Abstract
Purpose
The aim of this study was to investigate metabolic and textural parameters from pretreatment [18F]FDG PET/CT scans for the prediction of neoadjuvant radiation chemotherapy response and 3-year disease-free survival (DFS) in patients with locally advanced rectal cancer (LARC).
Methods
We performed a retrospective review of 74 patients diagnosed with LARC who were initially examined with [18F]FDG PET/CT, and who underwent neoadjuvant radiation chemotherapy followed by complete resection. The standardized uptake value (mean, peak, and maximum), metabolic volume (MV), and total lesion glycolysis of rectal cancer lesions were calculated using the isocontour method with various thresholds. Using three-dimensional textural analysis, about 50 textural features were calculated for PET images. Response to neoadjuvant radiation chemotherapy, as assessed by histological tumour regression grading (TRG) after surgery and 3-year DFS, was evaluated using univariate/multivariate binary logistic regression and univariate/multivariate Cox regression analyses.
Results
MVs calculated using the thresholds mean standardized uptake value of the liver + two standard deviations (SDs), and mean standard uptake of the liver + three SDs were significantly associated with TRG. Textural parameters from histogram-based and co-occurrence analysis were significantly associated with TRG. However, multivariate analysis revealed that none of these parameters had any significance. On the other hand, MV calculated using various thresholds was significantly associated with 3-year DFS, and MV calculated using a higher threshold tended to be more strongly associated with 3-year DFS. In addition, textural parameters including kurtosis of the absolute gradient (GrKurtosis) were significantly associated with 3-year DFS. Multivariate analysis revealed that GrKurtosis could be a prognostic factor for 3-year DFS.
Conclusion
Metabolic and textural parameters from initial [18F]FDG PET/CT scans could be indexes to assess tumour heterogeneity for the prediction of neoadjuvant radiation chemotherapy response and recurrence in LARC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant radiation chemotherapy (nRCT) in patients with locally advanced rectal cancer (LARC, clinical stage T3/4 with or without lymph node metastasis) has improved the local control rate and overall survival [1–3]. Complete or near-complete response to nRCT is associated with better outcomes [4–6]. Therefore, predicting tumour response during the initial staging assessment of LARC before the start of nRCT may have a great impact on patient management. Several imaging modalities are currently used for staging and monitoring the response to nRCT in patients with LARC. Together with conventional CT, MRI has been shown to be effective for local staging of rectal cancer by accurately determining the T4 stage with circumferential resection margin involvement [7]. However, these imaging modalities based on morphology fail in the evaluation of post-nRCT state [8, 9].
There has been several effort to predict tumour response in patients with LARC using [18F]FDG PET/CT functional molecular imaging [10–14]. Early changes in glucose metabolic parameters such as maximum standardized uptake value (SUVmax) assessed using sequential [18F]FDG PET/CT scans during nRCT could predict response to the therapy. Reassessment of stages using [18F]FDG PET/CT after nRCT has been shown to be beneficial in subgrouping of patients and in predicting outcome.
Recently in oncology, textural analysis, a mathematical method used to determine the relationships among pixels in medical images, has been applied to [18F]FDG PET imaging [15]. Possible applications of the assessment of intratumoral heterogeneity have been evaluated for oesophageal and lung cancer [16, 17]. Also, assessment of intratumoral heterogeneity of LARC using textural analysis in [18F]FDG PET imaging could be another biomarker for therapeutic response [18]. Although these are promising results, several rounds of [18F]FDG PET/CT scan acquisitions are required, and there is no consensus as to the time and frequency of PET/CT assessments in relation to preoperative nRCT duration.
The purpose of this study was to assess possible metabolic and textural parameters from pretreatment [18F]FDG PET/CT scans for the prediction of nRCT response and survival in patients diagnosed with LARC.
