Abstract
Radionuclide therapy using I-131 is commonly used for the treatment of benign thyroid diseases. The therapeutic dose to be administered is calculated based on the type of disease, the volume of the thyroid, and the measured uptake percentage. This methodology assumes a similar biological half-life of iodine, whereas in reality a large variation in biological half-life is observed. More knowledge about the actual biological half-life of iodine for individual patients will improve the quantification of the delivered radiation dose during radioiodine therapy and could aid the evaluation of the success of the therapy. In this feasibility study we used a novel measurement device [Collar Therapy Indicator (CoTI)] to measure the uptake curve of patients undergoing I-131 radioiodine therapy. The CoTI device is a light-weight wearable device that contains two independent gamma radiation detectors that are placed in a collar. By comparing results of thyroid uptake measurements with results obtained with a gamma camera, the precision of the system is demonstrated. Additionally, for three patients the uptake curve is measured during 48 h of admission in the hospital. The presented results demonstrate the feasibility of the new measurement device to measure the uptake curve during radioiodine therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The unique ability of the thyroid gland to concentrate iodine has been exploited to diagnose thyroid diseases and the first reports of successful treatment of hyperthyroidism using radionuclide therapy date back to 1941 [1, 2]. Since these first reports, I-131 radionuclide therapy has become a well-established option for the treatment of benign thyroid diseases, as well as thyroid cancer.
The planned radiation dose to the thyroid gland depends on the type of disease. In guidelines this is commonly converted to a required target concentration of I-131 in the thyroid (in MBq/mL) 24 h after administration. According to the recommendations of the Dutch Society of Nuclear Medicine (NVNG) patients with Graves’ disease and patients with euthyroid multinodular goitre are treated with an I-131 concentration of 4 MBq/mL 24 h after administration. Toxic adenoma is treated according to these recommendations with a fixed dose of 740 MBq or with a planned concentration of 8 MBq/mL. Patients with toxic multinodular goitre are recommended to be treated with a concentration of 4 or 8 MBq/mL, depending on other clinically relevant parameters.
The European Association of Nuclear Medicine (EANM) gives recommendations for the planned radiation dose for the specific thyroid diseases, following a similar approach [7]. For toxic or nontoxic multinodular goitre an absorbed radiation dose of 100–150 Gy is recommended, requiring about 3.7–5.5 MBq/mL I-131 at 24 h after administration. In patients with autonomous nodules, the recommended dose is 300–400 Gy. In patients with Graves’ disease, the dose with the aim of restoring a euthyroid status is approximately 150 Gy, whereas the dose to achieve complete ablation is in the range 200–300 Gy.
These guidelines assume a fixed biological half-life of iodine in the thyroid, whereas in reality a large variation in biological half-life is observed [3–6]. This missing parameter results in a large uncertainty in the actual delivered radiation dose to the thyroid.
According to these guidelines, the activity to be administered (D) can be calculated using information about the uptake percentage, the volume of the thyroid and the desired concentration of I-131 using the following formula:
where V is the volume (in mL), K the planned activity concentration in the thyroid (in MBq/mL multiplied by 100) and U the uptake of iodine (in % of the total administered dose) in the thyroid after 24 h. The uptake can be estimated by an uptake measurement, in which the measured number of counts in the patient’s thyroid are compared to a phantom measurement of a sample amount of I-131 or I-123 and the administered activity. The volume of the thyroid is determined by palpation, ultrasound imaging or scintigraphic imaging. [7]
Following the Dutch radiation protection regulations the patient can be discharged from the hospital when the external radiation dose rate is less than 20 μSv/h measured at a distance of 1 m. As a result, administration of a therapeutic dose of I-131 may require isolation of the patient in the hospital for radiation safety purposes if the administered dose is above 400 MBq.
Using the described method mentioned above and formula 1, the delivered radiation dose is calculated from the administered activity, the pre-therapeutic uptake measurement and the thyroid volume. One of the main assumptions in this approach is that the effective half-life of the iodine is the same for all patients with a similar thyroid disease, whereas in practice this can vary considerably [1]. To determine the actual delivered thyroid radiation dose it is, therefore, necessary to have information about the actual uptake curve of a patient. To determine the patient-specific activity uptake and discharge over time, repeating scans should be made. However, making SPECT or whole-body (WB) scans more frequently is time consuming and, therefore, expensive and challenging from a radiation safety point of view. Especially in the first 24 h after the administration of I-131 transporting the patient to the department of nuclear medicine is undesired.
Because in practice the uptake curve is often not measured during therapy, the actual delivered thyroid radiation dose is a missing parameter in cases where the radionuclide therapy was clinically unsuccessful or only partly successful. The actual delivered thyroid radiation dose could give useful information whether the patient should be given a new radionuclide therapy and with which dose.
