Abstract
Purpose
Typical and atypical carcinoids (TC and AC) represent 20 – 25 % of all neuroendocrine tumours. No standard therapeutic approach is available for patients with advanced disease. The aim of this phase II study was to investigate the efficacy and safety of peptide receptor radionuclide therapy with 177Lu-DOTATATE (Lu-PRRT) and the role of thyroid transcription factor 1 (TTF-1) and 18F-FDG PET as prognostic factors in patients with advanced TC or AC.
Methods
A total of 34 consecutive patients with radiologically documented progressive disease were treated with Lu-PRRT at a therapeutic cumulative activity of 18.5 or 27.8 GBq in four or five cycles according to the patient’s kidney function and bone marrow reserve. Information on TTF-1 was available in all patients. FDG PET studies prior to Lu-PRRT were available in 29 patients.
Results
The median follow-up was 29 months (range 7 – 69 months). The disease control rate (DCR) in patients with TC was 80 %: 6 % complete response, 27 % partial response and 47 % stable disease. The median progression-free survival (mPFS) was 20.1 months (95 % CI 11.8 – 26.8 months). Stable disease was achieved in 47 % of patients with AC with a mPFS of 15.7 months (95 % CI 10.6 – 25.9 months). No major acute or delayed toxicity occurred in either group or with either cumulative activity. mPFS in patients with TTF-1-negative TC was 26.3 months (95 % CI 12.9 – 45.2 months), but in patients with TTF-1-positive TC mPFS was 7.2 months (4.2 – 14.0 months; p = 0.0009). FDG PET was negative in 13 patients (10 TC and 3 AC) and positive in 16 patients (4 TC and 12 AC). The mPFS in the FDG PET-negative group was 26.4 months (95 % CI 14.2 – 48.9 months) and 15.3 months (11.7 – 31.1 months) in the FDG PET-positive group.
Conclusion
Lu-PRRT showed antitumour activity in terms of DCR and PFS and proved safe, even in patients with a higher risk of side effects. TTF-1 would appear to be a prognostic factor. FDG PET positivity in bronchial carcinoids is a hallmark of aggressive tumour and is more frequent in patients with AC than in those with TC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine tumours (NETs) of the lung represent 1 – 2 % of all lung cancers and 20 – 25 % of all NETs. The 2004 WHO classification [1] divides pulmonary NETs into typical and atypical carcinoids (TC and AC), large-cell neuroendocrine carcinomas and small-cell carcinomas. It is well known that patients with TC exhibit a better prognosis with a 5-year survival of up to 87 – 90 %. Patients with AC have a poorer prognosis with a 5-year survival ranging from 44 % to 78 %. Radical surgery is the therapeutic gold standard and the best long-term prognostic factor in limited disease. However, distant metastases may occur many years after apparently radical resection [2]. New diagnostic and therapeutic strategies are thus needed for recurrent disease. The histological diagnosis of these tumours is based on typical immunohistochemistry determination. Thyroid transcription factor 1 (TTF-1) is one of the most specific markers, being positive in up of 75 % of patients [3, 4].
Radiological diagnosis of pulmonary NETs depends on whether the tumour is centrally or peripherally located, and multidetector tomography is the most widely recommended investigation for both forms. Bronchoscopy with biopsies may also be performed for tumours with a central location. Whole-body nuclear medicine imaging plays a fundamental role in the detection of distant metastases. Given that about 80 % of lung NETs express somatostatin receptors, with a high prevalence of subtype 2, somatostatin receptor scintigraphy or 68Ga-DOTATATE/TOC PET (68Ga-PET) are useful for the diagnosis of well-differentiated lung tumours [5]. Moreover, diagnostic 18F-FDG PET may be positive in aggressive forms of the disease [6–9].
There are no prospective trials on lung NETs to guide treatment choices, and much of the data in the literature are based on mixed populations of patients with primary NET. In metastatic disease, the role of cytotoxic agents such as cisplatin, streptozocin, capecitabine, dacarbazine, 5-fluorouracil, temozolomide and interferon is still unclear. Temozolomide is the most widely studied agent in lung NETs and has an acceptable safety profile [2]. A number of studies of biological agents, such as mTOR-inhibitors or tyrosine kinase inhibitors, have shown objective response rates of 5 – 10 % [10]. Treatment with somatostatin analogues can be used for secreting forms with clinical symptoms and have been shown to produce a partial response (PR) in 5 – 10 % of patients, stable disease (SD) in 30 – 50 % and improved symptoms in 40 – 60 % [2].
