness:
-
a.
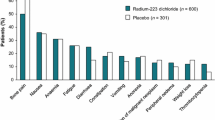
The most frequently observed adverse reactions (≥ 10%) in patients receiving radium-223 were diarrhoea, nausea, vomiting and thrombocytopenia. The most serious adverse reactions were thrombocytopenia and neutropenia (grade 3–4 according to Common Terminology Criteria for Adverse Events, CTCAE Version 3). There is an increased risk of haematological adverse reactions in patients with evidence of compromised bone marrow function, e.g. following prior chemotherapy or EBRT or in patients with advanced diffuse metastatic infiltration of the bone (see section on risk factors and precautions for use). Haematological adverse reactions may pose an increased risk of infection or bleeding and may require the use of blood or blood products.
-
b.
Very common: Diarrhoea, nausea, vomiting and thrombocytopenia.
-
c.
Common: Leukopenia, neutropenia, pancytopenia. Reactions at the injection site (e.g. erythema, pain and swelling).
-
d.
Uncommon: Lymphopenia.
-
e.
Uncommon: Development of osteonecrosis of the jaw (ONJ) in patients treated with bisphosphonates.
-
a.
-
Radium-223 contributes to a patient’s cumulative radiation exposure, which may be associated with an increased risk of cancer. In particular, the risk for osteosarcoma, myelodysplastic syndrome and leukaemias may be increased. There is much less if any concern in elderly patients (aged >75 years) regarding these risks. No cases of radium-223-induced cancer have been reported in clinical trials in follow-up of up to 3 years [2].
-
Extravasation can potentially cause serious injury, such as tissue necrosis.
-
After intravenous injection, radium-223 is rapidly cleared from the blood and is distributed primarily into bone and bone metastases. However, a significant amount of activity is excreted into intestine. Faecal excretion is the major route of elimination from the body. At 48 h after injection, the cumulative faecal excretion was 13% (range 0%–34%), and the cumulative urine excretion was 2% (range 1%–5%). Approximately 60% to 75% of the administered activity is excreted within a week [2]. Depending on the interval following injection, activity can be found in various bodily fluids or vomit. Written instructions encouraging good hygienic practices to reduce unnecessary radiation exposure and contamination of household members and caregivers should be provided to the patient, household members or caregivers. Catheterisation may be taken into consideration for in-patients with urinary incontinence directly before application of radium-223. The catheter should remain in place for 24 h after the treatment. Extraordinary precautions other than already mentioned do not seem justified with regard to the low external radiation exposure [19]. Thus, no restrictions regarding contact with other people after receiving radium-223 should be applied. However, general principles in radiation protection still apply.
Patient preparation and data required
-
A complete blood count should be performed within 10 days prior to radium-223 administration. Cell counts should not be lower than the limits specified in section 4.2. Lower cell counts increase the risk of infection or bleeding (among others). However, there are no data regarding if it is reasonable to increase cell count values by red blood cell transfusion or treatment with colony-stimulating factors before treatment (see also section 6). Thus, we propose the following approach for low cell counts: before therapy, the most likely cause of the condition has to be determined. The administration of radium-223 has to be delayed depending on the degree of decrease of the cell count. If necessary, the condition should be treated according to the standard of care. In general, further treatment with radium-223 should only be continued after a careful benefit/risk evaluation. It has to be taken into account that patients with significantly impaired bone marrow function exhibit a poor prognosis and reduced life expectancy [20, 21]. This is particularly important in view of the required number of cycles of radium-223 and accordingly required total time of the treatment. Close monitoring is recommended prior to the planned administration. In case there is no recovery in the affected blood count despite receiving standard of care we do not consider treatment with radium-223 as useful.
-
Fasting is not required prior to therapy. The patient should be well hydrated.
-
Medication should be taken as usual. Exceptions: Pausing supplementation with calcium, phosphates, or vitamin D should be considered about 4 days before and after each injection of radium-223. Concomitant medication with chemotherapy should be subject to a careful benefit/risk evaluation.
