Abstract
Purpose
In patients with metastatic differentiated thyroid carcinoma (DTC), fluorodeoxyglucose (FDG) uptake as well as age, tumor size and radioactive iodine (RAI) uptake are prognostic factors for survival. High FDG uptake is a poor prognostic factor and lesions with high FDG uptake are often considered aggressive, but the predictive value of FDG uptake for morphological progression is unknown. The principal aim of this retrospective single center study was to determine whether the intensity of FDG uptake was correlated on a per lesion analysis with tumor growth rate (TGR) expressed as the percentage of increase in tumor size during 1 year (1-year TGR).
Methods
Fifty five patients with DTC were included between July 2012 and May 2014 with the following criteria: (i) at least one distant metastasis measuring ≥ 1 cm in diameter on CT scan (ii) evaluation by FDG-positron emission tomography/computed tomography (PET/CT) performed at our center (iii) at least one CT or another FDG-PET/CT performed 3 to 12 months after the reference FDG-PET/CT in the absence of systemic or local treatment between the two imaging procedures.
Results
One hundred and fifty-six metastatic lesions located in lungs (63), neck lymph nodes (28), chest lymph nodes (42), bone (11), liver (2) and other sites (12) were studied. The median size was 16 mm, median SUVmax/lesion: 8.7; median metabolic tumor volume/lesion (Metab.TV/lesion): 3.7 cm3. The median 1-year TGR was 40.68 %. SUVmax and Metab.TV/lesion were not correlated to their 1-year TGR (p = 0.38 and p = 0.74 respectively). Among single patients with multiple lesions, the lesions with the highest SUVmax/lesion or the highest Metab.TV/lesion did not disclose the higher 1-year TGR.
Conclusion
The intensity of FDG uptake on a per lesion analysis is not correlated to its 1-year TGR and cannot be used as a surrogate marker of tumour progression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients with metastatic differentiated thyroid carcinoma (DTC) are first treated with radioactive iodine (RAI). When the disease is deemed to be refractory, RAI treatment is abandoned. Targeted molecular therapies are used in refractory patients with large tumor burden when radiological tumor progression is demonstrated or in case of symptomatic and/or threatening disease, when local treatments are not considered as a treatment option [1]. In patients with small tumor burden, in the absence of symptomatic and/or threatening disease, a wait and see policy is adopted with a close monitoring with computed tomography (CT) performed every 4 to 12 months until tumor progression is demonstrated. High FDG uptake is not by itself a reason to start systemic treatment. In patients with a small number of lesions that are slowly progressive, it may be important to recognize which lesion may be at risk of progression, because local treatments such as surgery, thermoablation or external beam radiation may then be considered [2, 3].
18F-Fluorodesoxyglucose (FDG) positron emission tomography (PET) is performed in metastatic DTC patients to assess both the location of distant metastases and their FDG status. In fact, high FDG uptake is a strong predictor of resistance to RAI treatment and a prognostic factor for shorter survival [4–6]. Other independent prognostic factors for survival include RAI status, age of the patients, histology and size of the distant metastases [7].
Given the prognostic value of FDG uptake in DTC, a lesion with a high FDG uptake is often considered aggressive. The 2015 American Thyroid Association recommendations suggest that these lesions with high FDG uptake should be considered for focal treatment modalities or for close monitoring [1]. However, to date, no data has shown that the intensity of FDG uptake in a lesion was predictive of its growth rate and that FDG-PET could be used as a surrogate marker of morphological tumor progression.
The primary aim of this retrospective single center study was to determine in patients with distant metastases of DTC whether the intensity of FDG uptake of distant metastases were correlated to their own tumor growth rate (TGR) expressed as the percentage of increase in tumor size during a 1 year-period (1-year TGR).
Materials and methods
Patients
Metastatic consecutive DTC patients referred to our center for FDG PET/computed tomography (CT) between July 2012 and May 2014 were included if they met the following criteria (i) DTC confirmed by our pathologist, (ii) measurable distant metastases of at least 1 cm in diameter on the CT scan of the FDG-PET/CT, (iii) availability of a second CT scan performed 3 to 12 months after the reference FDG PET/CT to assess tumour progression, and (iv) absence of systemic or local treatment between the two imaging procedures.