Peptide receptor radionuclide therapy (PRRT) is a promising treatment for patients with advanced or inoperable somatostatin receptor-positive tumours. Several studies have demonstrated the efficacy of PRRT in gastroenteropancreatic NETs (GEP-NETs), showing objective responses in more than 30 % of patients [11–16]. The few retrospective studies investigating the efficacy of PRRT in metastatic lung NETs have shown overall remission rates comparable, in some cases, to those in GEP-NETs [17, 18].
We report here the results of a prospective phase II trial investigating the role of PRRT with 177Lu-DOTATATE (Lu-PRRT) in patients with bronchial carcinoids (BC), focusing on those with advanced TC or AC. We evaluated its efficacy in terms of disease control rate (DCR), progression-free survival (PFS) and overall survival (OS), and safety profile. We also assessed the role of TTF-1 and FDG PET as predictive and prognostic factors in BC, respectively.
Materials and methods
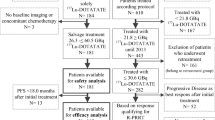
From April 2008 to March 2014, 34 consecutive patients with metastatic BC (TC and AC) were enrolled in a phase II disease-oriented study. Patients were eligible if they:
-
Were 18 years of age or older.
-
Had histological confirmation of pulmonary NET [1] and positive OctreoScan® scintigraphy according to the Krenning score.
-
Had radiological documentation of disease progression in the 12 months preceding enrolment.
-
Had an ECOG performance status ≤2.
-
Had adequate bone marrow, renal and hepatic function (WBC >2,5 × 109/l, haemoglobin >10 g/dl, platelets >100 × 109/l, bilirubin <2.5 mg/dl, and creatinine <2.0 mg/ dl).
Prior treatments including octreotide/lanreotide (≥4 weeks after long-acting preparations or >72 h after short-acting preparations) and cytotoxic chemotherapy or radiotherapy were allowed. Pregnant and lactating women, patients with a life expectancy of <6 months and those with known previous malignancies were excluded. All patients gave written informed consent. The protocol was approved by the Ethics Committee of the Wide Catchment Area of Romagna-IRST (CEIIAV) and by the competent Italian regulatory authorities. The study was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice guidelines. Although an FDG PET scan performed before Lu-PRRT was not a prerequisite for the study, it was taken into account as a prognostic factor if applicable [19]. Patients with a standardized uptake value >2.5 (arbitrary cut-off value), as reported elsewhere [20], were considered FDG-positive.
Study treatment
All patients were scheduled to receive four or five cycles of therapy at intervals of 6 – 8 weeks. The planned activity per cycle was 3.7 or 5.5 GBq of [177Lu-DOTA0, Tyr3]-octreotate according to kidney and bone marrow toxicity risk factors. Based on previous studies, patients were treated with a reduced activity of 3.7 GBq per cycle when at least one of the following risk factors was present: previous Y-PRRT with a cumulative activity of ≥9.25 GBq at least 6 months before Lu-PRRT, creatinine 1.5 – 2 mg/dl, morphological renal abnormalities, severe hypertension and insulin-dependent diabetes not controlled by drugs, previous platinum-based chemotherapy, and age >80 years [12, 13, 21–23].
Radiopeptide preparation
DOTA-Tyr3, Thr8-octreotide (DOTATATE) was purchased from piCHEM (Graz, Austria). The radioisotope was purchased from PerkinElmer (Waltham, MA). Preparation was carried out following the standard procedure [24].
Radiopeptide administration
The radiopharmaceutical was administered intravenously as a 30-min infusion using a dedicated pump system (patent US 7842023 B2). In order to protect the kidneys during excretion of the radiopeptide, patients were pretreated with intravenous amino acids (lysine 70 mEq in 500 ml saline, 250 cm3 over 30 min immediately before therapy, 250 cm3 during therapy; lysine 70 mEq in 500 ml saline during the first 3 h after therapy, and lysine 60 mEq in 500 ml saline over 1 h twice the following day) [13].