-
Catheterisation may be taken into consideration directly before application of radium-223 for in-patients with urinary incontinence. The catheter should remain in place for 24 h after the treatment. Absorbent pads, rubber sheets, or diapers may be used.
Administration
-
The treatment is usually performed on an outpatient basis. However, hospitalisation is at the discretion of the nuclear medicine specialist (with respect to national legislation and regulations). Hospitalisation is encouraged in cases of faecal incontinence or seriously ill patients (see section on relative contraindications). The patient can be discharged directly after administration of radium-223 (with respect to national legislation and regulations, see section on facility and personnel requirements). Written instructions to reduce unnecessary radiation exposure and contamination should be provided to the patient, family members or caregivers. Burial or cremation within a short interval after administration of radium-223 is subject to national legislation and regulations. However, significant radiation exposure of the public is unlikely.
-
Radium-223 is for intravenous use. It should be administered by slow injection (generally within 1 min) via an indwelling cannula or central venous access to ensure intravenous administration and to avoid extravasation. Proper placement and patency of the cannula/venous access must be confirmed directly before and monitored during injection. The infusion line should be flushed with saline prior to and at the end of the administration of radium-223 (e.g. with 100 mL). A three-way stopcock is recommended. Absorbent or water-impermeable material may be useful to avoid contamination of the patient's skin adjacent to the site of injection.
-
In the case of reasonable suspicion of extravasation the administration must be halted. Suspicion can be substantiated by measuring local activity (e.g. to image the injection site. The images give an opportunity to quantify the activity at the injection site and repeated imaging gives the opportunity to calculate the absorbed dose, which provides information on what adverse effects to expect). Extravasation can potentially cause serious injury, such as tissue necrosis, but no major effect due to extravasation has ever been observed. There is no specific therapy in the case of extravasation or infiltration. Using a clean syringe, aspirate as much of the radium-223 as possible from the tissue through the cannula. Application of local hyperthermia, elevation of the affected extremity, and gentle effleurage may promote lymphatic drainage reducing the local radiation dose. Documentation of the adverse event is required.
-
There is no specific antidote. In the event of adverse effects (e.g. nausea, vomiting, diarrhoea), symptomatic therapy and general supportive measures are the treatment of choice.
-
The room in which the injection was performed has to be checked for contamination with an appropriate radioactive surface contamination monitor. The monitor should permit an alpha and beta-gamma discrimination. Also, staff who performed the injection must be checked for contamination.
-
Radiation safety: Radiation protection precautions for handling and administration of radionuclides must be taken in accordance with national regulations. Although radium-223 is predominantly an alpha emitter, gamma and beta radiation is associated with the decay of radium-223 and its radioactive daughter isotopes. The external radiation exposure associated with handling of patient doses is considerably lower in comparison to other radiopharmaceuticals for therapeutic purposes as the administered radioactivity will usually be below 8 MBq. However, incorporated radium-223 possesses a high biological effectiveness. There is risk of contamination or incorporation from spills of bodily fluids such as of blood, vomiting, etc. Nevertheless, the most relevant path of incorporation is inhalation. In keeping with the ALARA (“As Low As Reasonably Achievable”) principle, it is recommended to minimise the time spent in radiation areas, to maximise the distance to radiation sources, and to use adequate shielding. Contamination or incorporation is strictly to be avoided. Thus, close proximity to the treated patient should be minimised. The treated patient should leave the facility without undue delay.
-
Incorporation measurements have to be performed in the case of suspected incorporation (e.g. stool sampling, whole body counting). Nevertheless, incorporation measurements of radium-223 are demanding.
-
Regular incorporation measurements may be necessary depending on national legislation and regulations. To minimise the risk of a single person to cumulative incorporate significant amounts of radium-223, dispensing, administration, and disposal may be carried out by different members of the staff.
