Abstract
Purpose
Neuroendocrine tumours of the pancreas (pNET) are observed in 8 – 17 % of patients with von Hippel-Lindau disease (vHLD), and 11 – 20 % of these patients develop metastatic disease. MRI and CT have a very high resolution; however, their sensitivity and specificity for the detection of pNET amongst cystic lesions in the pancreas of vHLD patients are generally considered insufficient. In contrast, 68Ga-DOTATOC PET/CT demonstrates a high sensitivity for the diagnosis and staging of neuroendocrine tumours. In this study we investigated the potential role of 68Ga-DOTATOC PET/CT in screening of patients with vHLD.
Method
68Ga-DOTATOC PET/three-phase contrast-enhanced CT was performed according to guidelines in all consecutive vHLD patients between January 2012 and November 2015. All patients underwent additional MRI imaging of the abdomen, spine, and head. Chromogranin A (CgA) was determined at the time of the PET/CT examination. A lesion seen on 68Ga-DOTATOC PET in the pancreas was defined as positive if the uptake was visually higher than in the surrounding tissues. Lesions were quantified using maximum SUV.
Results
Overall, 20 patients (8 men, 12 women; mean age 44.7 ± 11.1 years) were prospectively examined. Genetically, 12 patients had type 1 vHLD and 8 had type 2 vHLD. 68Ga-DOTATOC PET/CT detected more pNET than morphological imaging (CT or MRI): 11 patients (55 %; 8 type 1, 3 type 2) vs. 9 patients (45 %; 6 type 1, 3 type 2). The concentration of CgA was mildly elevated in 2 of 11 patients with pNET. The mean SUVmax of the pancreatic lesions was 18.9 ± 21.9 (range 5.0 – 65.6). Four patients (36.4 %) had multiple pNETs. The mean size of the lesions on CT and/or MRI was 10.4 ± 8.3 mm (range 4 – 38 mm), and 41.1 % were larger than 10 mm. In addition, somatostatin receptor-positive cerebellar and spinal haemangioblastomas were detected in three patients (SUVmax 2.1 – 10.1). One patient presented with a solitary somatostatin receptor-positive lymph node metastasis. pNETs were observed more frequently in vHLD type 1 than type 2 (66.7 % vs. 37.5 %, p = 0.089). None of the patients showed progressive disease during follow-up.
Conclusion
In this study, 68Ga-DOTATOC PET detected pNETs in a higher proportion of patients with vHLD than found in previous studies with 111In-octreoscan, the imaging method recommended by the NCCN. We therefore suggest 68Ga-DOTATOC PET/CT as the more sensible screening tool.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Von Hippel-Lindau disease (vHLD) is an autosomal, dominant heritable disorder characterized by the development of numerous endocrine and nonendocrine tumours and cysts in the central nervous system as well as in the visceral organs [1]. The incidence of vHLD is reported to be 1 per 36,000 live births [2]. The gene for vHLD is located on chromosome 3p25.5 [3]. Patients with vHLD often have retinal angioma, haemangioblastoma of the cerebellum and spinal cord, renal cell carcinoma and cysts, phaeochromocytoma, and benign or malignant tumours of the pancreas together with multiple pancreatic cysts [4–6]. The majority of pancreatic lesions in patients with vHLD are cystic and benign. However, 10 – 17 % of patients may harbour nonfunctioning pancreatic neuroendocrine tumour (pNET) [7–9]. The recently observed improved overall survival of patients with vHLD has been attributed to improved screening methods for haemangioblastoma, renal clear cell carcinoma and phaeochromocytoma. These highly sensitive screening methods may also increase the detection and thus the prevalence of pNET in vHLD [10].