Materials and methods
Patients
We retrospectively reviewed patients with clinically diagnosed LARC without distant metastases (i.e. stage II or III according to the AJCC Cancer Staging Manual (seventh edition) who underwent [18F]FDG PET/CT scans between April 2009 and December 2013 at Seoul National University Bundang Hospital. All patients received nRCT followed by R0 resection and/or adjuvant chemotherapy. Electronic medical records were reviewed and data were extracted using a clinical database warehouse [19].
All procedures were in accordance with the principles of the 1975 Declaration of Helsinki (2013 revision). Study design and exemption from informed consent were approved by the Institutional Review Board of Seoul National University Bundang Hospital.
Clinicopathological and survival data
Clinicopathological data that were considered potentially relevant for prognosis were collected from electronic medical records using a clinical database warehouse. Data included age at initial diagnosis, sex, clinical TNM stage (assessed using CT, [18F]FDG PET/CT, and/or MRI), nRCT regimen, and performance of adjuvant chemotherapy. Pathological data included histological subtype, histological differentiation grade, pathological staging after nRCT (ypTNM), involvement of circumferential margin, lymphangitic/venous/perineural tumour involvement, and tumour regression grade (TRG). The AJCC guidelines were used for the assessment of TRG. Patients with TRG 0 and 1 were considered responders and patients with TRG 2 and 3 nonresponders (Table 1).
We defined 3-year disease-free survival (DFS) as the time between the date of the pre-treatment [18F]FDG PET/CT scan and the date of any report of recurrence first identified using imaging (CT, [18F]FDG PET/CT or MRI) within 3 years. All tumour recurrences were confirmed by pathology or clinical follow-ups. Overall survival (OS) was defined as the time between the day of the pretreatment [18F]FDG PET/CT scan and death due to rectal cancer.
Acquisition of [18F]FDG PET/CT scans
[18F]FDG PET/CT scans were acquired using a Discovery VCT scanner (GE Medical Systems, Milwaukee, WI). All patients fasted for at least 6 h before the scan. After venous blood glucose levels were confirmed to be below 200 mg/dL (mean 101.5 mg/dL, range 68 – 175 mg/dL), [18F]FDG was injected intravenously (5.18 MBq/kg body weight for body weights less than 80 kg, a minimum of 444 MBq for body weights more than 80 kg, or a maximum dose of 518 MBq), and PET/CT scanning was performed 50 min later. CT scans were performed at 120 kVp for attenuation correction and anatomical information. PET scans were obtained from the skull base to the upper thigh using a 128 x 128 matrix. Images were reconstructed with an ordered subsets expectation maximization iterative algorithm (two iteration, eight subsets). The SUV was calculated using lean body mass.
Analysis of [18F]FDG PET/CT imaging
All PET/CT data were analysed using a commercially available system (Advantage Windows workstation and PET VCAR software; GE Medical Systems). Reference volumes of interest (VOI) were automatically placed on the right liver using a fixed size (volume 11.03 cm3) spherical VOI. All automatically drawn reference VOIs were reviewed and manually redrawn if the CT scan quality was compromised or the liver was displaced by other lesions. SUVmax, SUVpeak and SUVmean of the rectal cancer lesions were calculated. Metabolic volume (MV) and total lesion glycolysis (TLG) of rectal cancer lesions were calculated using the isocontour method. Various isocontour thresholds were applied: 30 % to 70 % of SUVmax, SUVmean of liver + two standard deviations (SDs), and SUVmean of liver + three SDs. Only the primary rectal cancer lesion was analysed, but peritumoral and perirectal lymph nodes that were too close to the primary lesion could be included in the VOI depending on the isocontour threshold.
Three-dimensional textural analysis of the [18F]FDG PET images was done using MaZda version 4.6 (Institute of Electronics, Technical University of Łódź, Poland) [20–22]. VOIs were manually drawn on the rectal cancer lesions and images were normalized with the limit of three SDs from the mean. Textural analysis including histogram-based grey level, absolute gradient, co-occurrence matrix (COM) and run-length matrix were calculated after grey level intensity resampling to 64 degrees. From each VOI 50 textural features were calculated. Table 2 shows the list of textural features used for analysis, and these textural features are described in detail in the MaZda manual [23].
