Abstract
Purpose
The L1 cell adhesion molecule (L1CAM) is considered a valuable target for therapeutic intervention in different types of cancer. Recent studies have shown that anti-L1CAM radioimmunotherapy (RIT) with 67Cu- and 177Lu-labelled internalising monoclonal antibody (mAb) chCE7 was effective in the treatment of human ovarian cancer xenografts. In this study, we directly compared the therapeutic efficacy of anti-L1CAM RIT against human ovarian cancer under equitoxic conditions with the radiolanthanide 177Lu and the potential alternative 161Tb in an ovarian cancer therapy model.
Methods
Tb was produced by neutron bombardment of enriched 160Gd targets. 161Tb and 177Lu were used for radiolabelling of DOTA-conjugated antibodies. The in vivo behaviour of the radioimmunoconjugates (RICs) was assessed in IGROV1 tumour-bearing nude mice using biodistribution experiments and SPECT/CT imaging. After ascertaining the maximal tolerated doses (MTD) the therapeutic impact of 50 % MTD of 177Lu- and 161Tb-DOTA-chCE7 was evaluated in groups of ten mice by monitoring the tumour size of subcutaneous IGROV1 tumours.
Results
The average number of DOTA ligands per antibody was 2.5 and maximum specific activities of 600 MBq/mg were achieved under identical radiolabelling conditions. RICs were stable in human plasma for at least 48 h. 177Lu- and 161Tb-DOTA-chCE7 showed high tumour uptake (37.8–39.0 %IA/g, 144 h p.i.) with low levels in off-target organs. SPECT/CT images confirmed the biodistribution data. 161Tb-labelled chCE7 revealed a higher radiotoxicity in nude mice (MTD: 10 MBq) than the 177Lu-labelled counterpart (MTD: 12 MBq). In a comparative therapy study with equitoxic doses, tumour growth inhibition was better by 82.6 % for the 161Tb-DOTA-chCE7 than the 177Lu-DOTA-chCE7 RIT.
Conclusions
Our study is the first to show that anti-L1CAM 161Tb RIT is more effective compared to 177Lu RIT in ovarian cancer xenografts. These results suggest that 161Tb is a promising candidate for future clinical applications in combination with internalising antibodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is a gynaecological malignancy with high mortality, because it is often diagnosed late. Approximately 75 % of the patients will have already developed metastases at the time of diagnoses, and debulking surgery is still the crucial step in ovarian cancer therapy [1]. Some success has been achieved in the treatment of ovarian cancer during the last few decades, but the largest percentage of patients will have a relapse with a median progression-free survival of 18 months [2]. New therapeutic approaches with antibodies and/or selective kinase inhibitors (targeted therapy) have not made any significant improvement for patients until now [3, 4]. Overall mortality due to ovarian cancer remains unchanged, and new therapeutic strategies to control the disease are very desirable.
The L1 cell adhesion molecule (L1CAM) is a highly glycosylated type I transmembrane protein that is overexpressed in various tumours and is considered a promising target for novel therapies (reviewed in: [5–9]). The aberrant expression of L1CAM is associated with tumour cell invasion and motility [7], and with poor prognosis and a high risk for progression in ovarian, uterine, and colorectal cancers [10–12]. The restricted expression of L1CAM in normal tissue [13] has led to the use of anti-L1CAM monoclonal antibodies in targeted ovarian cancer therapies [14–17]. To increase the efficacy of antibody-based L1CAM therapy, we introduced 67Cu [16] and 177Lu [15] for radioimmunotherapy (RIT) in ovarian cancer therapy models. The choice of the therapeutic low-߯-energy emitting nuclides for RIT matches the size of small residual disease found after tumour resection in ovarian cancer patients. The used monoclonal antibody chCE7 is a chimeric IgG1 molecule that binds with high affinity to human L1CAM. We could demonstrate that chCE7 binds near the RGD sequence in the sixth Ig-like domain of human L1CAM and can prevent the binding to integrins [18]. The antigen-antibody complex internalised by endocytosis [19] and metallic radionuclides like 67Cu or 177Lu were trapped intracellularly. Limiting factors for a more widespread use of 67Cu in preclinical and clinical evaluations is probably due to the limited availability of 67Cu and due to the production-related limited specific activity that can be reached.