-
Interruption of therapy, delay of treatment cycles
Relevant interruption of the treatment schedule (i.e. the treatment interval exceeds 4 weeks) due to medical or other reasons results in non-conformity with the approved dose regimen. However, evidence from previous studies on radium-223 suggests that a further delay by no more than 4 weeks (on top of the regular 4-week interval) does not impact benefit in overall survival [22]. There are no data on the effects of any further delay. In case of a cancellation of the treatment or a dosing delay greater than 4 weeks, the treating physician should take into consideration the patient's condition, the duration of the delay as well as alternative treatment options when determining the best care for the patient. A change in the therapy regimen or the continued treatment with radium-223 should be subject to a careful benefit/risk evaluation.
Radiopharmaceutical
The radionuclide is supplied in a ready to use solution for injection (Xofigo®), contained in an 11 mL plastic vial and stored in a lead pot. Each vial contains 6 mL of solution (6.6 MBq radium-223 dichloride at the reference date). Each millilitre of solution contains 1100 kBq radium-223 dichloride, corresponding to 0.58 ng radium-223 at the reference date. Radium is present in the solution as a free ion.
Posology and method of administration
-
55 kBq per kg body weight, given at 4-week intervals for six injections. Safety and efficacy beyond six injections of radium-223 have not been established.
-
The volume to be administered to a given patient should be calculated using:
-
Patient’s body weight in kilogramme (KG)
-
Dosage level (55 kBq/kg body weight)
-
Radioactivity concentration of the product at reference date (i.e. 1100 kBq/mL). The reference date is stated on the vial and lead pot label.
-
Decay correction (DC) factor to correct for physical decay of radium-223 between reference date and eventual application date. A table of DC factors is provided with each vial as part of the SmPC leaflet.
-
The total volume to be administered to a patient is calculated as follows \( \mathrm{V}\kern0.5em \left(\mathrm{ml}\right)=\frac{\mathrm{KG}\kern0.5em \left(\mathrm{kg}\right)\ast 55}{{\mathrm{DC}}^{\ast }1100} \):
-
-
The amount of radioactivity in the dispensed volume shall be confirmed by measurement in a properly calibrated activity or dose calibrator (radium-223 standard reference material can be requested from Bayer Healthcare for calibration purposes).
-
Adjustment of the activity to administer:
-
No overall differences in safety or efficacy were observed between elderly (aged ≥65 years) and younger patients (aged <65 years) in the phase III study. No adjustment of the activity to administer is considered necessary in elderly patients.
-
Safety and efficacy of radium-223 have not been studied in patients with hepatic impairment. Since it is neither metabolised by the liver nor eliminated via the bile, hepatic impairment is not expected to affect the pharmacokinetics of radium-223. Based on subgroup analyses in the phase III clinical trial, adjustment of the activity to administer is not needed in patients with mild hepatic impairment. No adjustment of the activity to administer can be recommended for patients with moderate or severe hepatic impairment due to lack of clinical data.
-
No dedicated renal impairment trial for radium-223 has been conducted. In subgroup analyses of the phase III clinical study, no relevant differences in safety or efficacy were observed between patients with mild renal impairment (creatinine clearance [CLCR]: 50 to 80 mL/min) and normal renal function. Limited data are available on patients with moderate (CLCR: 30 to 50 mL/min) renal impairment. No data are available on patients with severe (CLCR <30 mL/min) renal impairment or end-stage renal disease. However, since excretion in urine is minimal and the major route of elimination is via the faeces, renal impairment is not expected to affect the pharmacokinetics of radium-223. No adjustment of the activity to administer is considered necessary in patients with renal impairment. There is no experience with regard to efficacy or safety of treatment with radium-223 in patients undergoing haemodialysis. Wherever possible, dialysis should be performed no earlier than approximately 24 h after administration of radium-223 as less than 1% of the administered activity should remain in the blood at this time. For reasons of radiation safety, dialysis should be managed in close liaison of the nephrologist and the nuclear medicine specialist responsible for the treatment with radium-223. Depending on the interval between radionuclide administration and need for dialysis, the procedure may be performed in a nuclear medicine facility featuring a decay tank system.