Statistical analysis
Clinicopathological, TRG and PET parameters were analysed using a t test and binary logistic regression. For multivariate binary logistic regression, the backward conditional method (entry threshold P < 0.05; removal threshold P > 0.1) was applied. Survival analysis was performed using clinicopathological and PET parameters as covariates, and tumour recurrence within 3 years and death as endpoints. The duration of 3-year DFS and OS were calculated using the Kaplan-Meier method. Univariate Cox regression analysis was performed for each parameter and variable, and those with a P value less than 0.05 were included in the multivariate analysis. For multivariate Cox regression, the backward conditional method (entry threshold P < 0.05; removal threshold P > 0.1) was applied. SPSS software (version 18.0; SPSS Inc., Chicago, IL) was used for all statistical analyses.
Results
Patient characteristics
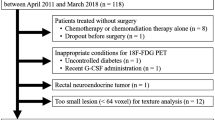
We initially enrolled 78 patients who fulfilled the inclusion criteria. The nRCT regimens included a 5-fluorouracil (5-FU) bolus with leucovorin (FL group, 30 patients) and capecitabine (capecitabine group, 48 patients) with radiation therapy of 50.4 Gy. Only one patient in the FL group did not finish the full chemotherapy course due to recurrent pneumonia. Two patients in the capecitabine group dropped the radiation therapy due to emergency surgery and underlying haematological disease; thus, they were excluded from the analysis. After nRCT, patients received complete R0 resection, except one patient who was excluded from the study because of the presence of liver metastasis which was detected during surgery. The final analysis included 74 patients (Fig. 1). There was no significant difference in age, sex and clinical T and N stages between the FL and capecitabine groups. After surgery, all patients underwent adjuvant chemotherapy, except for five patients who did not receive further chemotherapy because of poor performance status. There was no significant difference in survival between patients who underwent adjuvant chemotherapy and those who did not. Table 3 summarizes the demographics and tumour characteristics of the 74 enrolled patients. The t test was used to compare the difference of SUVs (SUVmax, SUVpeak, SUVmean) between the responder group and nonresponder group, and no significant difference was found between these groups.
Tumour regression grade and clinical/metabolic/textural parameters
Binary logistic regression was performed to evaluate the possible clinical, metabolic and textural parameters for the prediction of nRCT response. None of the clinical parameters was significantly associated with TRG. However, MVs calculated using the thresholds SUVmean of the liver + two SDs and SUVmean of the liver + three SDs were significantly associated with TRG (OR 1.031, 95 % confidence interval, CI, 1.001 – 1.063, P = 0.045; and OR 1.036, 95 % CI 1.001 – 1.071, P = 0.043, respectively). On the other hand, MVs calculated using other thresholds (SUVmax, SUVpeak and TLG; data not shown) were not significantly associated with TRG (Table 4).
Regarding textural parameters, the 50 %, 90 % and 99 % percentiles from the histogram-based analysis and sum entropy and entropy from the COM analysis were significantly associated with TRG. All of these parameters in the nonresponder group were significantly higher than in the responder group. However, the multivariate analysis showed no significant associations between metabolic or textural parameters and TRG.
Survival characteristics
Among the 74 patients, 12 (16.2 %) had tumour recurrence. The median follow-up duration in the 62 recurrence-free patients was 27.2 months (range 10 – 36 months). The mean 3-year DFS calculated using the Kaplan-Meier method in the 74 patients was 32.2 months (95 % CI 30.1 – 34.3 months). Sites of tumour recurrence were the liver (three patients), lung (six patients), and local area (three patients). One patient (1.3 %) died during follow-up as a result of progression of the primary cancer. The mean follow-up duration in the 73 surviving patients was 32.3 months (range 10 – 61 months). The mean OS in the 74 patients was 60.2 months (95 % CI 58.7 – 61.7 months). Because only one patient died, OS data were not used for analysis.