Among the 276 patients with distant metastases referred for FDG PET/CT during this period, 26 were excluded because of the absence of a second procedure imaging during the requested interval of time, 184 because treatments were given between the two imaging procedures, and 11 because of lesions under 1 cm. For the remaining 55 patients, we collected: sex, age, pathology (papillary, follicular, poorly differentiated, and their subtypes), Tumor Node Metastasis classification (TNM), size of the primary, and history of previous treatments.
Images acquisition and analysis
All patients underwent PET/CT in our nuclear medicine department using a time of flight General Electric DISCOVERY 690 PET/CT device. PET images were acquired 60 min after tracer injection (2–3 MBq/Kg of FDG) and recorded from the apex of the skull to the mid-thighs in 3-D mode, 7–10 beds, 2 min/bed position. Prior to PET acquisition, a low dose CT acquisition for attenuation correction and anatomical correlation was performed with the same longitudinal field of view. PET images were reconstructed on a 2562 matrix, using an iterative algorithm (Ordered-Subset Expectation Maximization - two iterations 24 subsets) and post filtering (3-points Z filter – weighting factors 1:2:1). CT images were reconstructed on a 5122 matrix using the usual Filtered Back Projection algorithm. After reconstruction, the final slice thickness was 3.27 and 2.5 mm regarding, respectively, PET and CT.
Patients fasted for 4–6 h before the exam and had a venous glycaemia with a median level of 5.3 mmol/L (range: 3.9–10.8). FDG PET/CT were performed under thyroxine treatment in all cases except one in which it was performed after thyroid hormone withdrawal. Median TSH level under thyroxine treatment was 0.06 μU/mL (range: 0.01–2).
Per lesion imaging analysis
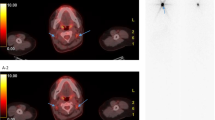
For each patient, a maximum of ten lesions with FDG uptake were studied with a maximum of two lesions per organ defined as lung, neck lymph node, mediastinal lymph node, bone, liver, pleura or other locations. Lesions had to be measurable on the CT of the FDG PET/CT (Fig. 1). For bone lesions, only those with a measurable soft tissue component on CT were taken into account, as defined by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria [8].
FDG-PET – Maximum Standardized Uptake Value of each target lesion (SUVmax/lesion) was measured by using a volume of interest with Standardized Uptake value (SUV) being expressed using the most commonly used definition of SUV (g/mL) = (Tissue activity (Bq/mL)/[(injected activity (Bq)/ body weight (g)]). FDG metabolic tumor volume of each lesion (Metab.TV/lesion), representing the volume measured in the volume of interest was determined using margin thresholds set at 40 % of maximal SUV (SUVmax). Both SUVmax and Metab.TV/lesion were automatically extracted using Advantage Window volume share 5 General Electric software dedicated to image analysis in routine. The Metab.TV/lesion could only be determined in patients with lesions with FDG uptake sufficiently different from surrounding tissue in order to correctly define the lesions.
CT scan – Lesions were initially measured on the CT of the FDG PET/CT. Target lesions measured on the first examination were measured on a subsequent examination performed 3 to 12 months after the first one. The subsequent examination was a CT from a PET/CT performed in our center in 48 cases or on a diagnostic CT in seven cases. If so, images were acquired and reconstructed in accordance with the best practice recommendations. The morphological tumor volume (Morpho.TV) could only be determined in patients with lesions with density sufficiently different from surrounding tissue in order correctly define the lesions.
Per patient imaging analysis
SUVmax/patient represents the highest SUVmax of all lesions in a given patient. FDG metabolic tumor volume of each patient (Metab.TV/patient) represents the volume of all lesions with FDG uptake for a given patient calculated by adding the metabolic tumor volume of all lesions present in this patient.
The Morphological Tumor Volume of each patient (Morpho.TV/patient) represents the sum of the morphological tumor volume of all lesions on CT scan in a given patient. All calculations (FDG PET and CT scan) have been performed using the same software package, as mentioned above.
Tumor growth rate
Tumor size (D) (defined as the longest diameter of target lesions) was measured at baseline (D1) and on a subsequent CT (D2), t being the interval of time between the two imaging procedures. The t was expressed in years in order to express TGR as a percentage of increase in tumor volume within 1 year, as previously described (9,10). Assuming that lesions are spheres, volume (V) of the lesions was calculated as V = 4πR3/3, where R (the radius of the sphere) equals D/2. Assuming the tumor growth follows an exponential law, Vt (the tumor at time t) is equal to Vt = V1 exp (TG*t), where V1 is the volume at the first procedure imaging and TG is the growth rate. Consecutively, TG = 3ln (D2/D1)/t. Finally in order to express TGR as a percentage increase in tumor volume during 1 year, the following transformation was applied, TGR = 100[exp(TG)-1], in which exp(TG) represents the exponent of TG. The 1-year TGR was calculated for each lesion.