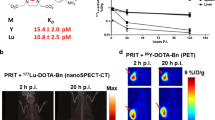
In this study, we directly compared the electron (߯) emitters 177Lu and 161Tb for RIT. Both radionuclides are similar with respect to half-life (6.7 and 6.9 days, respectively), ߯-energy (mean 134 keV vs. 154 keV), and chemical properties, and emit low-energy photons suited for gamma camera imaging. The main difference between these two lanthanides is the number of emitted conversion and Auger electrons (referred to as Auger electrons). 161Tb emits 16 times more Auger electrons per decay (3–50 keV) than 177Lu (Table 1).
Auger electrons produce short-range damage that may have an advantage for the eradication of small tumour nodules. In this study, we compared biodistributions and therapeutic anticancer efficacies of 177Lu- and 161Tb-DOTA-chCE7 immunoconjugates in human ovarian tumour-bearing nude mice under equitoxic conditions.
Materials and methods
Cell lines and antibodies
IGROV1 human ovarian carcinoma cells were obtained from Istituto Nazionale per lo Studio e la Cura dei Tumori (Milano, Italy). Cells were analysed by STR profiling (DSMZ, Braunschweig, Germany). Cell culture conditions and antibodies are described in Supplementary Information (SI).
L1CAM expression on IGROV1 cells
L1CAM expression on IGROV1 cells was analysed by flow cytometry (SI).
Conjugation of antibodies to DOTA and radiolabelling
Conjugation of antibodies to DOTA was performed as previously described [15]. The molar excess of p-SCN-Bn-DOTA (Macrocyclics, Dallas, TX, USA) was adapted individually for the different antibodies to achieve a similar number of DOTA-ligands coupled to the antibodies. The reaction mixture was adjusted to pH 9–10 using a saturated Na3PO4 solution and was incubated overnight (16 h) at 4 °C. Excess ligands were removed by gel filtration chromatography on a NAP-5 column (GE Healthcare, Glattbrugg, Switzerland) which was eluted with 0.25 M ammonium acetate (pH 5.5) for labelling. The number of chelators coupled per mAb molecule was determined by mass spectrometry [15]. Immunoconjugates were stored at −80 °C until use.
Carrier-free 161Tb was produced in-house by irradiation of highly enriched gadolinium-160 targets at the spallation-induced neutron source at Paul Scherrer Institute (PSI, Villigen, Switzerland) or at the high-flux nuclear reactor at the Institut Laue-Langevin (Grenoble, France) and separated by cation exchange chromatography as described before [20]. 177Lu was obtained from ITG (Garching, Munich, Germany). Both radionuclides were used for radiolabelling according to published procedures [21]. Briefly, 200–1,000 μg of the immunoconjugates in a total volume of 500 or 900 μL of 0.25 M ammonium acetate buffer (pH 5.5) was reacted with 100–600 MBq of 177Lu or 161Tb solution at 37 °C for 1 h. After incubation, EDTA was added to a final concentration of 5 mmol/L and the mixture was incubated for 5 min to complex unchelated lutetium or terbium. Purification of the labelled antibodies was achieved by fast protein liquid chromatography (FPLC) size exclusion chromatography on a Superose 12 column (GE Healthcare, Glattbrugg, Switzerland) in phosphate buffered saline (PBS) with a flow rate of 0.5 mL/min. Fractions of 500 μL were collected and the major peak fractions were pooled.
Quality control of radiolabelled preparations
The immunoreactive fraction of labelled antibody conjugates was measured by cell-binding assays and data were analysed according to the Lindmo method [22]. Stability of the labelled antibodies after incubation in human plasma at 37 °C was analysed by FPLC size exclusion chromatography on a TSKgel G3000Wxl column (Tosoh Bioscience, Stuttgart, Germany) with sodium phosphate buffer (0.3 M NaCl, 0.05 M Na2HPO4, pH 6.2) as mobile phase at a flow rate of 1 mL/min.