-
-
Dosimetry
Absorbed radiation doses for Xofigo®, considering its observed biodistribution and specific characteristics (according to the SmPC [2]) are presented in Table 5. For radium-223, as primarily an alpha emitter, additional assumptions were made for the intestine, red marrow and bone/osteogenic cells, to provide the best possible absorbed dose calculations for Xofigo®. The absorbed radiation dose calculation was performed based on clinical biodistribution data. Calculations of absorbed doses were performed using OLINDA/EXM (Organ Level Internal Dose Assessment/Exponential Modelling) based on the Medical Internal Radiation Dose (MIRD) algorithm. However, Chittenden et al. demonstrate a wide range of absorbed doses delivered to normal organs [23]. There is a growing body of literature on this complex topic [24,25,26,27,28]. The values have to be interpreted with caution as these absorbed dose values cannot be directly compared with those for beta emitters as there are well-known differences in therapeutic efficacy between alpha and beta emitters.
Handling and preparation of the radiopharmaceutical, storage, quality control
Preparation/dispensing
-
Radium-223 is a ready-to-use solution and should not be diluted or mixed with any other solution. Each vial is for single use only. Shelf life is 28 days.
-
Radium-223 has to be dispensed according to the most recent SmPC [2].
-
Storage should be in accordance with national regulation on radioactive materials. Store in the original container or equivalent radiation shielding. Store at room temperature, below 40 °C.
-
Radium-223 needs to be received, handled and administered only in facilities authorised for performing this treatment. The facilities requirements will depend on national legislation and regulations for the handling of alpha emitters (see section on facility and personnel requirements). Radium-223 needs to be handled by the user in a manner that satisfies both radiation safety (see section on administration) and pharmaceutical quality requirements. Appropriate aseptic precautions should be taken.
-
The syringe should be prepared in a fume hood as a small amount of radon-219 (Rn-219) in gaseous form will be present in the vial.
-
Adequate shielding of syringes is recommended. Shielding material may contain acrylic glass, tungsten, or lead.
-
Gripping devices such as tongs and tweezers are suggested, but it must be taken into account that if they are not properly used they may increase the risk of dropping syringes or vials.
Quality control
The vial/solution should be visually inspected before use. Radium-223 is a clear, colourless solution and should not be used in case of discolouration, the occurrence of particulate matter or a defective container.
Activity measurement
The gamma radiation associated with the decay of radium-223 allows for the radioactivity measurement with standard instruments. Nevertheless, activity measurements have to be performed in properly calibrated activity calibrators (radium-223 standard reference material can be requested from Bayer Healthcare for calibration purposes) accounting for measured volume and geometry.
Waste disposal
Any unused product or materials used in connection with the preparation or administration are to be treated as radioactive waste and should be collected separately from waste related to other radionuclides and disposed of in accordance with local regulations. Xofigo® may contain traces of long-lived impurities such as thorium-227 (Th-227) (half-life = 18.7 days) and actinium-227 (Ac-227) (half-life = 21.8 years), which could cause specific waste disposal even at relatively low activities of the latter radionuclides (maximum activity of actinium-227 in every delivered batch is provided by Bayer Healthcare on a regular basis and has to be considered in waste management).
Documentation and discharge letter
Documentation of biodistribution
Whole body scans may be performed to visualise biodistribution following therapy. Acquisition may be performed approximately 6 days after administration to minimise interfering intestinal activity. Imaging should then be performed in multiple energy windows. Radium-223 emits ten photons with a probability of emission greater than 1% with energies ranging from 12 to 338 keV [29]. The photons emitted at 81 and 84 keV have the highest probability of emission [29]. Further photon energies with high probabilities of emission and within the energy interval suitable for gamma camera imaging are 95 keV, 154 keV and 270 keV [29]. An energy window set at 82 keV with a 20% width to encompass counts from the 81-keV and the 84-keV emissions seems useful [23]. A medium energy collimator is recommended [29]. Count statistics are usually very poor due to both the low photon yield and the low remaining activity. Acquisition times should be adjusted appropriately. Thus, whole-body or spot views may need to be acquired for approximately 30 min [23].