Thyroglobulin (Tg) measurement
Tg was measured under levothyroxine treatment using a chemiluminescent immunoenzymatic « sandwich » assay (Access® Thyroglobulin, automated on UniCel® DxI 800 instruments, Beckman Coulter, Villepinte, France) The Tg was considered as not measurable in the presence of Tg antibodies (Access® Thyroglobulin Antibody II assay, Beckman Coulter). Tg was collected at the time of each imaging procedure: Tg1 for the first and Tg2 for the second procedure. The Tg doubling time (TgDT) was calculated as follows: TgDT = (0.693 x Dt)/[Ln (Tg2/Tg1))] with Dt being the interval of time between the two imaging procedures.
Follow-up
Following the two imaging procedures, patients were followed with clinical and imaging examinations every 4 to 12 months. The status of each patient (alive or dead) was collected at the end of the study.
Statistical analysis
Quantitative data were expressed in mean and standard deviation (STD), or median and range if non-normally distributed. Qualitative data were expressed in percentage. Correlations were searched between parameters (1-year TGR, SUVmax and Metab.TV) by using the Spearman test.
The overall survival was defined as the duration between the date of first FDG PET to death from any cause or to the date of the last follow-up and was estimated using the Kaplan-Meier method. The median follow-up was estimated by the reverse Kaplan-Meier method (Schemper’s method). The parameters significantly associated with overall survival were searched using univariate analysis (P < 0.05) by log rank tests. All tests were two sided.
Analyses were performed using SAS statistical software (SAS Institute Inc., Cary, NC). Significance level was 0.05, two sided.
Results
Patient’s characteristics (Table 1)
Fifty-five patients (26 women, 29 men; mean age: 65 years; range: 46–90 years) were included. Pathology was papillary TC in 33 cases (classic: 23 cases, tall cell: eight cases, trabecular: two cases), follicular TC in eight cases, and poorly DTC in 14 cases (with oncocytic cells in two cases). Mean primary tumor size was 37 mm (range: 6–90 mm STD 21.3). Clinical characteristics of the patients are shown in Table 1. Primary treatment consisted in a total thyroidectomy with or without neck dissection in 50 cases and RAI administration after thyroid hormone withdrawal in 49 cases. In five cases, thyroid tumor was not resectable because of local invasion and pathology was assessed on biopsy. Distant metastases were present at initial diagnosis in 26 patients (47 %) and were diagnosed during follow-up in the other patients after a median interval of time of 3.0 years (range: 0.75–8.5 years).
Among the 49 patients who received RAI, the median number of RAI administrations was 2 (range 1–8) with a median cumulated activity of RAI of 7400 MBq (range: 3700–29,600 MBq). RAI administration was not performed in the absence of total thyroidectomy in five cases and because of rapid tumor progression following surgery in one case. Forty seven (96 %) of the 49 patients treated by RAI were classified as RAI refractory because of distant metastases without RAI uptake on a post-therapeutic scan in 38 cases, because of persistent distant metastases after six courses of 3700 MBq of RAI in five cases or because of RECIST 1.1 disease progression within 14 months of a RAI treatment despite persistent RAI uptake in four cases (11,12). The median interval of time between last RAI treatment (for those that received RAI) and FDG PET was 24 months (range: 6–15).
The median SUVmax of each patient was 13.2 (range 3.6–66.2 STD 15.6). The median metabolic volume per patient (Metab.TV/patient), available in 48 patients, was 15.2 cm3 (range: 0.1–199.2 STD 27.9). The median morphological volume (Morpho.TV/patient), available in 52 patients was 15.5 cm3 (range: 0.4–254.8 STD 41.5). The median interval of time between two morphological examinations was 6 months (range: 3–12, STD 0.32).
After a median follow up of 77.4 weeks (range 13.9–234), 30 patients died, all from thyroid cancer. The 1 and 2-year survival rates were respectively 89 % (IC 76–96 %) and 70 % (IC 49–83 %), and the median survival was 30 months.
