Abstract
Purpose
To evaluate the concordance among 18F-FDG PET imaging, MR T2-weighted (T2-W) imaging and apparent diffusion coefficient (ADC) maps with diffusion-weighted (DW) imaging in cervical cancer using hybrid whole-body PET/MR.
Methods
This study prospectively included 35 patients with cervical cancer who underwent pretreatment 18F-FDG PET/MR imaging. 18F-FDG PET and MR images were fused using standard software. The percent of the maximum standardized uptake values (SUVmax) was used to contour tumours on PET images, and volumes were calculated automatically. Tumour volumes measured on T2-W and DW images were calculated with standard techniques of tumour area multiplied by the slice profile. Parametric statistics were used for data analysis.
Results
FDG PET tumour volumes calculated using SUVmax (14.30 ± 4.70) and T2-W imaging volume (33.81 ± 27.32 cm3) were similar (P > 0.05) at 35 % and 40 % of SUVmax (32.91 ± 18.90 cm3 and 27.56 ± 17.19 cm3 respectively) and significantly correlated (P < 0.001; r = 0.735 and 0.766). The mean DW volume was 30.48 ± 22.41 cm3. DW volumes were not significantly different from FDG PET volumes at either 35 % SUVmax or 40 % SUVmax or from T2-W imaging volumes (P > 0.05). PET subvolumes with increasing SUVmax cut-off percentage showed an inverse change in mean ADC values on DW imaging (P < 0.001, ANOVA).
Conclusion
Hybrid PET/MR showed strong volume concordance between FDG PET, and T2-W and DW imaging in cervical cancer. Cut-off at 35 % or 40 % of SUVmax is recommended for 18F-FDG PET/MR SUV-based tumour volume estimation. The linear tumour subvolume concordance between FDG PET and DW imaging demonstrates individual regional concordance of metabolic activity and cell density.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The newly introduced hybrid whole-body PET/MRI scanners have demonstrated huge potential in the oncological field, particularly since MRI is regarded as the first-line imaging procedure for oncological diagnosis [1], and one such example is cervical cancer. 18F-FDG PET is an established functional imaging technique for the evaluation of cervical cancer. Tumour FDG avidity and intratumoral metabolic heterogeneity are related to progression and survival [2, 3]. MRI is also used to evaluate cervical cancer. MR T2-weighted (T2-W) imaging provides improved tumour delineation [4]. The correlation between tumour volume measured by T2-W imaging and the metabolically active volume measured on FDG PET during hybrid PET/MR scanning has not been evaluated. One obvious benefit of obtaining an accurate cut-off percentage of the maximum standardized uptake value (SUVmax) is increased reliability and consistency of functional and anatomical image fusion with hybrid PET/MR scanners, especially when manual coregistration is demanded. Furthermore, it is not known if findings from hybrid PET/MR scanners are equivalent to those obtained separately (i.e. PET-only scanners) where various methods based on functional molecular information (e.g. FDG PET) for accurate definition of the radiation field before therapy have been used without any consensus [5, 6].
Diffusion-weighted (DW) imaging is a functional MRI technique that may provide information regarding tumour cellularity and aggressiveness. For example, tumour cellularity measured on DW imaging has been shown to be inversely correlated with the apparent diffusion coefficient (ADC) [7]. Various microstructures and pathophysiological states show different ADCs. DW-generated maps of ADCs have been used to differentiate benign from malignant cervical tissue and to monitor the treatment response of cervical cancer [8]. Notably, some recent studies comparing DW imaging and FDG PET/CT in cervical cancer have shown that the two techniques might be comparable for evaluating primary cervical cancer [9, 10]. For example, Ho et al. analysed the correlations of minimum ADC (ADCmin) and mean ADC (ADCmean) with SUVmax and mean SUV (SUVmean) within whole primary cervical tumours. They found an inverse correlation between the ADCmin/ADCmean ratio and the SUVmax/SUVmean ratio, except in squamous cell carcinoma and poorly differentiated tumours [9]. Olsen et al. found concordance between FDG PET/CT and DW imaging in the determination of volume and subvolume in squamous cell cervical carcinoma after pixel-by-pixel image segmentation [10]. There are some apparent incompatibilities between these two studies. Moreover, there was one further step in obtaining this functional volume concordance described by Olsen et al. with acceptable image resolution and an easy and quick method of image coregistration. Hybrid PET/MR offers advantages, and might be able to overcome these difficulties.