Three-year disease-free survival and clinical/metabolic/textural parameters
Univariate Cox regression analysis of the 3-year DFS data was performed to evaluate the prognostic potential of clinical, metabolic and textural parameters (Tables 5 and 6). Of the pathological parameters, circumferential margin and perineural involvement were associated with 3-year DFS. Stage T4 after neoadjuvant therapy (yT4) was more significantly associated with recurrence than yT0/T2. However, 3-year DFS was not significantly associated with clinical T or N stages or nRCT regimen. In addition, histological differentiation grade, N stage after neoadjuvant therapy (yN), lymphatic/venous involvement, and TRG were not significantly associated.
Regarding metabolic parameters, MV calculated using various thresholds was significantly association with 3-year DFS (Table 6). MV calculated using higher thresholds tended to be more strongly associated with 3-year DFS. TLG showed a borderline hazard ratio (HR) although statistically significant. Among 50 textural parameters, GrKurtosis was significantly associated with 3-year DFS (Table 6). Multivariate analysis revealed that GrKurtosis was a factor predicting DFS that was independent of pathological parameters such as circumferential margin and perineural involvement (Table 7, Fig. 2).
Representative pretreatment [18F]FDG PET/CT images. a A 75-year-old man with cT3N2M0 rectal cancer who had undergone nRCT with capecitabine. After surgery, the pathological stage was ypT3N1M0 with a negative circumferential resection margin and perineural involvement. He received adjuvant chemotherapy, and showed no evidence of disease for 21 months after initial diagnosis. The PET/CT image shows a primary rectal cancer lesion with homogeneous FDG uptake (arrow). b A 56-year-old man with cT3N2M0 rectal cancer who had undergone nRCT with capecitabine. After surgery, the pathological staging was ypT3N0M0 with a negative circumferential resection margin. He received adjuvant chemotherapy, but developed liver metastasis 8 months after the initial diagnosis. The PET/CT image shows a primary rectal cancer lesion with heterogeneous FDG uptake (arrow), for which GrKurtosis was high
Discussion
In this retrospective study we found that MV and textural parameters of rectal cancer calculated from pretreatment [18F]FDG PET/CT scans could potentially be used as imaging biomarkers for nRCT response, and have value for predicting recurrence in LARC. Previous studies have investigated the use of [18F]FDG PET/CT monitoring for the prediction of nRCT response in patients diagnosed with LARC. The subject is not completely new, but previous studies assessed only conventional metabolic values (SUVmax, SUVmean), and none investigated the use of pretreatment FDG PET imaging [10–14]. Oku et al. studied rectal cancer patients who received neoadjuvant radiation therapy alone, and found that SUVmean from pretreatment [18F]FDG PET/CT scans was not significantly related to therapeutic response or recurrence [24]. In our study, SUVmax, SUVpeak and SUVmean were not significantly associated with nRCT response or recurrence, which was consistent with the findings of previous studies.
SUVmax, SUVpeak and SUVmean represent only some part of the tumour. Therefore, SUV itself provides limited information on tumour lesion, and heterogeneity might not be shown by the SUV. However, MVs calculated using the thresholds SUVmean of the liver + two SDs and SUVmean of the liver + three SDs, that generally include the entire cancer lesion were significantly associated with nRCT response. This result might be consistent with the findings of previous studies showing that the volume of rectal cancer measured clinically or CT imaging is significantly related to tumour downstaging after nRCT [25, 26]. In contrast to MV, TLG (calculated as the product of MV and SUVmean) did not show a significant correlation with nRCT response. This result could be because SUVmean prediction of recurrence in LARC, MV could be a promising imaging biomarker. MV calculated using higher thresholds showed a positive correlation with 3-year DFS. In this study, the threshold used for the calculations was not the fixed SUV level, but the percentage of SUVmax in individual tumours in each patient. Since the absolute value of SUVmax or SUVpeak did not show any correlation with the clinical data, a better measure reflecting the character of the tumour might be considered, since these results might have been due to individual variations in glucose metabolism. MV calculated using various tumour percentage thresholds could be an easily evaluated semiquantitative measure of tumour textural heterogeneity, and could also be an easy way to minimize individual variations. Henriksson et al. found intratumoral heterogeneity in FDG uptake which correlated with histopathological findings in nude mice [27]. In solid cancers other than rectal cancer, a higher SUV could also represent more aggressiveness of cancer cells [28–31]. Therefore, MV calculated using a higher threshold might be a representative marker for tumour aggressiveness in primary rectal cancer. From this point of view, an initial assessment of rectal cancer using [18F]FDG PET/CT could be a more sophisticated method to accurately assess clinical stage.