Biodistribution and SPECT/CT imaging
Animal studies were conducted in compliance with the Swiss laws on animal protection. All experiments were approved by the cantonal committee on animal experiments and permitted by the responsible cantonal authorities (permission numbers 75528 and 75535). Housing and animal husbandry was conducted according to local law on animal protection. Female CD1-foxn1nu mice, 4–5 weeks old (Charles River, Sulzfeld, Germany) were inoculated subcutaneously (right shoulder) with 7 × 106 IGROV1 cells and biodistribution studies and SPECT/CT (NanoSPECT/CT, Bioscan Europe; Paris, France) imaging were performed 14 days later. For biodistribution studies 1–3 MBq of 177Lu- or 161Tb-DOTA-chCE7 (30 μg) was injected into a tail vein of tumour-bearing nude mice and mice were euthanised at the indicated time points. Tumours and major organs were collected, weighed, and counted for radioactivity together with an aliquot of the injected solution in a gamma counter. Each group with IGROV1 xenografts consisted of five mice. Results are expressed as percentage of injected activity per gram (%IA/g). In vivo imaging was done 72 h post i.v. injection of 6 MBq 177Lu-DOTA-chCE7 or 161Tb-DOTA-chCE7 (38 μg) or of 6 MBq isotope matched 177Lu-DOTA control IgG or 161Tb-DOTA control IgG (38 μg). SPECT data were acquired by Nucline software (version 1.02, Bioscan, Washington DC, USA). SPECT data were reconstructed iteratively with HiSPECT software (version 1.4.3049, Scivis, Göttingen, Germany). SPECT and CT data were automatically co-registered as both modalities shared the same axis of rotation. The fused datasets were analysed with the InVivoScope postprocessing software (version 1.44, Bioscan, Washington DC, USA).
Dose escalation study
Groups of four non-tumour-bearing nude mice (female CD1-foxn1nu, Charles River, Sulzfeld, Germany) were treated intravenously with escalating doses of 177Lu-DOTA-chCE7 (8, 10, 12, and 14 MBq; each 65 μg mAb) or 161Tb-DOTA-chCE7 (6, 8, 10, and 12 MBq; each 65 μg mAb). After injection, mice were weighed every other day and monitored daily for humane endpoint. After 2 weeks, we changed the inspection intervals to every third day until the end of the experiment (47 days). The maximum tolerated dose (MTD) is defined as the first dose level below the dose leading to >20 % decrease in total body weight in at least one of the mice, or an early reaching of a defined endpoint of at least one mouse [23]. Peripheral blood mononuclear cell (PBMC) viability was analysed 13 days post i.v. injection of RICs in whole peripheral blood using the Guava ViaCount assay (Guava Technologies, Cardiff, UK) measured on a guava easyCyte flow cytometer (Millipore, Zug, Switzerland) according to the instructions given by the supplier.
Radioimmunotherapy experiments
Based on the results of the dose escalation studies, RIT studies were performed in female nude mice (eight per group) with subcutaneous tumours (IGROV1 human ovarian carcinoma, mean volume 133 ± 50 mm3, 8 days after cell inoculation) and a 50 % MTD of 177Lu-DOTA-chCE7 or 161Tb-DOTA-chCE7 (30 μg). Equivalently labelled, unspecific matched control IgGs and PBS were used as controls. All injections were done i.v. via the lateral tail vein. Tumour growth and the weight of the mice were evaluated every 2–3 days. Humane endpoint criteria were defined as weight loss of more than 20 % of the initial body weight or a tumour volume of more than 1,000 mm3 or ulceration of the tumours. The tumour volume (V) was calculated using the following equation: V = (L × W2)/2, where W is the width of the tumour (small diameter), and L the length (larger diameter), both in millimetres. The relative tumour volume (RTV) of each individual tumour was calculated as Vx/Vo (Vx = tumour volume at a given time, Vo = tumour volume at the start of therapy).
Statistical analysis
Excel software (Microsoft Office 2003) was used for statistical analyses as described in Supplementary Information.
Results
Conjugation of antibodies to DOTA and labelling of the conjugates with 177Lu and 161Tb
The average number of ligands per mAb chCE7 was 2.5 determined by mass spectrometry. For the control antibodies we obtained a similar number of ligands per molecule. For both radionuclides, 177Lu and 161Tb, maximum specific activities of 600 MBq/mg antibody have been achieved under identical reaction conditions.
Aggregates, small fragments, and free radionuclides were separated from the radiolabelled antibody fraction by FPLC size exclusion chromatography. The immunoreactivity of the antibodies analysed by cell binding assays ranged from 78 to 84 %. Both radioimmunoconjugates were stable in human plasma at 37 °C for at least 48 h. No sign of degradation, release of radioactivity or aggregation was observed (SI).