The objectives of this hybrid PET/MR study in primary cervical cancer were as follows. First, we aimed to compare anatomical volume concordance between PET and T2-W imaging by obtaining an optimal SUVmaxcut-off percentage. Then, functional volume concordance between PET and DW imaging was assessed by comparing their volumes at this cut-off level. Finally, we further evaluated changes in ADCmean within different subvolumes defined at different SUVmax cut-off levels. We considered that quantification of the appropriate SUV cut-off value would help demonstrate hybrid PET/MRI as a reliable and accurate method for cervical cancer imaging. Good anatomical and functional volume concordance in PET/MRI of cervical cancer, hypothesized in this study, would benefit clinical decision making including preoperative staging and treatment planning, radiochemotherapy target definition and therapy follow-up. Functional and molecular imaging studies involving PET and DW imaging could also be obtained by hybrid PET/MRI. These are ongoing issues at our institution which we sought to address by performing a large prospective study of the use of hybrid PET/MRI in patients with primary cervical cancer.
Materials and methods
Patients
This study prospectively included 35 women treated with definitive radiotherapy for cervical cancer. The patient and imaging characteristics are shown in Table 1. The patients’ mean age was 52.50 years (median 53 years, range 33 – 70 years). The inclusion criterion was histologically proven cervical cancer with an advanced clinical stage (stage II or worse). Clinical stage was determined according to the guidelines of the International Federation of Gynecology and Obstetrics (FIGO). The FIGO stages of the patients included in the study were: IIA (4 patients), IIB (13 patients), IIIA (5 patients), IIIB (12 patients), and IV (1 patient). The exclusion criteria were previous treatment and medical or psychological condition that would have precluded investigations or treatment. Tumours less than 2 cm in diameter on MRI were excluded from statistical analysis to avoid partial volume effects on the SUV measurements [11]. This prospective study was approved by the Institutional Review Board of Shengjing Hospital of China Medical University. Informed consent was obtained from all patients.
PET/MRI data acquisition
Pelvic PET/MRI was performed on an Ingenuity TF PET/MR scanner (Philips, Cleveland, OH), which combines time-of-flight (TOF) PET imaging and 3-T MRI with multiple transmission capabilities. The PET and MR subsystems run identically to those of the corresponding Gemini TF PET(/CT) and stand-alone Achieva 3.0 T MR systems [12]. The patients were injected with between 240 and 350 MBq 18F-FDG depending on body weight. After an uptake period of 60 ± 12 min, all patients underwent a pelvic plus a whole-body PET/MR scan. The protocol for the pelvic PET/MR scan consisted of a fast 3-D T1-weighted gradient-echo MR (atMR) sequence used for the computation of the attenuation map (49 s), followed by a pelvic PET emission acquisition (one bed position, 4-min acquisition) and then followed by diagnostic MR sequences (total diagnostic MR time was approximately 30 min, and no MR contrast medium was administrated). The whole-body PET scan included atMR and a whole-body PET emission acquisition (10 to 12 bed positions, 2 min each). A Q-Body coil was used for the atMR image acquisitions. For pelvic investigation, a Torso-XL local coil was additionally connected but only used for the acquisition of diagnostic MR sequences following the pelvic PET scans. The average total acquisition time of this protocol was approximately 1 h. The parameters for pelvic T2-W and DW imaging were as follows:
-
Axial T2-W turbo spin-echo (TSE): TR/TE 2,583/90 ms, voxel size 0.36/0.36/5.00 mm, thickness/interval 5.0/1.0 mm, number of signal averages (NSA) 2
-
Coronal T2-W TSE: TR/TE 1,587/100 ms, voxel size 0.83/0.84/5.00 mm, thickness/interval 5.0/1.0 mm, NSA 2
-
Sagittal T2-W TSE: TR/TE 3,408/60 ms, voxel size 0.49/0.49/5.00 mm, thickness/interval 5.0/1.0 mm, NSA 2
-
Axial echo-planar DW imaging: TR/TE 5,000/100 ms, voxel size 1.41/1.04/8.00 mm, thickness/interval 8.0/1.0 mm, NSA 3, b-values 0, 200, 500, and 1,000 s/mm2
Axial images were acquired from the vaginal canal to the iliac crests. Throughout the entire examination the patients remained motionless while the bed was able to move automatically between the MR and PET gantries. The examinations in all patients were performed in arms-down and free-breathing mode.