Imaging
The gamma emission of 177Lu (113 and 208 keV, relative abundance 6 % and 11 %, respectively) allowed the monitoring of radiopharmaceutical biodistribution during the therapeutic phase. Anterior and posterior whole-body images were acquired 24 h after Lu-PRRT administration with a 256 × 1,024 matrix using a dual-headed gamma camera (Infinia Hawkeye; GE Healthcare, Milwaukee, WI) equipped with a low-energy high-resolution collimator with the energy window set on 177Lu peaks. A SPECT study was acquired (64 projections, 360°) in selected patients to better document tumour uptake. Tumour evaluation with multiphase CT and/or MRI was performed 3, 6, 12, 18 and 24 months after the end of treatment and every 6 – 12 months thereafter. Tumour response rate was evaluated according to SWOG criteria [25, 26]. Toxicity was assessed according to CTCAE criteria, version 3 [27].
Statistical analysis
The main objective of this study was to evaluate the DCR with Lu-PRRT in patients with advanced progressive BC. Secondary objectives were safety, PFS and OS. DCR, defined as the percentage of patients who achieved complete response (CR), PR or SD, was evaluated according to SWOG criteria. PFS was defined as the time from the start of Lu-PRRT to the date of the first documented evidence of disease progression or death due to any cause or to the last date of tumour evaluation. OS was defined as the time from the start of treatment to the time of death from any cause or last follow up. Time to event data (PFS and OS) and their 95 % confidence intervals (CI) were evaluated using the Kaplan-Meier method [28] and compared with the log-rank test [29]. P values were based on two-sided testing. Statistical analyses were carried out using SAS statistical software, version 9.3 (SAS Institute, Cary, NC).
Results
A total of 34 consecutive patients (17 men and 17 women) with radiologically documented progressive disease [25] were treated with Lu-PRRT at two different therapeutic cumulative activities of 18.5 or 27.8 GBq in four or five cycles according to each patient’s kidney function and bone marrow reserve (IRST protocol no. 100.01). The median cumulative activity was 21.5 GBq (12.9 – 27.8 GBq) and the interval between cycles was 6 – 8 weeks in both groups. Of the 34 patients, 15 (44 %) had a histopathological diagnosis of TC and received a median cumulative activity of 22.2 GBq (range 14.8 – 27.4 GBq), and 19 (56 %) had a diagnosis of AC and received a median cumulative activity of 20.3 GBq (range 12.9 – 27.4 GBq). The median follow-up was 29 months (range 7 – 69 months). Information on TTF-1 was available in all patients: 80 % of patients with TC were TTF-1-negative and 79 % of those with AC were TTF-1-positive. FDG PET imaging prior to Lu-PRRT was available in 29 patients (85 %). FDG PET was negative in 13 patients (45 %), 10 (77 %) with TC and 3 (23 %) with AC. FDG PET was positive in 16 patients (55 %), 4 (28 %) with TC and 12 (80 %) with AC. With regard to treatment, 22 patients (65 %) were treated surgically, 29 (85 %) received somatostatin analogues and 13 (38 %) underwent chemotherapy before Lu-PRRT, 9 patients (26 %) had previously responded to Y-PRRT, 13 patients (30 %) had received other treatments and only 2 (6 %) had never undergone any treatment. Patient characteristics are summarized in Table 1.
Response to therapy
Overall, in the 34 evaluable patients 1 (3 %) showed CR, 4 (12 %) PR and 16 (47 %) SD, with a DCR of 62 %. The median PFS (mPFS) of the entire group was 18.5 months (95 % CI 12.9 – 26.4 months) and the median OS (mOS) was 48.6 months (95 % CI 26.4 – 68.9 months). With regard to outcome for each histopathological type, DCR in patients with TC was 80 % (6 % of patients with CR, 27 % with PR and 47 % with SD). The mPFS was 20.1 months (95 % CI 11.8 – 26.8 months). In patients with AC the DCR was 47 % (all SD) and the mPFS was 15.7 months (10.6 – 25.9 months). The mOS was 48.6 months (95 % CI 26.0 months – not reached) and 37 months (95 % CI 18.7 – 68.9 months) in the patients with TC and those with AC, respectively. No major acute or delayed toxicity (CTCAE grade 3 or 4) occurred in either group or with either therapeutic cumulative activity.