According to the literature, these pNETs metastasize in 11 – 20 % of patients. The only curative treatment option for patients with pNET is surgery, that is feasible only in the early nonmetastasized stage. The repeated use of highly sensitive and accurate imaging methods for prognosis, in combination with other predictors of malignancies such as germline mutation in the vHLD gene located on exon 3, and prognostic parameters such as tumour size and doubling time are critically needed [11]. The best imaging method for screening for pNETs is still under discussion [10]. Multiple cystic pancreatic lesions pose a challenge in the detection of small solid pNETs on CT or MRI [1, 8, 9, 12]. Previous studies have shown that there is a very limited or no role for 18F-FDG, 18F-DOPA and 11C-5-HTP PET in the detection of pNET in vHLD [10, 12]. In vHLD the National Cancer Network (NCCN) guidelines recommend three-phase CT or MRI for the diagnosis of pNET [10, 13]. For further characterization of pancreatic lesions somatostatin receptor (SR) scintigraphy as a functional imaging tool and tumour markers chromogranin A (CgA) and/or pancreatic polypeptide may be considered.
SR scintigraphy with 111In-octreoscan is a well-established imaging method for staging and restaging of neuroendocrine tumours [14]. However, it has a much lower sensitivity and diagnostic accuracy than state-of-the-art 68Ga-DOTATOC or 68Ga-DOTATATE PET/CT. DOTATOC or DOTATATE are synthetic SR agonists with high affinity for SR subtype 2a [15]. Furthermore, 68Ga is a positron emitter with a short half-life of 68 min, resulting in lower radiation burden than from 111In-octreoscan [16]. Both 68Ga-DOTATOC and 68Ga-DOTATATE have achieved orphan drug designation. Their lower radiation burden in patients with genetic mutations in combination with high sensitivity for NETs (>90 %) makes them ideal radiopharmaceuticals for screening in vHLD.
The aim of this study was to evaluate the potential role of 68Ga-DOTATOC PET/three-phase CT in the screening of patients with vHLD for pNETs.
Material and methods
Patient selection
This cross-sectional prospective investigation evaluated the results of the annual follow-up of vHLD patients cared for by the Interdisciplinary Centre of Metabolism at the Charité-Universitätsmedizin Berlin. All patients older than 18 years and referred for 68Ga-DOTATOC PET/CT as part of the screening protocol from January 2012 to November 2015 were included. The Ethics Committee of Charité-Universitätsmedizin Berlin approved the analysis of the data (EA2/089/15). vHLD patients are screened annually for progression of the variable disease manifestations by MRI of the head, spine and abdomen. In addition, the plasma concentration of CgA was determined using the Kryptor assay (Brahms, Hennigsdorf, Germany). Values above the upper limit of normal (>150 mg/L) were considered pathological.
68Ga-DOTATOC PET/CT
68Ga was eluted from a 68Ge/68Ga generator and was used to label DOTATOC according to the standard labelling procedure described elsewhere [17]. The selection of DOTATOC for imaging was purely based on the availability of the compound due to patent regulations. 68Ga-DOTATOC PET/CT was performed according to EANM guidelines [18]. Mean radioactivity injected was 1.7 MBq/kg body weight and the acquisition was performed 45 – 60 min after injection of the radiotracer. All PET scans were acquired in a three-dimensional acquisition mode on a Gemini TF 16 PET/CT system (Philips Medical Systems) [19]. The standard three-dimensional line of response algorithm of the system software was used with default parameter settings to reconstruct transaxial slices of 144 × 144 voxels with 4.0 × 4.0 × 4.0 mm with 10 – 12 bed positions each with an acquisition time of 1.5 min. CT was used for attenuation correction for both scanners. If contrast-enhanced multiphase CT was performed at the time of PET/CT, 70 – 100 ml Ultravist 370 (Bayer Schering Pharma, Berlin, Germany) was injected intravenously. and images were acquired using bolus tracking methodology with delays of 30 s for the arterial phase, 50 s for the portovenous phase and 70 s for the venous phase, with a collimation of 0.75 mm and 16 slices of thickness 0.75 mm for the arterial and portovenous phases and 16 slices of thickness 1.5 mm for the venous phase.
The SUV was measured only for those lesions that were definitely positive by visual assessment, i.e. the uptake of the lesion was higher than the uptake of the immediate surrounding normal tissue.