Per lesion analysis (Table 2)
One hundred and fifty-six lesions were studied. The median number of lesions per patient was two (range: 1–6). There were 63 lung metastases, 28 neck lymph nodes, 42 mediastinal lymph nodes, 11 bone metastases, two liver metastases and 12 metastases in other locations. Their median SUVmax/lesion was 8.7 (range: 0.7–66.2 STD 12.19) with a median Metab.TV/lesion of 3.7 cm3 (range: 0.6–184 STD 19.3) and a median size of 16 mm (range: 7–96 STD 14.7). No correlation was found between the subtype of pathology and the mean SUVmax/lesion considering classic, tall cell variant, trabecular, follicular and poorly differentiated subtypes (p = 0.49) or considering poorly differentiated subtype versus others (p = 0.47).
Their median 1-year TGR rate was 40.68 % (range: 0–1451,308 STD 116,278). SUVmax/lesion, Metab.TV/lesion and size according to each location are shown in Table 2.
The SUVmax/lesion was not correlated to its 1-year TGR (p = 0.38) (Fig. 2a). The Metab.TV/lesion was not correlated to its 1-year TGR (p = 0.74) (Fig. 2b). Furthermore, the location of the lesions did not influence the lack of correlation between the FDG uptake and TGR, in neck lymph nodes (p = 0.65), in mediastinal lymph nodes (p = 0.34), in lung lesions (p = 0.50), in bone lesions (p = 0.89), nor in other lesions (p = 0.48). The initial size of the lesions did not influence the lack of correlation between the FDG uptake and TGR (p = 0.83)). Finally, among patients with five lesions or more, the lesions with the highest SUVmax/lesion or the highest Metab.TV/lesion did not disclose the highest 1-year TGR (Fig. 3).
Per patient analysis
Patients with a SUVmax < 5 had a 1 and 2-year survival of 100, % whereas patients with SUVmax ≥5 had a 1-year survival of 87 % (IC 73–95 %) and a 2-year survival of 66 % (IC 45–81 %) (p = 0.18 and 0.16, respectively) (Fig. 4a).
The SUVmax/patient was correlated to Morpho.TV/patient (p = 0.02) but not to the Metab.TV/patient (p = 0.34). Otherwise the SUVmax/patient was not correlated to gender p = 0.57.
Survival was correlated to the Metab.TV/patient (p = 0.005) and to the Morpho.TV/patient (p = 0.005) (Fig. 4b and c). The 1- and 2-year survival were 100 % and 93 % in patients with a Metab.TV/patient below 15.2 cm3 and 87 % and 66 % in patients with a Metab.TV/patient equal to or above 15.2 cm3. The 1- and 2-year survival were 100 % and 92 % in patients with a Morpho.TV/patient below 15.5 cm3 and 88 and 61 % in patients with a Morpho.TV/patient equal to or above 15.5 cm3. Otherwise, no correlation was found between survival and gender (p = 0.17) or pathology (p = 0.60). Age was not tested, all patients being older than 45 years. The median TgDT, available in 39 cases was 101 days (range 0–1611 STD 349.26) and was not correlated to the SUVmax/patient (p = 0.47) nor to the overall survival (p = 0.10).
Discussion
A wait and see policy to determine the natural history and tumour growth rate before any treatment decision, in a patient with distant metastases that can impact survival or quality of life, is not easily accepted by patients. However, because tumour progression of DTC can vary widely from one patient to another and because of the toxicities of treatments, systemic treatment is initiated only in patients with progressive disease. Furthermore, we may propose, in case of small tumour burden with few distant metastases, local treatments such as thermal ablation or stereotaxic beam radiation therapy, in order to postpone systemic treatment. The identification of a tool that predicts tumour morphological RECIST progression and to identify the lesions at highest risk of progression could be useful in the management of metastatic RAI refractory DTC patients. In patients where CT scan are not performed at the same interval of time, it is possible to use TGR to evaluate tumour growth, since it takes into account the interval of time between CT evaluations [9, 10]. TGR has been validated in previous studies and was correlated to survival even though its calculation makes the assumptions that lesions are a sphere and that their growth is exponential, which might not be true in all cases [9, 10]. A lesion measuring 20 mm on a first CT scan and 25 mm on a second CT performed at 4–6 months, has an increase in size of 25 % and is considered as progressive within 4–6 months according to RECIST 1.1 criteria. If the control CT scan is performed at 12 months, the lesion is considered as progressive within 12 months [8]. Using TGR to evaluate this progression, the lesion has a 1 year-TGR of 281 % in the first case and of 95 % in the second case.