Comparative biodistribution of 177Lu-DOTA-chCE7 and 161Tb-DOTA-chCE7
In order to compare the biological behaviour of the DOTA conjugated chCE7 antibodies in 177Lu- and 161Tb-labelled form biodistribution experiments were performed on nude mice with human IGROV1 ovarian carcinoma xenografts. The L1CAM expression on the cell surface of IGROV cells was confirmed by FACS analysis (SI). Thirty micrograms of RICs were injected i.v. and accumulation of radioactivity in all major organs and tumours was measured (Table 2). Uptake of radioactivity in tumours was high after 72 h (31.5–33.4 %IA/g) and increased to 37.8–39.0 %IA/g after 144 h. Both RICs showed almost identical behaviour in vivo.
SPECT/CT imaging with 177Lu- and 161Tb-DOTA-chCE7 allowed an excellent visualization of implanted L1CAM-expressing ovarian carcinomas (Fig. 1). Subcutaneous IGROV1 tumours (7 × 106 tumour cells, inoculated 14 days in advance) were visualised with high resolution 72 h after injection of 6 MBq (38 μg) of 177Lu- and 161Tb-labelled RICs. The images showed low blood activities and low activities in liver and other organs which leads to the low background. The labelled unspecific isotope-matched antibodies did not accumulate at the tumour side (Fig. 1b, d).
Whole-body SPECT/CT imaging of tumour-bearing nude mice. The images were taken 72 h after i.v. injection of six MBq radioimmunoconjugates (38 μg). a 177Lu-DOTA-chCE7, b 177Lu-DOTA-matched-control IgG, c 161Tb-DOTA-chCE7, d 161Tb-DOTA-matched-control IgG. The mice are had one IGROV1 tumour on the right shoulder
Determination of the maximum tolerated dose
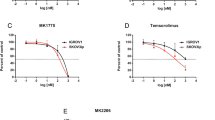
A direct comparison of the therapeutic effects of 177Lu- and 161Tb-labelled mAb chCE7 can be based on experimentally determined maximum tolerated dose (MTD). The dose escalation study was done in groups of four non-tumour bearing nude mice treated with 8, 10, 12, or 14 MBq 177Lu-DOTA-chCE7 (Fig. 2a) and 6, 8, 10, or 12 MBq 161Tb-DOTA-chCE7 (Fig. 2b). The amount of the injected RICs was adjusted to 65 μg. Within the first 10 days, one mouse died unexpectedly in the 12 MBq 177Lu-DOTA-chCE7 group. This death was not regarded as related to the application of the RIC (no signs of distress, no change in behaviour, and no radiation syndrome), and therefore, it was not taken into the evaluation.
Dose escalation studies with a 177Lu-DOTA-chCE7 or b 161Tb-DOTA-chCE7 in nude mice. Mice were injected i.v. with increasing doses of 177Lu- or 161Tb-labelled antibody chCE7 (n = 4 per dose). The amounts of injected antibodies were constant at 65 μg for each dose. Body-weight and health of mice were monitored for 47 days. The maximum tolerated dose was 12 MBq for 177Lu-DOTA-chCE7 and 10 MBq for 161Tb-DOTA-chCE7
Twelve to 14 days after administration of the highest dose of radioactivity (14 MBq of 177Lu-labelled and 12 MBq of 161Tb-labelled immunoconjugate) we observed a significant decrease in body weight for almost all mice in these treatment groups (Fig. 2a, b). The mice looked pale; their agility dropped, and mice showed signs of distress and acute radiation syndrome. A representative picture of such a mouse is shown in (SI). At the same time, the number of red blood cells and the viability of the peripheral white blood cells dropped (Table 3). The MTD for the 177Lu-labelled compound was found to be higher (12 MBq) compared to the 161Tb-labelled compound (10 MBq).