With regard to TTF-1 status, TTF-1-positive patients had a mPFS of 13.2 months (95 % CI 7.2 – 20.2 months) and TTF-1-negative patients had a mPFS of 26.4 months (95 % CI 14.2 – 45.2 months; p = 0.074). Of the patients with TC, 12 (80 %) were TTF-1-negative and showed a mPFS of 26.3 months (95 % CI 12.9 – 45.2 months), and 3 (20 %) were TTF-1-positive and showed a mPFS of 7.2 months (95 % CI 4.2 – 14.0 months; p = 0.0009); Fig. 1, Table 2).Of the patients with AC, 15 (79 %) were TTF-1-positive and showed a mPFS of 15.3 months (95 % CI 6.1 – 25.9 months), and 4 (21 %) were TTF-1-negative and showed a mPFS of 33.8 months (95 % CI 12.1 – 48.9 months; Table 3).
Overall, FDG PET-positive patients had a mPFS of 15.3 months (95 % CI 11.7 – 31.1 months) and FDG PET-negative patients had a mPFS of 26.4 months (95 % CI 14.2 – 48.9 months; p = 0.201). Of the patients with TC, 4 (29 %) were FDG PET-positive and had an mPFS of 12.9 months (95 % CI 4.2 – 45.2 months), and 10 (71 %) were FDG PET-negative and had a mPFS of 26.4 months (95 % CI 12.9 – 26.8 months; Table 2). Of the patients with AC, 12 (80 %) were FDG PET-positive and had a mPFS of 15.7 months (95 % CI 6.1 – 31.1 months), and 3 (20 %) were FDG PET-negative and had a mPFS of 48.9 months (95 % CI 18.7 – 48.9 months; Table 3). Finally, 13 patients who were both TTF-1-negative and FDG PET-negative had a mPFS of 26.4 months (95 % CI 14.2 – 48.9 months, and 13 patients who were both TTF-1-positive and FDG PET-positive had a mPFS of 15.7 months (95 % CI 6.1 – 31.1 months; Fig. 2).
Discussion
The main objective of this study was to investigate the role of Lu-PRRT in metastatic BC in terms of DCR and PFS. The majority of patients with BC have advanced disease at diagnosis, requiring a multidisciplinary approach to their management. Furthermore, no standard therapy is currently available for metastatic disease. Several studies have analysed the response to treatment with cytotoxic chemotherapeutic agents, and have shown in general an objective response of 20 – 30 % with an mOS of 24.3 months [30]. New agents such as temozolomide provide better clinical benefit than classic standard treatments. The first international randomized study dedicated to lung NETs (LUNA trial) that compared everolimus alone, everolimus in association with pasireotide and pasireotide alone is still ongoing. Our results are in line with the results of the RADIANT II trial in patients with BC, in which 33 patients in the treatment arm had a mPFS 8 months longer than 11 patients in the placebo arm [10].
Retrospective studies have shown that Lu-PRRT has antitumour activity in BC [17] and this treatment currently represents almost third-line therapy after surgery and chemotherapy [2]. Of the 34 patients treated with Lu-PRRT, disease control was achieved in 21 (62 %), with a mPFS of 18.5 months and a mOS of 48.6 months. In 15 patients with TC, disease control was achieved in 12 (80 %), with a mPFS of 20.1 months and a mOS of 48.6 months. In 19 patients with AC, disease control was achieved in 9 (47 %), with a mPFS of 15.7 months and a mOS of 37 months.
In our opinion, Lu-PRRT compares well with other therapies and has acceptable levels of toxicity. Indeed, no patient showed acute or delayed haematological toxicity. All patients were treated with lysine before, during and after Lu-PRRT, optimizing renal protection.
There are three further aspects that merit attention:
-
1.
FDG PET would seem to be an important prognostic factor. The difference in terms of PFS between FDG PET-positive and FDG PET-negative patients is in line with data reported for GEP-NETs [20]. It is important to remember that FDG PET studies are as valuable as nuclear receptor imaging for the good clinical management of patients with BC. FDG PET positivity is a hallmark of more aggressive tumours and was more frequent in patients with AC than in those with TC. In particular, of patients with AC FDG PET-negative patients had better mPFS and mOS than FDG PET-positive patients.