FDG PET/CT is a powerful tool in DTC. FDG uptake is correlated to tumor dedifferentiation and is prognostic for survival. In contrast to what is frequently believed, our study clearly showed that lesions with the highest FDG uptake do not have the greatest TGR, since we did not find any correlation in a per lesion analysis between SUVmax/lesion and its TGR. This was not influenced by the location of the lesion nor by its size or by the pathology. Furthermore, in a given patient, lesions with the highest SUVmax/lesion are not those that will disclose the highest TGR.
These results are obtained in a retrospective design and on a small number of patients, which is an obvious limitation of the study. They only apply to the population selected: DTC patients with lesions above 1 cm who are on a wait and see policy in order to determine RECIST progression and who were in most of the cases refractory to RAI. This is, indeed, the population for which, in clinical practice we discuss local treatments depending on the number, the size and location of the lesions.
A weakness of this study regards the accuracy of measures on the CT scan to calculate the TGR. First, we measured CT size on the CT of a PET/CT for the first determination and for 87 % of the second determination (the last 13 % being measured on the diagnostic CT) which is due to the retrospective design of the study. Second, the reproducibility of CT measurements is known to be lower that the reproducibility of SUVmax determination [11]. This is indeed why only lesions of more than 1 cm are taken into account for RECIST criteria, and why we only considered lesions above 1 cm.
Of note the definition used to define refractory to RAI is consensual for 42 of the 47 patients classified as refractory [1]: distant metastases without RAI uptake on a post-therapeutic scan or RECIST disease progression within 14 months of a RAI treatment despite persistent RAI uptake. The last criteria (persistent distant metastases after six courses of 3700 MBq of RAI) is more controversial, but only represent 10 % of the patients in this study [1, 12, 13].
We did not perform FDG PET/CT after TSH stimulation, which is known to slightly increase FDG uptake, since the patients had already known distant metastasis [14]. A TSH stimulation before the PET would not have had any impact on their management. Whether TSH stimulated SUV values would correlate to TGR cannot be answered in this retrospective study.
Because of the surprising absence of correlation between SUVmax/lesion and its TGR, we checked in our population whether FDG uptake was correlated to survival on a per patient analysis, as previously described [4–6]. One and 2-year survivals of patients with SUVmax < 5 was 100 % compared to 87 % and 66 % in patients with SUVmax ≥5. The difference in survival was not statistically significant, even though it appeared clinically significant. It might be due to the small size of the sample in conjunction with the absence of events in the group of patients with SUXmax < 5, or to the selection of the patients. Indeed, the patients selected here do not represent at all the whole spectrum of metastatic refractory RAI patients but a selected population under a watch and see management. We found similar data to what was previously reported [4–6]. The initial data from Robbins et al. [5] had previously shown SUVmax > 4.6 as being a prognostic factor for DTC patients. In a previous study from our group we also found SUXmax >5 as being prognostic [4]. Even though SUVmax are not reproducible from one camera to another one, even though, they only represent a small portion of one metastasis in a patient, it was identified in two retrospective studies from two different centers. We must admit that it is a simple reproducible criterion that has a striking impact on prognosis.
In this study, SUVmax/lesion was not correlated to TGR on a per lesion analysis. On the other side, Metab.TV/patient and Morpho.TV/patient were correlated to survival. A correlation was also found between SUVmax/patient and Morpho.TV/patient. Survival is known to be correlated to tumour growth rate and to tumour volume [15]. Whether the association between SUVmax/patient and survival is real by itself or just due to the correlation between Morpho.TV/patient and survival is questioned. Morpho.TV/patient could be, in that case, a confounding factor between SUVmax and survival. This cannot be sorted out by this study, and the question remains to be elucidated. Interestingly, a recent study did not find a correlation between metabolic tumour volume and progression free survival in a group of 37 patients with DTC [16]. Of note, in a few patients, Metab.TV and Morpho.TV could not be determined because of technical issue of lesions with low FDG uptake or lesions with a density close to the surrounding tissue preventing delimitation of the lesions with the software used. The use of scan with intravenous contrast administration could have overcome this problem for Morpho.TV but not for Metab.TV.
The correlation between Metab T.V. and survival could be interesting in clinical practice both to evaluate prognosis and treatment efficacy. Studies are however necessary to confirm this correlation exists in all metastatic DTC patients, and that it is an independent prognostic factor taking into account other known prognostic factors such as SUVmax, pathology, age, size of the lesions.