Image analyses
The PET/CT images were analysed by experienced board-certified physicians, primarily by a radiologist (T.D.), and a nuclear medicine physician (V.P.). For reevaluation of images in this study, consensus of the two main readers (V.P. T.D.), was considered sufficient. On CT images, lesions were recorded as solid lesions when they demonstrated positive contrast enhancement in the arterial phase. Data were related to their clinical context by the attending treating physicians (U.P. and N.T.). Lesions seen on PET/CT were characterized as tumour tissue or metastases only if all the physicians achieved consensus. Any lesions for which there was discrepancy in characterization among the readers were followed up with CT and/or MRI and by the clinical course. Tracer accumulation on PET images was defined as positive uptake by visual assessment by the two observers V.P. and T.D. Lesions detected only by one modality (CT or PET) were considered positive or negative based on follow-up or complementary imaging modalities such as MRI and/or CT.
Statistical analysis
An independent sample t test was used to compare differences in mean CgA levels between patients with type 1 and with type 2 vHLD and also between patients with and without pNET. In addition, the nonparametric Spearman’s rho test was performed to determine the significance of the correlation in the occurrence of pNET between type 1 vHLD and type 2 vHLD.
Results
Patient characteristics
A total of patients (8 men, 12 women; mean age 44.7 ± 11.1 years) were prospectively examined. Genetically, 12 patients had type 1 vHLD and 8 patients had type 2 vHLD. Additional manifestations of vHLD were present: phaeochromocytoma in 8 patients; paraganglioma in 3 patients; retinal angioma in 16 patients; cerebellar and/or spinal haemangioblastoma in 16 and 18 patients, respectively; renal cell carcinoma in 9 patients; and renal, pancreatic and/or testicular cysts in 17, 17 and 4 patients, respectively (Table 1). Detailed genetic information was available for 12 patients (Table 1).
Chromogranin A
The plasma CgA concentrations (mean ± SD) in patients with vHLD type 1 and vHLD type 2 were 65.2 ± 47.8 mg/L and 51.6 ± 57.3 mg/L, respectively (p not significant). However, the CgA concentrations were significantly higher in patients with pNET than in those without (74.9 ± 6.1 vs. 42.8 ± 22.9, p < 0.05). Individually 2 of 11 patients with pNET had a pathologically increased CgA concentration, whereas CgA concentration was not increased in patients without pNET.
CT and MRI results
In nine patients (45 %; six type 1, three type 2), both MRI and CT demonstrated solid, contrast-enhancing lesions in the pancreas suggestive of pNET. The mean size of the lesions on CT and/or MRI was 10.4 ± 8.3 mm (range 4 – 38 mm), and 41.1 % of the lesions were larger than 10 mm (Table 2).
68Ga-DOTATOC PET/CT
68Ga-DOTATOC PET/CT detected SR-positive lesions in the pancreas suggestive of pNET in 11 of the 20 patients (55 %; Table 2). pNET were more frequent in patients with type 1 than in those with type 2 vHLD (8 patients, 66.7 %, vs. 3 patients, 37.5 %; p = 0.089; Table 2). In all patients, CT and/or MRI information was needed for correct localization of the tumour. The mean SUVmax of the pancreatic lesions was 18.9 ± 21.9 (range 5.0 – 65.6). Four patients (36.4 %) had multiple pNETs (Fig. 1). In addition, SR-positive haemangioblastomas were detected in the cerebellum and spine in three patients (SUVmax 2.1 – 10.1). One of the 11 pNET patients (9.1 %) presented with SR-positive lymph node metastasis.