Comparative therapy study of 177Lu-DOTA-chCE7 and 161Tb-DOTA-chCE7
An experiment comparing the therapeutic efficacy of anti-L1CAM RIT using 177Lu-DOTA-chCE7 and 161Tb-DOTA-chCE7 was performed in nude mice with subcutaneous tumour xenografts (IGROV1 human ovarian carcinoma, mean volume 133 ± 50 mm3, 8 days after cell inoculation). Radiolabelled antibodies were injected i.v. A 50 % MTD dose of 161Tb-DOTA-chCE7 (5 MBq) induced prominent tumour growth retardation compared with mice that received corresponding control treatments (Fig. 3). The effect of 50 % MTD of 177Lu-DOTA-chCE7 (6 MBq) was less prominent. After a short delay phase, the mean relative tumour volume (RTV) in all control groups increased roughly linearly and increased three times compared to therapy started after a tumour growth delay (TGD) of 40 days (in the case of 161Tb-DOTA-unspecific matched control IgG), 47 days (in the case of 177Lu-DOTA-unspecific matched control IgG) and 54 days (in the case of PBS). In both anti-L1CAM targeted therapy groups, a mean three-fold increase of the start RTV was not reached, because the first mouse in these groups attained the endpoint before. Therefore, we calculated the TGD in these groups with a RTV increase of 2.3 for 177Lu-DOTA-chCE7 (TGD 69 days) and a RTV increase of 2.5 for 161Tb-DOTA-chCE7 (TGD 118 days). This fact underestimates the tumour growth delay for both therapies.
Therapeutic efficacy of 177Lu- and 161Tb-DOTA-chCE7 RIT in nude mice bearing subcutaneous IGROV1 tumours. Tumour-bearing nude mice were treated i.v. with 50 % MTD of 177Lu- or 161Tb-labelled anti-L1CAM mAb chCE7 or labelled control antibodies or PBS. Data represent the mean relative tumour volumes ± SD. Tumour growth curves were stopped when the first tumour in a treatment group reached 1,000 mm3. The dashed line represents a three-fold increase of the RTV. The dotted lines represent a) a 2.3-fold increase of the RTV (for the 177Lu-DOTA chCE7 treatment) or b) a 2.5-fold increase of the RTV (for the 161Tb-DOTA-chCE7 treatment)
The average RTV was reduced significantly in the targeted 177Lu- and 161Tb-DOTA-chCE7 therapy groups compared to all controls (p-values 0.004 to <0.00001). The average RTV dropped significantly (p < 0.05) in the 161Tb-RIT group compared to the 177Lu-RIT group. A tumour growth inhibition (TGI) of 67.5–85.7 % was obtained for 177Lu anti-L1CAM RIT and was higher than 94 % for the 161Tb-RIT in comparison to control treatments. The TGI of 161Tb-RIT was better by 82.6 % compared to 177Lu-RIT.
Discussion
In this study, we investigated the efficacy of anti-L1CAM radioimmunotherapy using mAb chCE7 labelled with 177Lu or 161Tb. For both radiolanthanides, we used the same labelling procedures and obtained stable radiolabelled DOTA conjugated antibodies with high specific activities. Biodistribution studies showed that both radioconjugates distributed similarly in tumour-bearing nude mice. The immunoreactivity of both radioconjugates was equal. Terbium and lutetium have similar ionic radii (Tb: 0.923 Å; Lu: 0. 861 Å) for six-coordinate complexes [24, 25], and both lanthanides build extremely stable complexes with DOTA ligands [26], which is reflected in the low uptake of radioactivity in bones. It has been described that nude mice exhibit often-different endogenous IgG concentrations that can influence blood pool, spleen and liver uptake of injected mAbs [27, 28]. To compensate for most of these effects we injected 30 μg IgG per mouse in the biodistribution experiments. We cannot exclude that such effects influenced the biodistribution of 177Lu-DOTA-chCE7 in one mouse at 144 h post injection. The mouse showed unusually low activity in tumour and organs (SI). We assume that this low activity was due to a small amount of administered antibody. This mouse was not considered for statistical analysis. The clear SPECT/CT images confirmed the biodistributions we obtained for both radioconjugates. Therefore, both radiolanthanides are suitable in the same way for imaging in small animals. The low gamma ray energies of 161Tb may limit the clinical application for diagnostic purposes.