Discharge letter
The report should contain relevant patient data, date of therapy, activity of radium-223, number of cycles, and details on necessary aftercare/follow-up (see section on monitoring, visits between cycles and intermediate- and longterm follow-up).
Quality control
-
Institutional quality management programs are recommended.
-
A close liaison of the health care professionals involved in the treatment of the patient is recommended with regard to documentation and evaluation of the outcome of the treatment, as well as adverse effects and results from aftercare / follow-up.
-
Therapy prerequisites (see sections on indications, contraindications, and therapy prerequisites, data required) must be checked at baseline and prior to every dose of radium-223.
Sources of error
-
Miscalculation of therapy activity. Miscalculation can result in over- or undertreatment of the patient. The accurate measurement of radium-223 activity is assured by calibration of the dose calibrators using a NIST-traceable reference standard, which is provided by Bayer Healthcare to the end user.
-
Paravascular placement of the venous access with consecutive extravasation. Proper placement and patency of the cannula/venous access must be confirmed directly before and monitored during injection (see section on administration).
Monitoring, visits, aftercare, follow-up
Monitoring, visits, aftercare and follow-up may be delegated to physicians involved in the care of the patient (preferably uro-oncologists or oncologists, depending on national legislation and regulations). Forwarding of relevant data to the nuclear medicine specialist who is responsible for the therapy is mandatory. Regular meetings with discussion of the clinical course are encouraged in this setting.
Monitoring, visits between cycles
-
A single complete blood count within 10 days prior to the next cycle of radium-223 seems sufficient in the majority of cases. However, a complete blood count may be performed 2 to 3 weeks after the last cycle (most often representing the nadir) depending on the initial blood count and the condition of the patient (e.g. compromised bone marrow function). Blood count may be performed more frequently if clinically necessary. In the case of cell counts below the limits specified in section on relative contraindications the administration of radium-223 has to be delayed depending on the degree of decrease of the cell count. A treatment should be performed according to the standard of care. If there is no recovery to reasonable values within 6 to 8 weeks after the last administration of radium-223, the treatment should be continued only after a careful benefit-risk assessment (see section on patient preparation and data required). Nonetheless, further treatment results in non-conformity with the approved dose regimen (see section on interruption of therapy, delay of treatment cycles).
-
Patient condition.
-
Currently, there are no well-defined markers of response to radium-223 treatment and treatment should not be discontinued on the basis of individual bone scans, PSA or ALP levels, or pain responses [18], as the most important aim of the treatment is prolonging survival. Variability or lack of evidence makes these markers of response unreliable:
-
(a) PSA levels may vary in patients during radium-223 treatment; however, this is not considered a reliable marker of response or non-response, and the decision to continue or withdraw treatment should not be based on this marker alone [18].
-
(b) Improvements in pain are often rapid and marked; however, pain relief is not experienced by every patient and cannot be considered a reliable indicator of treatment response and survival benefit [18].
-
(c) Patients receiving radium-223 may show a decrease in ALP levels, suggesting a meaningful treatment effect [30]; however, there is currently no solid evidence for the use of ALP as a biomarker to alter the recommended dosing schedule [18].
Until more robust markers of response to radium-223 treatment are identified, nuclear medicine centres should strive to maintain the recommended dosing schedule and complete all six treatment cycles in order to optimise outcomes [18]. Thus, to avoid uncertainties, checking of biomarkers or imaging should only be initiated if deemed necessary.
-
In the case of a significant deterioration of the general condition of the patient (reduction of the Karnofsky score to <50% or increase of ECOG performance status to >2) imaging (e.g. bone scan, PET/CT, CT, MRI, see also section on therapy prerequisites, data required) and checking of biomarkers are recommended. In the case of unequivocal progression (e.g. appearance of new metastases not attributable to flare reaction (interpretation criteria see Scher et al. [31]) the treatment should be continued only after a careful benefit-risk assessment.