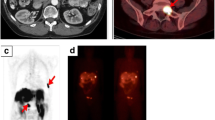
68Ga-DOTATOC PET/CT images in a patient (patient 8) who presented with multiple (seven) hypervascular somatostatin receptor-positive pNET; this patient had a heterozygote R161Q mutation. a, b The CT images (left) show hypervascular lesions (yellow arrows). On the PET images (centre) the lesions show somatostatin receptor expression (red arrows). The fused images (right) clearly show the lesions (light green arrows)
Follow-up
For ethical reasons, none of the suspected pNETs was biopsied. However, all patients with positive pNET results were followed-up with conventional imaging for at least 6 months. These investigations showed in identical radiological features. Of the 11 patients with pNET, 3 (33.3 %) were followed up with 68Ga-DOTATOC PET/CT. None of the patients showed progressive disease on CT or MRI.
Discussion
In this explorative study we demonstrated the possible pivotal role of 68Ga-DOTATOC PET/CT for the screening of patients with vHLD. This is the single largest investigation of the value of SR PET/CT with 68Ga-DOTATOC in the detection of pNET in this rare inherited disease. There are several advantages of performing 68Ga-DOTATOC PET/three-phase CT in comparison with SR scintigraphy with 111-octreoscan, as recommended by the NCCN guidelines [15, 16]. First, in patients with genetic mutations, radiation exposure is always a matter of concern. The radiation exposure with 68Ga-DOTATOC PET is normally in the range of 2 – 3 mSv, i.e. three to four times less than that from 111In-octreoscan. Second, 68Ga-DOTATOC has a higher sensitivity than 111In-octreoscan, allowing detection of smaller lesions [16]. As sensitivity is important for the detection of small lesions 68Ga-DOTATOC PET/CT is the imaging tool of choice for screening pNETs instead of the combination of 111In-octreoscan with MRI or CT. Unfortunately, the lower availability of 68Ga-DOTATOC, despite having achieved orphan drug designation, is still an important hurdle for routine use in several countries.
Another important recommendation of the NCCN guidelines as well as of the ENETS guidelines is the assessment of CgA as a tumour marker for pNET [20]. Previous studies have shown that CgA is elevated in patients with pNET [13, 17]. In our study cohort we observed a trend towards a higher mean CgA concentration in patients with pNET than in those without. However, no data are available for the use of CgA as a tumour marker in vHLD. The increase of CgA in patients on proton pump-inhibiting (PPI) drugs as well as in patients with renal insufficiency has to be taken into account. [13, 17]. Thus, the value of CgA as a screening and prognostic tool for pNET in vHLD is not known. In our series 2 of 11 patients (8.2 %) with pNET showed CgA concentrations above the upper normal limit. One of the patients (patient 6) had grade III/IV kidney insufficiency and was on PPI drugs, whereas the other patient (patient 4) was not on PPI drugs and had normal kidney function.
One of the primary aims of screening in vHLD is not only to detect but also to diagnose pNETs at an early stage so that surgery is a curative option. The probability of pNETs metastasizing in vHLD patients is 0.3 % [8]. Based on radiographic and genetic criteria, Blansfield et al. suggested three criteria for prediction of metastases: (1) pNET ≥3 cm in size, (2) presence of mutation in exon 3, and (3) tumour diameter doubling time <500 days [8]. In patients with none of these criteria, follow-up with CT/MRI every 2 – 3 years is sufficient. Those patients meeting only one criterion should be followed-up more frequently (every 6 – 12 months), whereas in patients meeting two or more criteria, surgical resection might be considered. None of our patients with pNET on SR PET/CT was found to have progressive disease (Table 2). However, one patient showed lymph node metastases on 68Ga-DOTATOC PET/CT (Fig. 2). Although in our patient cohort we could not recognize any consistent pattern in the genetic analyses and frequency of pNET (Table 1), interestingly pNET tended to occur more frequently in patients with type 1 vHLD than in those with type 2 vHLD. We could not find any literature to support this observation. In vHLD, due to the genetic aetiology of the disease, multiple pNETs can be observed. In our patient cohort 4 of 11 patients (36.36 %) had multiple sites of SR-positive solid hypervascularized lesions. In the presence of multiple cysts, it is often a challenge to identify multiple pNETs on CT and MRI. The high target/non-target ratio of 68Ga-DOTATOC in pNETs can help in detecting small lesions missed by CT or MRI on initial evaluation. Indeed, in 2 of the 11 patients, CT and MRI were able to detect pNETs only retrospectively after diagnosis using the PET images. In both patients, the lesions were smaller than 1 cm and were detected on PET/CT fusion images only retrospectively. This may highlight the advantage of 68Ga-DOTATOC PET/CT as it allows the delineation of tumours among multiple pancreatic cysts, a well-known difficulty with conventional imaging. Both the patients were followed up with conventional imaging and did not show any disease progression. This emphasizes the synergistic values of these two imaging tools. Indeed, with PET/MRI machines now clinically available, future vHLD screening should be performed with 68Ga-DOTATOC PET/MRI rather than 68Ga-DOTATOC PET/CT to reduce the radiation exposure in genetically susceptible patients.