Textural analysis could maximize the information obtained from standard clinical images [15]. In this study, GrKurtosis was an independent factor predicting 3-year DFS. GrKurtosis is a measure of the grey-level variation across the image, and represents the relationship between the neighbourhood pixels [32]. It is also a measure of “peakedness” of the gradient in the images, and higher GrKurtosis indicates a peaked distribution, which tends to be more random and heterogeneous. Patients who had a significantly higher GrKurtosis value showed a higher risk of recurrence. Gigher Grkurosis was significantly correlated with 3-year DFS. Randomness of FDG uptake could represent intratumoral heterogeneity, and a greater degree of intratumoral heterogeneity was associated with a poorer clinical outcome. Uptake of FDG by tumours has been shown to correlate with biological markers such as GLUT-1, as well as glycolysis-related and hypoxia-related markers in lung and oral cancers [33, 34]. Hypoxia is a well-known factor in tumour radioresistance [35].
Textural parameters from [18F]FDG PET imaging could be indicators of intratumoral heterogeneity, especially hypoxia. In the present study, various textural parameters were significantly associated with TRG and 3-year DFS. Among these textural parameters, entropy and the sum of entropy are indicators of the randomness of FDG uptake distribution [36]. We hypothesize that these textural parameters, which were significantly higher in the nonresponder group than in the responder group, could predict response to radiation therapy because they reflect the microenvironment of the tumour, including hypoxia. Further studies are necessary to determine the exact relationA limitation of this study in relation to the evaluation of TRG was the relatively small number of patients. Furthermore, the patients were divided into two groups according to TRG and the patients in each group showed a wide range of TRG. The value of TRG in predicting recurrence and survival is well known [4–6]. In this study, the chi-squared test showed that there was no significant difference in TRG between the disease-free group and recurrence group (P = 0.057 according to Fisher’s exact test). However, TRG itself was not significantly associated with 3-year DFS. This can be explained by iin terms of the two limitations discussed above. Furthermore, all 12 patients who had tumour recurrence were nonresponders. Further study in a larger group of patients is needed. Also, to validate the textural analysis, a large-scale study including other malignancies such as soft-tissue sarcoma is needed. In addition, two chemotherapy regimens (based on 5-FU and capecitabine) were used in this study. However, there is no clinical difference between two regimens [37]. Lastly, the method of delineation of the tumour could have been a confounding factor in the textural analysis but the rectum is a less mobile organ than other bowel structures, so this limitation might have been of minimal effect [38]. In addition, to overcome this limitation we used CT-based segmentation to reduce variability.
Conclusion
We retrospectively evaluated pretreatment [18F]FDG PET/CT scans in patients diagnosed with LARC in relation to their clinical outcomes, and found that MV and textural parameters from histogram-based and COM analysis were significantly associated with response to nRCT. Furthermore, MV calculated using a higher threshold tended to be more strongly associated with 3-year DFS. Also, multivariate analysis showed that textural parameters and MV were useful for predicting recurrence. Consequently, metabolic and textural parameters from pretreatment [18F]FDG PET/CT scans could be used as imaging biomarkers for measuring tumour heterogeneity for the prediction of response to nRCT and recurrence in patients diagnosed with LARC.