-
Appropriate biomarkers (e.g. PSA, LDH, CRP, ALP) may be checked during the course of the treatment if deemed necessary. A “mid-treatment” check prior to the forth cycle of radium-223 might be a reasonable time point. However, fluctuation of biomarkers is common in the course of the treatment (see above). Increasing values do not necessarily indicate an insufficient response to therapy. In case of strikingly increasing biomarkers, imaging (e.g. bone scan, PET/CT, CT, MRI, see also section on therapy prerequisites, data required) seems reasonable. In the case of unequivocal progression (e.g. appearance of new metastases not attributable to flare reaction; interpretation criteria see Scher et al. [31]) the treatment should be continued only after a careful benefit-risk assessment.
-
Intermediate and long-term follow-up
Intermediate and long-term follow-up should be decided and personalised by the treating physician based on each particular case and on the patient’s following treatments. Taking this into account, some general recommendations are:
-
A complete blood count may be performed every 3 months for 1 year and then every 6 months. However, blood count may be performed more frequently if clinically necessary.
-
Imaging (e.g. bone scan, PET/CT, CT, MRI, see also section on therapy prerequisites, data required) should be performed periodically depending on clinical symptoms, duration of disease, tumour biology, and previous course of the disease, but no later than 3 months after the last cycle of radium-223. Imaging is commonly repeated in semi-annual or annual intervals.
-
Monitoring of appropriate biomarkers (e.g. PSA, LDH, CRP, ALP) should be performed periodically depending on clinical symptoms, duration of disease, tumour biology, and previous course of the disease. Common are 3- to 6-month intervals in the beginning and annual intervals in the long-term.
-
Clinical follow-up should be performed periodically depending on clinical symptoms, duration of disease, tumour biology, and previous course of the disease. Common are 3- to 6-month intervals in the beginning and annual intervals in the long-term. Special attention should be paid to the possible development of adverse effects in the long-term, in particular to bone marrow suppression.
Evidence tables
No systematic review explicitly addressing prostate cancer and radium-223 was identified, but there were three randomised controlled trials done with radium-223 and another randomised controlled trial done with strontium-89 (Table 6).
Issues requiring further clarification
-
Safety and efficacy of radium-223 in predominantly osteolytic bone metastases.
-
Effect of concurrent use of calcium, phosphates or vitamin D on safety and efficacy of radium-223.
-
Effect of concurrent use of other life prolonging drugs (e.g. abiraterone, enzalutamide, chemotherapy) on safety and efficacy of radium-223.
-
Optimal therapeutic sequence of radium-223 and other life prolonging drugs (e.g. abiraterone, enzalutamide, chemotherapy).
-
Safety and efficacy of radium-223 in patients with blood cell counts below the limits specified in the section on relative contraindications. Reasonability to increase values before treatment, e.g. by red blood cell transfusion or treatment with colony-stimulating factors.
-
Efficacy of radium-223 using an incomplete number of cycles or a dosing delay exceeding 4 weeks (see section on interruption of therapy, delay of treatment cycles).
-
Interim determination of non-responders in whom continuation of treatment would be futile.
-
Safety and efficacy of re-treatment with radium-223 in patients who had received full six cycles.
-
Role of radium-223 imaging and patient specific dosimetry for treatment planning and verification.
Disclaimer
The EANM has written and approved guidelines to promote the cost-effective use of high-quality nuclear medicine therapeutic procedures. These generic recommendations cannot be rigidly applied to all patients in all practice settings. The guidelines should not be deemed inclusive of all proper procedures or exclusive of other procedures reasonably directed to obtaining the same results. Advances in medicine occur at a rapid rate. The date of a guideline should always be considered in determining its current applicability. The recommendations should be taken in the context of good practice of nuclear medicine and do not substitute for national and international legal or regulatory provisions.