68Ga-DOTATOC PET/CT images in a patient (patient 4) who presented with two small primary pNET and two peripancreatic lymph nodes; this patient had exon 1: CD86, CCC → TCC, Pro → Ser; CD87, GTA → TTA mutation. a The CT image (left) shows the hypervascular primary in the pancreas tail (yellow arrow). The PET image (centre) also shows focally increased somatostatin receptor expression in the pancreas tail (red arrow). The fused image (right) shows the lesion much more clearly (light green arrow). b The CT image (left) shows centrally hypodense, peripherally contrast-enhancing lymph nodes (yellow arrow). The PET image (centre) also shows the lymph nodes with increased somatostatin receptor expression (red arrow). The fused image shows the metastatic lymph nodes much more clearly (light green arrow)
Endosonography (EUS) is another imaging tool that has been shown to be superior to octreoscan and 11C-5-HTP PET/CT in the detection of pNETs in vHLD [10]. The major advantage of EUS is that it is radiation-free and allows simultaneous fine-needle aspiration cytology for confirmation of pNET. However, the major disadvantage is its high dependence on the experience of the observer and the invasive nature of the investigation. In a previous study, octreoscan has been shown to detect pNET in 3 of 22 patients (13.6 %); in comparison EUS detected pNET in 10 of 22 patients (45.6 %). In our patient cohort SR imaging with 68Ga-DOTATOC showed pNET in 55 % of the patients, almost in concordance with the rate of detection by EUS. EUS and 68Ga-DOTATOC PET/CT may be able to play a synergistic role, particularly if there is suspicion of pNET in the processus uncinatus of the pancreas. The normally high physiological uptake of 68Ga-DOTATOC in the processus uncinatus can sometimes hide small lesions seen on EUS. If there is suspicious focal uptake on PET in the processus uncinatus of the pancreas, additional EUS is advisable to confirm or rule out pNET.
We demonstrated a higher frequency of pNET on 68Ga-DOTATOC PET/CT than has previously been reported. This may have been due to the high sensitivity of 68Ga-DOTATOC PET/CT, indicated by the small diameters (<10 mm) of these lesions. During follow-up no progressive disease was observed, and this is not unexpected as progression has been shown to correlate with tumour size (>3 cm) and an exon 3 mutation. For final conclusions on the clinical impact of early detection of pNET in vHLD, long-term follow up of these small lesions should provide further data.
The advantage of using SR PET/CT in comparison to other radiopharmaceuticals such as 18F-DOPA, 18F-FDG or 11C-5-HTP is that most of grade 1 and grade 2 pNETs express SRs [17]. 18F-DOPA and 11C-5-HTP, both markers for the serotonin production pathway, are hampered by the fact that not all pNETs produce serotonin [10, 12]. 18F-FDG PET is normally not indicated for the evaluation of grade 1 or grade 2 pNETs [17].
Conclusion
In this study, 68Ga-DOTATOC PET detected pNETs in a higher proportion of patients with vHLD than found in previous studies with 111In-octreoscan, the imaging method recommended by the NCCN. We therefore suggest 68Ga-DOTATOC PET/CT as the more sensible screening tool.
References
Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. Von Hippel-Lindau disease. Lancet. 2003;361:2059–67.