References
Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumdar M, Enker W, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241(5):829–36.
Kaminsky-Forrett M-C, Conroy T, Luporsi E, Peiffert D, Lapeyre M, Boissel P, et al. Prognostic implications of downstaging following preoperative radiation therapy for operable T3-T4 rectal cancer. Int J Radiat Oncol Biol Phys. 1998;42(5):935–41.
Theodoropoulos G, Wise W, Padmanabhan A, Kerner B, Taylor C, Aguilar P, et al. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;45(7):895–903.
García-Aguilar J, de Anda EH, Sirivongs P, Lee S-H, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46(3):298–304.
Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo L-J, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–44.
Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. Clin Oncol. 2005;23(34):8688–96.
Brown G, Davies S, Williams G, Bourne M, Newcombe R, Radcliffe A, et al. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer. 2004;91(1):23–9.
Kahn H, Alexander A, Rakinic J, Nagle D, Fry R. Preoperative staging of irradiated rectal cancers using digital rectal examination, computed tomography, endorectal ultrasound, and magnetic resonance imaging does not accurately predict T0, N0 pathology. Dis Colon Rectum. 1997;40(2):140–4.
Suppiah A, Hunter I, Cowley J, Garimella V, Cast J, Hartley J, et al. Magnetic resonance imaging accuracy in assessing tumour down‐staging following chemoradiation in rectal cancer. Colorectal Dis. 2009;11(3):249–53.
Calvo FA, Domper M, Matute R, Martinez-Lazaro R, Arranz JA, Desco M, et al. 18F-FDG positron emission tomography staging and restaging in rectal cancer treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys. 2004;58(2):528–35.
Capirci C, Rampin L, Erba PA, Galeotti F, Crepaldi G, Banti E, et al. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging. 2007;34(10):1583–93.
Cascini GL, Avallone A, Delrio P, Guida C, Tatangelo F, Marone P, et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med. 2006;47(8):1241–8.
Guillem JG, Moore HG, Akhurst T, Klimstra DS, Ruo L, Mazumdar M, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining longterm outcomes of rectal cancer. J Am Coll Surg. 2004;199(1):1–7.
Konski A, Hoffman J, Sigurdson E, Haluszka O, Engstrom P, Cheng JD, et al. Can molecular imaging predict response to preoperative chemoradiation in patients with rectal cancer? A Fox Chase Cancer Center prospective experience. Semin Oncol. 2005;32(6 Suppl 9):S63–7.
Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJ. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging. 2013;40(1):133–40.
Ha S, Choi H, Cheon GJ, Kang KW, Chung J-K, Kim EE, et al. Autoclustering of non-small cell lung carcinoma subtypes on 18F-FDG PET using texture analysis: a preliminary result. Nucl Med Mol Imaging. 2014;48(4):278–86.
Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges J-P, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52(3):369–78.
Bundschuh RA, Dinges J, Neumann L, Seyfried M, Zsótér N, Papp L, et al. Textural parameters of tumor heterogeneity in 18F-FDG PET/CT for therapy response assessment and prognosis in patients with locally advanced rectal cancer. J Nucl Med. 2014;55(6):891–7.
Yoo S, Kim S, Lee K-H, Jeong CW, Youn SW, Park KU, et al. Electronically implemented clinical indicators based on a data warehouse in a tertiary hospital: its clinical benefit and effectiveness. Int J Med Inform. 2014;83(7):507–16.
Strzelecki M, Szczypinski P, Materka A, Klepaczko A. A software tool for automatic classification and segmentation of 2D/3D medical images. Nucl Instrum Methods Phys Res A. 2013;702:137–40.
Szczypiński PM, Strzelecki M, Materka A. MaZda - a software for texture analysis. International Symposium on Information Technology Convergence. ISITC 2007. 23–24 November 2007, Jeonju, Korea. p. 245–9. doi:10.1109/ISITC.2007.15.