For direct comparison of the therapeutic efficacy of the 177Lu- or 161Tb-labelled chCE7 antibodies, we determined the MTD for both radioimmunoconjugates. Tumour-free mice were chosen for the dose escalation study to simulate the worst case, i.e. no tumour accumulation of the antibody. Consequently, the RICs follow their biological half-life in blood and distribute without accumulation at a tumour site in the body. For the dose escalation study we used 65 μg of labelled antibody per mouse (resulting from the highest dose that was given) to exclude the influence of endogenous IgG concentrations and to hold the conditions for the whole dose range constant. The higher radiotoxicity of mAb 161Tb-DOTA-chCE7 (MTD: 10 MBq) compared to 177Lu-DOTA-chCE7 (MTD: 12 MBq) is certainly caused in part by the higher ߯ energy emitted by 161Tb. This is in agreement with observations of Brouwers et al. [29] who found a low MTD of 5.6 MBq for a 90Y-labelled internalising chimeric antibody in nude mice. Yttrium-90 has mean ߯ energy of 900 keV. It is most likely that the additional Auger and conversion electrons emitted by 161Tb influenced the radiotoxicity too. The short-range electrons exert their greatest toxicity effects only after internalisation or on the cell membrane of the target cell [30–33]. Inside the cell, Auger electrons are able to deposit high energy around the decay site which leads to ionisation and excitations, chemical transmutations, local effects of charged species, and nuclear recoil [30, 34]. The biological effects induced by Auger electrons are, for the most part, indirectly caused [30]. The significance of intracellular position near the cell nucleus has been discussed [30, 34–36]. In this context, the group of Reilly reported that nuclear localizing sequences coupled to an 111In-labelled antibody promoted specific nuclear uptake in tumour cells and enhanced the radiotoxicity of the antibody [37, 38]. On the other hand, Guo et al. [39] showed that an increased nuclear uptake of the Auger emitter 64Cu in a radioimmunotherapy regime of HCT116 tumour-bearing nude mice had no advantage. In this comparative RIT study between 177Lu- and 161Tb-labelled monoclonal antibodies using 50 % MTD we could clearly demonstrate that the 161Tb-labelled mAb chCE7 performed much better than the 177Lu-labelled counterpart. The differences were significant and tumour growth inhibition was 82.6 % higher for 161Tb-DOTA-chCE7 vs. 177Lu-DOTA-chCE7. We assume that the better therapeutic efficacy of 161Tb-RIT is the result of both the higher ߯ energy and the 16-fold higher emission rate of conversion and Auger electrons. More than half of these electrons (3–50 keV) emitted by 161Tb have an energy higher than 16 keV. This could explain why a nuclear localization is not necessary for the great effect of 161Tb. It is known that Auger electrons induce bystander effects, which can harm neighbouring cells [40]. This may also have influenced the therapeutic efficacy of the RIT. At present, dosimetric estimations are not able to reflect all radiobiological responses induced by conversion and Auger electrons [35].
Conclusions
Our study is, to our knowledge, the first to show that 161Tb-RIT is more effective in an ovarian cancer model under equitoxic conditions in comparison to 177Lu-RIT. For both radiolanthanides the same well established chemistry can be used and biodistributions of the RICs were similar. The 161Tb-labelled antibody showed a slightly higher radiotoxicity in a dose escalation study compared to the 177Lu-labelled mAb. Overall, 161Tb is potentially the better candidate for RIT with internalisation antibodies. Next, we will investigate if we can optimise the RIT with 161Tb in combination with radiosensitising agents, and we will analyse the exact molecular mechanisms of the radiobiological effects induced by the Auger electrons.
References
Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9:167–81.
Bukowski RM, Ozols RF, Markman M. The management of recurrent ovarian cancer. Semin Oncol. 2007;34:S1–15.
Hirte HW. Profile of erlotinib and its potential in the treatment of advanced ovarian carcinoma. Onco Targets Ther. 2013;6:427–35.
Schilder RJ, Sill MW, Lee RB, Shaw TJ, Senterman MK, Klein-Szanto AJ, et al. Phase ii evaluation of imatinib mesylate in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a gynecologic oncology group study. J Clin Oncol. 2008;26:3418–25.
Raveh S, Gavert N, Ben-Ze’ev A. L1 cell adhesion molecule (L1CAM) in invasive tumors. Cancer Lett. 2009;282:137–45.
Weidle UH, Eggle D, Klostermann S. L1-CAM as a target for treatment of cancer with monoclonal antibodies. Anticancer Res. 2009;29:4919–31.