-
2.
TTF-1 appears to be a prognostic factor in BC. In particular, we examined the role of TTF-1 as an indicator of response to Lu-PRRT in patients with AC, which is associated with a poorer prognosis. TTF-1-negative patients with AC had a better mPFS and mOS after Lu-PRRT than TTF-1-positive patients. One explanation could be that TTF-1 positivity is associated with a higher mitotic index and thus lower cell differentiation.
-
3.
Patients who were both TTF-1 negative and FGD-PET-negative had the best outcome after Lu-PRRT. FDG PET and TTF-1 positivity indicates the need for more aggressive treatments combining Lu-PRRT and chemotherapy to reduce the impact of tumour heterogeneity on treatment efficacy and patient outcome [31, 32].
In conclusion, Lu-PRRT showed antitumour activity in BC in terms of both DCR and PFS. It proved a safe treatment, even in patients previously treated with chemotherapy. TTF-1 should always be analysed in this disease setting. FDG PET positivity in BC, as in GEP-NETs, is a hallmark of an aggressive tumour and is more frequent in patients with AC than in those with TC.
References
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (editors) World HealthOrganization Classification of Tumours. Pathology and genetics of tumours of thelung, pleura, thymus and feart. Lyon: IARC Press; 2004. http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb10/BB10.pdf.
Caplin ME, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26:1604–20.
Sturm N, Lantuéjoul S, Laverrière MH, Papotti M, Brichon PY, Brambilla C, et al. Thyroid transcription factor 1 and cytokeratins 1, 5, 10, 14 (34betaE12) expression in basaloid and large-cell neuroendocrine carcinomas of the lung. Hum Pathol. 2001;32:918–25.
Folpe AL, Gown AM, Lamps LW, Garcia R, Dail DH, Zarbo RJ, et al. Thyroid transcription factor-1: immunohistochemical evaluation in pulmonary neuroendocrine tumors. Mod Pathol. 1999;12:5–8.
Righi L, Volante M, Tavaglione V, Billè A, Daniele L, Angusti T, et al. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinicopathologic and immunohistochemical study of 218 ‘clinically aggressive’ cases. Ann Oncol. 2010;21:548–55.
Granberg D, Sundin A, Janson ET, Oberg K, Skogseid B, Westlin JE. Octreoscan in patients with bronchial carcinoid tumors. Clin Endocrinol. 2003;59:793–9.
Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–18.
Rivera MP, Detterbeck F, Mehta AC. Diagnosis of lung cancer: the guidelines. Chest. 2003;123(1 Suppl):129S–36.
Lim E, Goldstraw P, Nicholson AG, Travis WD, Jett JR, Ferolla P, et al. Proceedings of the IASLC International Workshop on Advances in Pulmonary Neuroendocrine Tumors 2007. J Thorac Oncol. 2008;3:1194–201.
Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–12.
Kwekkeboom DJ, Mueller-Brand J, Paganelli G, Anthony LB, Pauwels S, Kvols LK, et al. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med. 2005;46 Suppl 1:62S–6.
Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38:2125–35.
Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging. 2008;35:1847–56.
Ezziddin S, Khalaf F, Vanezi M, Haslerud T, Mayer K, Al Zreiqat A, et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:925–33.
Sabet A, Dautzenberg K, Haslerud T, Aouf A, Sabet A, Simon B, et al. Specific efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in advanced neuroendocrine tumours of the small intestine. Eur J Nucl Med Mol Imaging. 2015;42:1238–46.
Sabet A, Ezziddin K, Pape UF, Reichman K, Haslerud T, Ahmadzadehfar H, et al. Accurate assessment of long-term nephrotoxicity after peptide receptor radionuclide therapy with (177)Lu-octreotate. Eur J Nucl Med Mol Imaging. 2014;41:505–10.
van Essen M, Krenning EP, Bakker WH, de Herder WW, van Aken MO, Kwekkeboom DJ. Peptide receptor radionuclide therapy with 177Lu-octreotate in patients with foregut carcinoid tumors of bronchial, gastric and thymic origin. Eur J Nucl Med Mol Imaging. 2007;34:1219–27.