Maher ER, Neumann HP, Richard S. Von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet. 2011;19:617–23.
Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–20.
Lamiell JM, Salazar FG, Hsia YE. Von Hippel-Lindau disease affecting 43 members of a single kindred. Medicine (Baltimore). 1989;68(1):1–29.
Neumann HP, Wiestler OD. Clustering of features of von Hippel-Lindau syndrome: evidence for a complex genetic locus. Lancet. 1991;337(8749):1052–4.
Hes FJ, Feldberg MA. Von Hippel-Lindau disease: strategies in early detection (renal-, adrenal-, pancreatic masses). Eur Radiol. 1999;9(4):598–610.
Hammel PR, Vilgrain V, Terris B, Penfornis A, Sauvanet A, Correas JM, et al. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d’Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119(4):1087–95.
Blansfield JA, Choyke L, Morita SY, Choyke PL, Pingpank JF, Alexander HR, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs). Surgery. 2007;142(6):814–8.
Neuzillet C, Vullierme MP, Couvelard A, Sauvanet A, Levy P, Richard S, et al. Difficult diagnosis of atypical cystic pancreatic lesions in von Hippel-Lindau disease. J Comput Assist Tomogr. 2010;34(1):140–5.
van Asselt SJ, Brouwers AH, van Dullemen HM, van der Jagt EJ, Bongaerts AH, Koopmans KP, et al. Potential value of EUS in pancreatic surveillance of VHL patients. Eur J Endocrinol. 2016;174(5):611–20.
Marcos HB, Libutti SK, Alexander HR, Lubensky IA, Bartlett DL, Walther MM, et al. Neuroendocrine tumors of the pancreas in von Hippel-Lindau disease: spectrum of appearances at CT and MR imaging with histopathologic comparison. Radiology. 2002;225(3):751–8.
Kitano M, Millo C, Rahbari R, Herscovitch P, Gesuwan K, Webb RC, et al. Comparison of 6-18F-fluoro-L-DOPA, 18F-2-deoxy-D-glucose, CT, and MRI in patients with pancreatic neuroendocrine neoplasms with von Hippel-Lindau disease. Surgery. 2011;150(6):1122–8.
Kulke MH, Benson 3rd AB, Bergsland E, Berlin JD, Blaszkowsky LS, Choti MA, et al. Neuroendocrine tumors. J Natl Compr Cancer Netw. 2012;10(6):724–64.
Pavel M, O’Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R, et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology. 2016;103(2):172–85.
Baum RP, Prasad V, Hommann M, Hörsch D. Receptor PET/CT imaging of neuroendocrine tumors. Recent Results Cancer Res. 2008;170:225–42.
Schreiter NF, Brenner W, Nogami M, Buchert R, Huppertz A, Pape UF, et al. Cost comparison of 111In-DTPA-octreotide scintigraphy and 68Ga-DOTATOC PET/CT for staging enteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39(1):72–82.
Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, et al. Processing of generator-produced 68Ga for medical application. J Nucl Med. 2007;48(10):1741–8.
Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37(10):2004–10.
Surti S, Kuhn A, Werner ME, Perkins AE, Kolthammer J, Karp JS. Performance of Philips Gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nucl Med. 2007;48(3):471–80.
Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103(2):153–71.
Authors’ contributions
Vikas Prasad and Ursula Plöckinger were involved in the conception of the study, in the writing and editing, and in the analysis of the results. Nikolaus Tiling and Timm Denecke helped in the analysis of the results and in the writing. Winfried Brenner helped in the analysis of the results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflicts of interest
None.
Ethical approval
The analysis was performed in accordance with the ethical standards of the institutional research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients.
Rights and permissions
About this article
Cite this article
Prasad, V., Tiling, N., Denecke, T. et al. Potential role of 68Ga-DOTATOC PET/CT in screening for pancreatic neuroendocrine tumour in patients with von Hippel-Lindau disease. Eur J Nucl Med Mol Imaging 43, 2014–2020 (2016). https://doi.org/10.1007/s00259-016-3421-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3421-6