A number of methods have been developed to measure the I-131 uptake curve in the thyroid, which are all based on repetitive measurements using existing procedures. The uptake of I-131 is generally determined using a gamma camera or a gamma probe. Using a gamma camera (either planar images or SPECT) the time-dependent uptake curve can be measured by repeating these measurements at different time intervals after administration [8]. These repetitive measurements have only been used to study the average uptake curves on a (small) set of patients, because of practical limitations. An alternative approach is to use a gamma probe, positioned in front of the patient’s neck, which can give similar results as those obtained with the gamma camera [9, 10]. To determine the uptake curve, the measurement is repeated at different time intervals after administration [3, 4]. Even though this is practically feasible and reduces the costs, this has not been routinely used to determine the individual uptake curve for a patient.
We have used a new and simple measuring device that measures the I-131 uptake in the thyroid. The device can easily be attached with a collar around the neck and can be used to measure continuously or to perform a sequence of measurements. The additional advantage of the system is that the measurements can be performed by the patients and no presence of staff is required, thereby eliminating the issue of staff radiation exposure. In this paper we will describe the Collar Therapy Indicator (CoTI) system and present the first results of a feasibility study of the system.
Material
CoTI (Collar Therapy Indicator) is a new type of device that provides gamma count information from a patient undergoing radionuclide therapy. It is a small, light-weight wearable device that has up to 4 independent gamma radiation detectors that can be placed anywhere on the body. The CoTI device that was used in this study is a prototype and is fabricated by AG Medical (Saint-Aubin, France).
Each CoTI gamma radiation detector contains a single silicon photo-multiplier (SiPM) device with a CsI(Tl) scintillation crystal. The active detection surface is 3x3 mm2. Data acquisition electronics within the small detector read and condition the photon pulse signal from the SiPM to produce the number of counts per second. This data is digitised and the detector’s on-board processor communicates this information to the CoTI Control Unit. The Control Unit stores this data in non-volatile memory and sends it via a wireless connection to the CoTI handheld tablet, which collects and displays the data. In the current version, no energy discrimination is included in the detectors, measurements are performed with an open energy window.
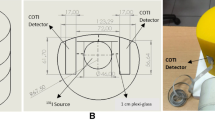
Two detectors are placed in a disposable collar at predefined positions. After wrapping the detectors in disposable plastic sleeves and an additional soft wrap around the collar, the collar can be positioned around the patient’s neck to perform the measurements, as demonstrated in Fig. 1.
The measurement protocol of the device is adjustable and can be used to perform a single set of measurements or a multi-phase set of measurements. For a single set of measurements, the measurement time can be set (in minutes), and the options are two, three, four, or five measurements per minute. For a multi-phase set of measurements, one can define the total length, the interval time between measurements and the integration time of each measurement for every phase. The battery lifetime of the control unit depends mainly on the amount of data points that are acquired (standby time usage is very low), in practice a full battery storage can easily accommodate measurements of a therapy patient until discharge from the hospital (typically after 48 h).
Methods
Reproducibility and accuracy measurements using I-123
To determine the accuracy of uptake measurements, we used the CoTI to perform I-123 uptake measurements and compared this with the standard clinical protocol to determine the uptake value based on a planar gamma camera measurement (Ecam, Siemens). The CoTI based uptake measurement was performed with 20 patients who underwent an uptake measurement. On each patient-day a reference measurement using a standard activity of 8 MBq of I-123 was performed with the CoTI to calculate the sensitivity of the CoTI system. The activity was positioned in a cylindrical phantom that resembles the thickness and attenuation properties of the patients neck. This procedure is similar to the routine method using the Siemens Ecam camera with a pinhole collimator. The reproducibility of the measurement system is determined by determining the variation of six subsequent sensitivity measurements using the phantom. The CoTI uptake measurement consisted of short acquisitions of 3 min and was performed on 20 patients who were scheduled for I-123 uptake measurements.
Linearity measurements using I-131
Linearity measurements were performed using an I-131 capsule dissolved in water, measurements were repeated over a period of 7 weeks until the linear regime was reached. At the start of the measurements the activity of I-131 was approximately 500 MBq, which is expected to be the maximum activity that will be measured in the thyroid during therapy.
Feasibility and reproducibility using I-131
To show the feasibility of this system to measure the activity uptake curve, three patients who were clinically treated with I-131 radionuclide therapy were monitored. These patients underwent repeated measurements with the CoTI during their clinical admission. In addition to the activity uptake curve, also practical experience was obtained to further study the feasibility of this method for the measurement of uptake curves.