A correlation between TgDT and FDG PET results has previously been demonstrated, but it was not the case in our study [17, 18]. The selection of the patients as discussed previously might have impacted the results. The TgDT was, however, only available in 39 patients precluding any definitive conclusion.
Conclusion
In metastatic RAI refractory DTC, the intensity of FDG uptake on a per lesion analysis cannot be used as a surrogate marker of morphological tumor progression, and within the same patient, lesions with the highest FDG uptake are not the most progressive. Since we cannot rely on the intensity of FDG uptake to predict tumour growth, the results should prompt us not to preferentially treat lesions with a high FDG uptake.
References
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off J Am Thyroid Assoc. 2016;26:1–133.
Cazzato RL, Bonichon F, Buy X, Godbert Y, de Figuereido BH, Pointillart V, et al. Over ten years of single-institution experience in percutaneous image-guided treatment of bone metastases from differentiated thyroid cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2015;41:1247–55.
de Baère T, Aupérin A, Deschamps F, Chevallier P, Gaubert Y, Boige V, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2015;26:987–91.
Deandreis D, Al Ghuzlan A, Leboulleux S, Lacroix L, Garsi JP, Talbot M, et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr Relat Cancer. 2011;18:159–69.
Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91:498–505.
Wang W, Larson SM, Fazzari M, Tickoo SK, Kolbert K, Sgouros G, et al. Prognostic value of [18F]fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000;85:1107–13.
Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–9.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Oxf Engl. 2009;45:228–47.
Gomez-Roca C, Koscielny S, Ribrag V, Dromain C, Marzouk I, Bidault F, et al. Tumour growth rates and RECIST criteria in early drug development. Eur J Cancer Oxf Engl. 2011;47:2512–6.
Ferté C, Koscielny S, Albiges L, Rocher L, Soria J-C, Iacovelli R, et al. Tumor growth rate provides useful information to evaluate sorafenib and everolimus treatment in metastatic renal cell carcinoma patients: an integrated analysis of the TARGET and RECORD phase 3 trial data. Eur Urol. 2014;65:713–20.
Jacene HA, Leboulleux S, Baba S, Chatzifotiadis D, Goudarzi B, Teytelbaum O, et al. Assessment of interobserver reproducibility in quantitative 18F-FDG PET and CT measurements of tumor response to therapy. J Nucl Med Off Publ Soc Nucl Med. 2009;50:1760–9.
Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014;2:356–8.
Sacks W, Braunstein GD. Evolving approaches in managing radioactive iodine-refractory differentiated thyroid cancer. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2014;20:263–75.
Leboulleux S, Schroeder PR, Busaidy NL, Auperin A, Corone C, Jacene HA, et al. Assessment of the incremental value of recombinant thyrotropin stimulation before 2-[18F]-Fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography imaging to localize residual differentiated thyroid cancer. J Clin Endocrinol Metab. 2009;94:1310–6.
Tubiana MLH. Gray medal lecture: cell kinetics and radiation oncology. Int J Radiat Oncol Biol Phys. 1982;8:1471–89.
Masson-Deshayes S, Schvartz C, Dalban C, Guendouzen S, Pochart J-M, Dalac A, et al. Prognostic value of (18)F-FDG PET/CT metabolic parameters in metastatic differentiated thyroid cancers. Clin Nucl Med. 2015;40:469–75.
Giovanella L, Trimboli P, Verburg FA, Treglia G, Piccardo A, Foppiani L, et al. Thyroglobulin levels and thyroglobulin doubling time independently predict a positive 18F-FDG PET/CT scan in patients with biochemical recurrence of differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2013;40:874–80.
Kelders A, Kennes LN, Krohn T, Behrendt FF, Mottaghy FM, Verburg FA. Relationship between positive thyroglobulin doubling time and 18F-FDG PET/CT-positive, 131I-negative lesions. Nucl Med Commun. 2014;35:176–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written consent was obtained from all individual participants included in the study.
Financial support
No financial support to declare.
Rights and permissions
About this article
Cite this article
Terroir, M., Borget, I., Bidault, F. et al. The intensity of 18FDG uptake does not predict tumor growth in patients with metastatic differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 44, 638–646 (2017). https://doi.org/10.1007/s00259-016-3551-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3551-x