References
Shore ND. Radium-223 dichloride for metastatic castration-resistant prostate cancer: the urologist's perspective. Urology. 2015;85:717–24.
Bayer AG. Xofigo Summary of Product Characteristics (SmPC). 2016.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–79.
Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–94.
Pandit-Taskar N, Larson SM, Carrasquillo JA. Bone-seeking radiopharmaceuticals for treatment of osseous metastases, part 1: alpha therapy with 223Ra-dichloride. J Nucl Med. 2014;55:268–74.
Hoskin P, Sartor O, O'Sullivan JM, Johannessen DC, Helle SI, Logue J, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014;15:1397–406.
Nilsson S, Franzen L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–94.
Nilsson S, Strang P, Aksnes AK, Franzen L, Olivier P, Pecking A, et al. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer. 2012;48:678–86.
Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23.
Parker CC, Pascoe S, Chodacki A, O'Sullivan JM, Germa JR, O'Bryan-Tear CG, et al. A randomized, double-blind, dose-finding, multicenter, phase 2 study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol. 2013;63:189–97.
Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014;15:738–46.
Saad F, Carles J, Gillessen S, Heidenreich A, Heinrich D, Gratt J, et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17:1306–16.
Sartor O, Hoskin P, Coleman RE, Nilsson S, Vogelzang NJ, Petrenciuc O, et al. Chemotherapy following radium-223 dichloride treatment in ALSYMPCA. Prostate. 2016;76:905–16.
Crawford ED, Petrylak DP, Shore N, Saad F, Slovin SF, Vogelzang NJ, et al. The role of therapeutic layering in optimizing treatment for patients with castration-resistant prostate cancer (prostate cancer radiographic assessments for detection of advanced recurrence II). Urology. 2017;104:150–9.
Du Y, Carrio I, De Vincentis G, Fanti S, Ilhan H, Mommsen C, et al. Practical recommendations for radium-223 treatment of metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1671–8.
Pezaro CJ, Omlin A, Lorente D, Nava Rodrigues D, Ferraldeschi R, Bianchini D, et al. Visceral disease in castration-resistant prostate cancer. Eur Urol. 2014;65:270–3.
Cook G Jr, Parker C, Chua S, Johnson B, Aksnes AK, Lewington VJ. 18F-fluoride PET: changes in uptake as a method to assess response in bone metastases from castrate-resistant prostate cancer patients treated with 223Ra-chloride (Alpharadin). EJNMMI Res. 2011;1:4.
Oyen WJ, Sundram F, Haug AR, Kalevi K, Lewington V, Mäenpää H, et al. Radium-223 dichloride (Ra-223) for the treatment of metastatic castration-resistant prostate cancer: Optimizing clinical practice in nuclear medicine centers. J Oncol Pathol. 2015:1–25. https://doi.org/10.13032/tjop.2052-5931.100121.
Dauer LT, Williamson MJ, Humm J, O'Donoghue J, Ghani R, Awadallah R, et al. Radiation safety considerations for the use of (2)(2)(3)RaCl(2) DE in men with castration-resistant prostate cancer. Health Phys. 2014;106:494–504.
Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–21.
Nieder C, Haukland E, Pawinski A, Dalhaug A. Anaemia and thrombocytopenia in patients with prostate cancer and bone metastases. BMC Cancer. 2010;10:284.
Wegener R, Schmickler M-R. Wichtige Informationen zu Xofigo®: temporärer Arzneimittelengpass 2014.
Chittenden SJ, Hindorf C, Parker CC, Lewington VJ, Pratt BE, Johnson B, et al. A phase 1, open-label study of the biodistribution, pharmacokinetics, and dosimetry of 223Ra-dichloride in patients with hormone-refractory prostate cancer and skeletal metastases. J Nucl Med. 2015;56:1304–9.
Carrasquillo JA, O'Donoghue JA, Pandit-Taskar N, Humm JL, Rathkopf DE, Slovin SF, et al. Phase I pharmacokinetic and biodistribution study with escalating doses of (2)(2)(3)Ra-dichloride in men with castration-resistant metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40:1384–93.