To study the reproducibility of the system, repeated measurements were performed on one patient. To simulate the measurements with therapy patients, the patient was asked to position the collar as accurately as possible in the centre of the patient’s neck. In between the subsequent measurements, the collar was taken off and repositioned. These results are a measure for the reproducibility of the measurements, including variations in repositioning the collar.
Results
Reproducibility and accuracy measurements using I-123
To verify the reproducibility of the results, the different sensitivity measurements with the phantom are compared as shown in Fig. 2. The uncertainty of the data points shown in Fig. 2 is 3%, determined from the observed fluctuations during the measurements. From these results a mean value of the sensitivity of 2.2 × 102 cps/MBq is obtained with a standard deviation of 15 cps/MBq. Because for these measurements the activity is determined with a dose calibrator, the error from the dose calibrator is included in this standard deviation. The observed standard deviation results in a coefficient of variation of 0.07, indicating that the phantom can be measured with a relatively good precision of 7%.
The results from the CoTI plotted against the uptake values of the gamma camera, are shown in Fig. 3. The uncertainty of these measurements are calculated by adding the errors from the fluctuations during the measurements and the uncertainty from the sensitivity measurements. The specific uptake values cover almost the entire range between 0 and 100%. The coefficient of determination between the two methods is 0.87, which is generally seen as a good agreement between two methods.
After demonstrating the capability of measuring the activity in the thyroid for I-123 uptake measurements, the next step is to show the possibility to use CoTI for monitoring during I-131 therapy. Before monitoring clinically treated patients with CoTI, the two main difficulties are studied. First of all, linearity of the detectors is tested for the higher count rates that are expected due to the higher administered activity for I-131 treatment. Additionally, repeated measurements of the same patients are taken to show the reproducibility of repositioning the collar and performing subsequent measurements.
Linearity measurements using I-131
The results of the linearity measurements are plotted in Fig. 4. The inaccuracy of these individual data points is approximately 3%, as determined from the fluctuations of the measurements. From these results it is concluded that the linear regime extends to a count rate of approximately 2000 cps. By (linear) interpolation of this curve, a correction factor is determined that is used to obtain the actual count rate for I-131 measurements with a count rate above 2000 cps.
Feasibility and reproducibility using I-131
To determine the reproducibility of the measurements, a therapy patient was asked to repeat the same measurement six times and take the collar off after each measurement. This takes variations of the position of the collar, as well as deviations from the measurement device into account and gives an estimate of the reproducibility of the measurements. The measurements show very similar values and the standard deviation is 346 cps, corresponding to a coefficient of variation of 0.02. From these results it is concluded that when the patients are correctly instructed, repositioning of the collar is possible with a relatively good precision.
To demonstrate the feasibility of the CoTI system to measure individual uptake curves, three clinically treated patients were included. Patient 1 was treated for Graves’ disease and patients 2 and 3 were treated for toxic multinodular goitre. Because the most dynamic situation is expected during the first hours after administration, the frequency of measurements was highest in the first 6 h. For patient comfort, no measurements were taken during the night. By monitoring this part of the uptake curve, it is demonstrated that the uptake of iodine in the thyroid can be measured easily, as shown in Fig. 5. The inaccuracy of the data points is 8%, as determined from the fluctuations during the measurements. After reaching a maximum level of activity in the thyroid, the remainder of the monitored 48 h the activity remains relatively constant or is slowly decreasing. Even though only a limited number of individual uptake curves have been measured, these preliminary results demonstrate the feasibility of the CoTI system to measure thyroid uptake curves.
Discussion
The CoTI system was developed to enable the measurement of the actual delivered radiation dose to the thyroid. In this study the feasibility of the system was evaluated.
The patients experienced no particular issues during the short measurements of 3 min. Placing the collar around the neck was done by the therapy patients themselves and this was found to be rather easy after a brief explanation before the start of the therapy. Leaving the collar around the patients neck for a longer period was in some cases problematic, where patients reported a feeling of discomfort and would prefer to do several short measurements rather than leaving the collar around the neck for several hours. From these experiences it is concluded that short measurements are the best approach and that a sufficient amount of data points can easily be obtained by these short measurements. Combining these results it is concluded that from a technological, as well as from a patient perspective the CoTI system is a promising approach to obtain thyroid uptake curves during I-131 therapy.
From the obtained results it is concluded that the CoTI system is feasible to study the thyroid uptake behaviour and obtain uptake curves; however, there are several limitations to the current study. First, to obtain more complete data, intermediate data points are required. The intervals missing during the evening and night, were not included in this study due to practical limitations. In addition to these intervals, information about the retained activity after the first 48 h, when the patient is discharged from the hospital, is also missing. This latter phase is essential to calculate the biological half-life of the I-131 and, therefore, the absorbed thyroid radiation dose.