Szczypiński PM, Strzelecki M, Materka A, Klepaczko A. MaZda – a software package for image texture analysis. Comput Methods Programs Biomed. 2009;94(1):66–76.
Materka A SMPSs. MaZda manual. 2006.
Oku S, Nakagawa K, Momose T, Kumakura Y, Abe A, Watanabe T, et al. FDG-PET after radiotherapy is a good prognostic indicator of rectal cancer. Ann Nucl Med. 2002;16(6):409–16.
Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the MD Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44(5):1027–38.
Willett CG, Warland G, Coen J, Shellito PC, Compton CC. Rectal cancer: the influence of tumor proliferation on response to preoperative irradiation. Int J Radiat Oncol Biol Phys. 1995;32(1):57–61.
Henriksson E, Kjellen E, Wahlberg P, Ohlsson T, Wennerberg J, Brun E. 2-Deoxy-2-[18F] fluoro-D-glucose uptake and correlation to intratumoral heterogeneity. Anticancer Res. 2007;27(4B):2155–9.
Halfpenny W, Hain S, Biassoni L, Maisey M, Sherman J, McGurk M. FDG-PET. a possible prognostic factor in head and neck cancer. Br J Cancer. 2002;86(4):512–6.
Higashi K, Ueda Y, Ayabe K, Sakurai A, Seki H, Nambu Y, et al. FDG PET in the evaluation of the aggressiveness of pulmonary adenocarcinoma: correlation with histopathological features. Nucl Med Commun. 2000;21(8):707–14.
Ngeow J, Quek R, Ng D, Hee S, Tao M, Lim L, et al. High SUV uptake on FDG–PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG-PET/CT staging in lymphoma. Ann Oncol. 2009;20(9):1543–7.
Pasquali C, Rubello D, Sperti C, Gasparoni P, Liessi G, Chierichetti F, et al. Neuroendocrine tumor imaging: can 18F-fluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J Surg. 1998;22(6):588–92.
Castellano G, Bonilha L, Li LM, Cendes F. Texture analysis of medical images. Clin Radiol. 2004;59(12):1061–9.
van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer. 2007;43(9):1392–8.
Yamada T, Uchida M, Kwang-Lee K, Kitamura N, Yoshimura T, Sasabe E, et al. Correlation of metabolism/hypoxia markers and fluorodeoxyglucose uptake in oral squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2012;113(4):464–71.
Hall EJ, Giaccia A. The oxygen effect and reoxygenation. Radiobiol Radiologist. 1994;4:133–52.
Pires A, Rusinek H, Suh J, Naidich DP, Pass H, et al. Clustering of lung adenocarcinomas classes using automated texture analysis on CT images. Proc. SPIE 8669, Medical Imaging 2013: Image Processing, 866925. doi:10.1117/12.2007154
Hofheinz R-D, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13(6):579–88.
Orlhac F, Soussan M, Maisonobe J-A, Garcia CA, Vanderlinden B, Buvat I. Tumor texture analysis in 18F-FDG PET: relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J Nucl Med. 2014;55(3):414–22.
Compliance with ethical standards
ᅟ
Funding
This research was supported by Korea Mouse Phenotyping Project (2013M3A9D5072560) of the Ministry of Science, ICT and Future Planning through the National Research Foundation.
Conflicts of interest
None.
Ethical approval
All procedures were performed in accordance with the principles of the 1975 Declaration of Helsinki (2000 revision). The study design and exemption from informed consent were approved by the Institutional Review Board of Seoul National University Bundang Hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bang, JI., Ha, S., Kang, SB. et al. Prediction of neoadjuvant radiation chemotherapy response and survival using pretreatment [18F]FDG PET/CT scans in locally advanced rectal cancer. Eur J Nucl Med Mol Imaging 43, 422–431 (2016). https://doi.org/10.1007/s00259-015-3180-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3180-9