Lassmann M, Nosske D. Dosimetry of 223Ra-chloride: dose to normal organs and tissues. Eur J Nucl Med Mol Imaging. 2013;40:207–12.
Pacilio M, Ventroni G, Cassano B, Ialongo P, Lorenzon L, Di Castro E, et al. A case report of image-based dosimetry of bone metastases with Alpharadin ((223)Ra-dichloride) therapy: inter-fraction variability of absorbed dose and follow-up. Ann Nucl Med. 2016;30:163–8.
Pacilio M, Ventroni G, De Vincentis G, Cassano B, Pellegrini R, Di Castro E, et al. Dosimetry of bone metastases in targeted radionuclide therapy with alpha-emitting (223)Ra-dichloride. Eur J Nucl Med Mol Imaging. 2016;43:21–33.
Yoshida K, Kaneta T, Takano S, Sugiura M, Kawano T, Hino A, et al. Pharmacokinetics of single dose radium-223 dichloride (BAY 88-8223) in Japanese patients with castration-resistant prostate cancer and bone metastases. Ann Nucl Med. 2016;30:453–60.
Hindorf C, Chittenden S, Aksnes AK, Parker C, Flux GD. Quantitative imaging of 223Ra-chloride (Alpharadin) for targeted alpha-emitting radionuclide therapy of bone metastases. Nucl Med Commun. 2012;33:726–32.
Sartor O, Coleman RE, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol. 2017;28:1090–7.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–18.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Preamble
The European Association of Nuclear Medicine (EANM) is a professional nonprofit medical association that facilitates communication worldwide between individuals pursuing clinical and research excellence in nuclear medicine. The EANM was founded in 1985. EANM members are physicians, technologists, and scientists specialising in the research and practice of nuclear medicine.
The EANM will periodically define new guidelines for nuclear medicine practice to help advance the science of nuclear medicine and to improve the quality of service to patients throughout Europe. Existing practice guidelines will be reviewed for revision or renewal as appropriate, on their fifth anniversary or sooner, if indicated.
Each practice guideline, representing a policy statement by the EANM, has undergone a thorough consensus process in which it has been subjected to extensive review. The EANM recognises that the safe and effective use of diagnostic nuclear medicine imaging requires specific training, skills, and techniques, as described in each document.
The EANM has written and approved these guidelines to promote the use of nuclear medicine procedures with high quality. These guidelines are intended to assist practitioners in providing appropriate nuclear medicine care for patients. They are not inflexible rules or requirements of practice and are not intended, nor should they be used, to establish a legal standard of care.
The ultimate judgement regarding the propriety of any specific procedure or course of action must be made by medical professionals taking into account the unique circumstances of each case. Thus, there is no implication that an approach differing from the guidelines, standing alone, is below the standard of care. To the contrary, a conscientious practitioner may responsibly adopt a course of action different from that set forth in the guidelines when, in the reasonable judgement of the practitioner, such course of action is indicated by the condition of the patient, limitations of available resources, or advances in knowledge or technology subsequent to publication of the guidelines.
The practice of medicine involves not only the science but also the art of dealing with the prevention, diagnosis, alleviation, and treatment of disease. The variety and complexity of human conditions make it impossible to always reach the most appropriate diagnosis or to predict with certainty a particular response to treatment. Therefore, it should be recognised that adherence to these guidelines will not ensure an accurate diagnosis or a successful outcome. All that should be expected is that the practitioner will follow a reasonable course of action based on current knowledge, available resources, and the needs of the patient to deliver effective and safe medical care. The sole purpose of these guidelines is to assist practitioners in achieving this objective.
Rights and permissions
About this article
Cite this article
Poeppel, T.D., Handkiewicz-Junak, D., Andreeff, M. et al. EANM guideline for radionuclide therapy with radium-223 of metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 45, 824–845 (2018). https://doi.org/10.1007/s00259-017-3900-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3900-4