If measurements are extended beyond this period, the complete thyroid uptake curve can be measured. In a next version the device will be made more user-friendly such that even measurements by the patient him/herself at home are feasible. The aim of this extended version is to measure the complete uptake curve even after patient discharge. If this information can be converted to activity in the thyroid by (individual) calibration of the CoTI for I-131 and additional information about the thyroid volume is used, the CoTI measurements enable one to estimate the actual radiation dose to the thyroid for individual patients.
Several sources of inaccuracy of the current setup were identified that need to be addressed if this information is to be used for accurate dose calculations. First of all the positioning of the collar around the patients neck. It is shown that this can be done in a reproducible manner for an individual patient, but variations in thyroid size and position between patients, as well as the presence of hot and cold nodules within the thyroid, will lead to variations. This variation partly explains the observed individual differences in the I-123 uptake measurements, compared to the gamma camera results. By individual calibration (for example by using scintigraphic information) of the sensitivity of the CoTI this inaccuracy could be addressed. The non-linearity of the dectectors is a second source of inaccuracy of the CoTI. This is only a minor issue and this is already corrected for using linearity correction factors. A third source of inaccuracy originates from the lack of energy discrimination of the detectors, resulting in the detection of scattered photons in addition to the measured primary photons. The influence of this scatter is not quantitatively investigated, but an improved detector system using energy discrimination would reduce the amount of scattered photons in the detected signal.
Conclusions
This feasibility study has shown that the CoTI system is accurate (as shown by uptake measurements), the measurements are reproducible (as shown by repetitive measurements) and that the system is able to measure uptake curves, as demonstrated by the successful measurement of uptake curves for three therapy patients.
In conclusion, the CoTI system is shown to be a feasible tool to measure the activity in the thyroid as a function of time, resulting in a thyroid uptake curve. To calculate the thyroid radiation dose, additional information including thyroid volume, and measurement of the thyroid uptake curve for several days to weeks will need to be performed. With this additional information, the CoTI system will enable the calculation of the actual I-131 delivered radiation dose to the thyroid tissue.
References
Czepczynski R. Nuclear medicine in the diagnosis of benign thyroid diseases. Nucl Med Rev Cent East Eur. 2012;15:113–9.
Becker DV, Sawin CT. Radioiodine and thyroid disease: the beginning. Semin Nucl Med. 1996;26:155–64.
Kobe C, Eschner W, Wild M, Rahlff I, Sudbrock F, Schmidt M, et al. Radioiodine therapy of benign thyroid disorders: what are the effective thyroidal half-life and uptake of 131I? Nucl Med Commun. 2010;31:201–5.
Willegaignon J, Sapienza MT, Coura Filho GB, Traino AC, Buchpiguel CA. Determining thyroid (131)I effective half-life for the treatment planning of Graves’ disease. Med Phys. 2013;40:022502.
Berg GE, Michanek AM, Holmberg EC, Fink M. Iodine-131 treatment of hyperthyroidism: significance of effective half-life measurements. J Nucl Med. 1996;37:228–32.
Flower MA, Schlesinger T, Hinton PJ, Adam I, Masoomi AM, Elbelli MA, et al. Radiation dose assessment in radioiodine therapy. 2. Practical implementation using quantitative scanning and PET, with initial results on thyroid carcinoma. Radiother Oncol. 1989;15:345–57.
Stokkel MP, Handkiewicz Junak D, Lassmann M, Dietlein M, Luster M. EANM procedure guidelines for therapy of benign thyroid disease. Eur J Nucl Med Mol Imaging. 2010;37:2218–28.
Hanscheid H, Canzi C, Eschner W, Flux G, Luster M, Strigari L, et al. EANM Dosimetry Committee series on standard operational procedures for pre-therapeutic dosimetry II. Dosimetry prior to radioiodine therapy of benign thyroid diseases. Eur J Nucl Med Mol Imaging. 2013;40:1126–34.
Benjamin RS, Amro A, El-Desouki MI. Measurement of iodine-123 thyroid uptake using a gamma camera with LEAP collimator. J Nucl Med Technol. 1999;27:215–9.
Balon HR, Silberstein EB, Meier DA, Charkes ND, Royal HD, Sarkar SD, Donohoe KJ. Society of Nuclear Medicine Procedure Guideline for Thyroid Uptake Measurement. Society of Nuclear Medicine Procedure Guidelines 2006.
Acknowledgments
The authors acknowledge AG Medical for supplying the CoTI device for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Informed consent was obtained from all individual participants in this study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Brinks, P., Van Gils, K., Kranenborg, E. et al. Measuring the actual I-131 thyroid uptake curve with a collar detector system: a feasibility study. Eur J Nucl Med Mol Imaging 44, 935–940 (2017). https://doi.org/10.1007/s00259-016-3595-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3595-y