Bodei L, Cremonesi M, Kidd M, Grana CM, Severi S, Modlin IM, et al. Peptide receptor radionuclide therapy for advanced neuroendocrine tumors. Thorac Surg Clin. 2014;24:333–49.
Binderup T, Knigge U, Loft A, Federspiel B, Kjaer A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin Cancer Res. 2010;16:978–85.
Severi S, Nanni O, Bodei L, Sansovini M, Ianniello A, Nicoletti S, et al. Role of 18FDG PET/CT in patients treated with 177Lu-DOTATATE for advanced differentiated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:881–8.
Sandström M, Garske-Román U, Granberg D, Johansson S, Widström C, Eriksson B, et al. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med. 2013;54(1):33–41.
Paganelli G, Sansovini M, Ambrosetti A, Severi S, Monti M, Scarpi E, et al. 177Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: results from a phase II study. Eur J Nucl Med Mol Imaging. 2014;41(10):1845–51.
Bergsma H, Konijnenberg MW, Kam BL, Teunissen JJ, Kooij PP, de Herder WW, et al. Subacute haematotoxicity after PRRT with 177Lu-DOTA-octreotate: prognostic factors, incidence and course. Eur J Nucl Med Mol Imaging. 2015. doi:10.1007/s00259-015-3193-4
Breeman WA, de Blois E, Bakker WH. Radiolabeling DOTA peptides with 90Y and 177Lu to a high specific activity. In: Chinol M, Paganelli G, editors. Radionuclide peptide cancer therapy. New York: Taylor & Francis; 2006. p. 119–26.
Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs. 1992;10:239–53.
van Vliet EI, Krenning EP, Teunissen JJ, Bergsma H, Kam BL, Kwekkeboom DJ. Comparison of response evaluation in patients with gastroenteropancreatic and thoracic neuroendocrine tumors after treatment with [177Lu-DOTA0, Tyr3] octreotate. J Nucl Med. 2013;54:1689–96.
Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS31 March 2003. 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
Kaplan EL, Meier P. Nonparametric estimation for incomplete observation. J Am Stat Assoc. 1958;53:457–81.
Lawless JS. Statistical models and methods for life-time data. New York: Wiley; 1982.
Granberg D, Eriksson B, Wilander E, Grimfjärd P, Fjällskog ML, Oberg K, et al. Experience in treatment of metastatic pulmonary carcinoid tumors. Ann Oncol. 2001;12:1383–91.
Claringbold PG, Brayshaw PA, Price RA, Turner JH. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38:302–11.
Kashyap R, Hofman MS, Michael M, Kong G, Akhurst T, Eu P, et al. Favourable outcomes of 177Lu-octreotate peptide receptor chemoradionuclide therapy in patients with FDG-avid neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2015;42(2):176–85.
Acknowledgments
The authors thank Ursula Elbling for editing the manuscript. This study was partially supported by AIRC – Associazione Italiana per la Ricerca sul Cancro (grant number: IG10679).
Authors’ contributions
S.S. coordinated the various stages of the project and was involved in the preparation, treatment and follow-up of patients. A.I., M.S., S.N., C.M.G., K.M. were involved in the enrolment, preparation, treatment and follow-up of patients. P.C. performed the diagnostic and therapeutic scintigraphy scans. A.B. and L.A. were involved in the preparation, treatment and follow-up of patients. V.D.I. prepared the radiopharmaceutical. A.S. was responsible for imaging quality control. M.M. was responsible for data collection. E.S. performed the statistical analysis. A.I. and G.P. drafted the manuscript. G.P. reviewed the manuscript for important intellectual content. All authors read and approved the final version of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
None.
Ethical approval
The protocol was approved by the Ethics Committee of the Wide Catchment Area of Romagna-IRST (CEIIAV) and by the competent Italian regulatory authorities. The study was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice guidelines.
Informed consent
All patients gave written informed consent.
Rights and permissions
About this article
Cite this article
Ianniello, A., Sansovini, M., Severi, S. et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE in advanced bronchial carcinoids: prognostic role of thyroid transcription factor 1 and 18F-FDG PET. Eur J Nucl Med Mol Imaging 43, 1040–1046 (2016). https://doi.org/10.1007/s00259-015-3262-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3262-8