Kiefel H, Bondong S, Hazin J, Ridinger J, Schirmer U, Riedle S, et al. L1CAM a major driver for tumor cell invasion and motility. Cell Adhes Migr. 2012;6:374–84.
Gavert N, Ben-Shmuel A, Raveh S, Ben-Ze’ev A. L1-CAM in cancerous tissues. Expert Opin Biol Ther. 2008;8:1749–57.
Novak-Hofer I. The L1 cell adhesion molecule as a target for radioimmunotherapy. Cancer Biother Radiopharm. 2007;22:175–84.
Boo YJ, Park JM, Kim J, Chae YS, Min BW, Um JW, et al. L1 expression as a marker for poor prognosis, tumor progression, and short survival in patients with colorectal cancer. Ann Surg Oncol. 2007;14:1703–11.
Fogel M, Gutwein P, Mechtersheimer S, Riedle S, Stoeck A, Smirnov A, et al. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet. 2003;362:869–75.
Kaifi JT, Reichelt U, Quaas A, Schurr PG, Wachowiak R, Yekebas EF, et al. L1 is associated with micrometastatic spread and poor outcome in colorectal cancer. Mod Pathol. 2007;20:1183–90.
Huszar M, Moldenhauer G, Gschwend V, Ben-Arie A, Altevogt P, Fogel M. Expression profile analysis in multiple human tumors identifies L1 (CD171) as a molecular marker for differential diagnosis and targeted therapy. Hum Pathol. 2006;37:1000–8.
Arlt MJE, Novak-Hofer I, Gast D, Gschwend V, Moldenhauer G, Grünberg J, et al. Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res. 2006;66:936–43.
Fischer E, Grünberg J, Cohrs S, Hohn A, Waldner-Knogler K, Jeger S, et al. L1-CAM-targeted antibody therapy and 177Lu-radioimmunotherapy of disseminated ovarian cancer. Int J Cancer. 2012;130:2715–21.
Knogler K, Grünberg J, Zimmermann K, Cohrs S, Honer M, Ametamey S, et al. Copper-67 radioimmunotherapy and growth inhibition by anti-L1-cell adhesion molecule monoclonal antibodies in a therapy model of ovarian cancer metastasis. Clin Cancer Res. 2007;13:603–11.
Wolterink S, Moldenhauer G, Fogel M, Kiefel H, Pfeifer M, Luttgau S, et al. Therapeutic antibodies to human L1CAM: functional characterization and application in a mouse model for ovarian carcinoma. Cancer Res. 2010;70:2504–15.
Friedli A, Fischer E, Novak-Hofer I, Cohrs S, Ballmer-Hofer K, Schubiger PA, et al. The soluble form of the cancer-associated L1 cell adhesion molecule is a pro-angiogenic factor. Int J Biochem Cell Biol. 2009;41:1572–80.
Novak-Hofer I, Amstutz HP, Morgenthaler JJ, Schubiger PA. Internalization and degradation of monoclonal-antibody chCE7 by human neuroblastoma-cells. Int J Cancer. 1994;57:427–32.
Lehenberger S, Barkhausen C, Cohrs S, Fischer E, Grünberg J, Hohn A, et al. The low-energy beta(-) and electron emitter Tb-161 as an alternative to Lu-177 for targeted radionuclide therapy. Nucl Med Biol. 2011;38:917–24.
Grünberg J, Novak-Hofer I, Honer M, Zimmermann K, Knogler K, Bläuenstein P, et al. In vivo evaluation of Lu-177- and Cu-67/64-labelled recombinant fragments of antibody chCE7 for radioimmunotherapy and PET imaging of L1-CAM-positive tumors. Clin Cancer Res. 2005;11:5112–20.
Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA. Determination of the immunoreactive fraction of radiolabeled monoclonal-antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984;72:77–89.
Foltz CJ, Ullman-Cullere M. Guidelines for assessing the health and condition of mice. Lab Anim. 1999;28:28–32.
Shannon RD. Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A. 1976;32:751–67.
Viola-Villegas N, Doyle RP. The coordination chemistry of 1,4,7,10-tetraazacyclododecane-n, n′, n″, n′″-tetraacetic acid (h(4)DOTA): structural overview and analyses on structure-stability relationships. Coord Chem Rev. 2009;253:1906–25.
Corneillie TM, Whetstone PA, Fisher AJ, Meares CF. A rare earth-DOTA-binding antibody: probe properties and binding affinity across the lanthanide series. J Am Chem Soc. 2003;125:3436–7.
Reddy N, Ong GL, Behr TM, Sharkey RM, Goldenberg DM, Mattes MJ. Rapid blood clearance of mouse IgG2a and human IgG1 in many nude and nu/+mouse strains is due to low IgG2a serum concentrations. Cancer Immunol Immunother. 1998;46:25–33.
van Gog FB, Brakenhoff RH, Snow GB, van Dongen G. Rapid elimination of mouse/human chimeric monoclonal antibodies in nude mice. Cancer Immunol Immunother. 1997;44:103–11.
Brouwers AH, van Eerd JE, Oosterwijk E, Oyen WJ, Corstens FH, Boerman OC. Preparation, characterization and application of I-131, Re-186, Y-90 and Lu-177-labeled cG250 for radioimmunotherapy of renal cell carcinoma. J Nucl Med. 2002;43:268P–9.
Kassis AI. The amazing world of Auger electrons. Int J Radiat Biol. 2004;80:789–803.
Pouget JP, Santoro L, Raymond L, Chouin N, Bardies M, Bascoul-Mollevi C, et al. Cell membrane is a more sensitive target than cytoplasm to dense ionization produced by Auger electrons. Radiat Res. 2008;170:192–200.
Behr TM, Behe M, Lohr M, Sgouros G, Angerstein C, Wehrmann E, et al. Therapeutic advantages of Auger electron- over beta-emitting radiometals or radioiodine when conjugated to internalizing antibodies. Eur J Nucl Med. 2000;27:753–65.
Paillas S, Boudousq V, Piron B, Kersual N, Bardies M, Chouin N, et al. Apoptosis and p53 are not involved in the anti-tumor efficacy of I-125-labeled monoclonal antibodies targeting the cell membrane. Nucl Med Biol. 2013;40:471–80.
Boswell CA, Brechbiel MW. Auger electrons: lethal, low energy, and coming soon to a tumor cell nucleus near you. J Nucl Med. 2005;46:1946–7.
Kassis AI. Molecular and cellular radiobiological effects of Auger emitting radionuclides. Radiat Prot Dosim. 2011;143:241–7.
Buchegger F, Perillo-Adamer F, Dupertuis YM, Delaloye AB. Auger radiation targeted into DNA: a therapy perspective. Eur J Nucl Med Mol Imaging. 2006;33:1352–63.
Chen P, Wang J, Hope K, Jin LQ, Dick J, Camron R, et al. Nuclear localizing sequences promote nuclear translocation and enhance the radiotoxicity of the anti-CD33 monoclonal antibody hum195 labeled with In-111 in human myeloid leukemia cells. J Nucl Med. 2006;47:827–36.
Costantini DL, Chan C, Cai ZL, Vallis KA, Reilly RM. In-111-labeled trastuzumab (herceptin) modified with nuclear localization sequences (nls): an Auger electron-emitting radiotherapeutic agent for her2/neu-amplified breast cancer. J Nucl Med. 2007;48:1357–68.
Guo YJ, Parry JJ, Laforest R, Rogers BE, Anderson CJ. The role of p53 in combination radioimmunotherapy with Cu-64-DOTA-cetuximab and cisplatin in a mouse model of colorectal cancer. J Nucl Med. 2013;54:1621–9.
Boyd M, Ross SC, Dorrens J, Fullerton NE, Tan KW, Zalutsky MR, et al. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with alpha-, beta-, and Auger electron-emitting radionuclides. J Nucl Med. 2006;47:1007–15.
Acknowledgments
This work was supported by the Swiss Cancer Research Foundation (Project No. KFS-2546-02-2010) to Jürgen Grünberg
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1391 kb)
Rights and permissions
About this article
Cite this article
Grünberg, J., Lindenblatt, D., Dorrer, H. et al. Anti-L1CAM radioimmunotherapy is more effective with the radiolanthanide terbium-161 compared to lutetium-177 in an ovarian cancer model. Eur J Nucl Med Mol Imaging 41, 1907–1915 (2014). https://doi.org/10.1007/s00259-014-2798-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2798-3