Image analysis
PET volume calculation
PET images were reconstructed using a TOF, line-of-response (LOR) ordered subsets expectation maximization (TOF-LOR-OSEM) algorithm. SUVs were calculated and tumour uptake was delineated according to different SUVmax percentages using an Extended Brilliance Workspace (EBW) workstation (Philips Healthcare, Cleveland, OH). SUVmax cut-off percentages were from 20 % to 60 % (at intervals of 5 %) were applied, and the PET volumes for different SUVmax percentages were noted. Another set of cut-off points from 20 % to 90 % (at intervals of 10 %) were applied and the PET images were fused with ADC maps.
T2-W imaging volume calculation
Cervical tumours were identifiable as high-signal intensity on T2-W images. T2-W image tumour volumes were determined in a consensus interpretation by two competent radiologists, using three-dimensional volumetric measurements according to the Simpson rule with modification [13]. Primary tumour volume was defined the gross mass visualized at the cervix and parametrium. Tumour mass was outlined on all contiguous axial images on the EBW workstation. The area of tumour in each slice was multiplied by the slice profile (5.0 mm slice thickness plus 1.0 mm intersection gap), and the total tumour volume was automatically calculated as the sum of adjacent volumes. Studies have shown that the diameter-based and contour-base volumes are similar on MRI, although both methods are considered to overestimate the volume [14].
DW imaging volume calculation
ADC maps were generated from each DW imaging examination and ADC values were then reconstructed for each pixel using the standard software on the accompanied console (Extended MR Workspace, Philips Healthcare, Cleveland USA). Cervical cancers are more diffusion restricted than uninvolved nearby normal tissue. Tumour contours were, therefore, delineated manually for each ADC map, according to the border between the tumour (dark) and normal tissue. Two-dimensional tumour area and mean ADC values for each slice were measured in cervical tumours. Tumour volume was calculated as the sum of all region of interest areas on transaxial slices multiplied by 8 mm. For each tumour, the whole tumour mean ADCmean was calculated as the average of the mean ADC values in all slices [15].
Statistical analysis
All data are expressed as means ± SD. Pearson correlation coefficients were calculated to assess the relationships between SUV and ADC (SUVmax and ADCmin, SUVmean and ADCmean). Tumour volumes from T2-W images were correlated with volumes on the FDG PET scans at 20 % to 60 % (at intervals of 5 %) of SUVmax in all patients using Pearson correlation analysis. Figure 1 shows an example of axial PET/T2-W image fusion with changes in contour and volume at different thresholds of SUVmax on the PET scan. PET-defined metabolic tumour volumes (based on 35 % and 40 % of SUVmax), and volumes from T2-W and DW images were compared using a paired t test. Figure 2 shows an example of subvolume delineation on an axial ADC map at different FDG PET thresholds from 20 % to 90 % (at intervals of 10 %) of SUVmax. Then ADCmean values within the different subvolumes were evaluated using analysis of variance (ANOVA). All statistical analyses were performed using the SPSS software package (version 17.0; SPSS, Chicago, IL).
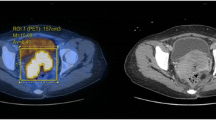
Representative axial fusion PET and T2-W images at various SUVmax cut-off percentages, and a T2-W image in the same patient. Volume of interest contours for the particular cut-off values are superimposed on the fusion images, and all volume of interest contours are superimposed on T2-W image for comparison
Representative axial PET and DW (ADC map) images at various SUVmax cut-off percentages in the same patient. Volume of interest contours for the particular cut-off values are superimposed on the ADC map. All volume of interest contours were finally superimposed on the PET image and ADC map for comparison
Results
Tumour volume comparison between PET and T2-W imaging
The mean T2-W tumour volume was 33.81 ± 27.32 cm3. Table 2 shows PET volumes for different SUVmax percentages and correlations with the T2-W volumes. With an increasing SUVmax cut-off percentage, there was a decrease in the autocontoured volume on FDG PET. The mean T2-W volume was correlated significantly (P < 0.001) with PET volumes delineated using SUVmax cut-off percentages more than 35 %. The mean difference between PET and T2-W volumes was not significant (P > 0.05) with SUVmax cut-off percentages of 30 %, 35 % and 40 %, with the least difference found with 35 % cut-off percentage. Thus, PET and mean T2-W volumes not only were similar (P > 0.05), but also showed significant correlations (P < 0.001) with the 35 % and 40 % of SUVmax cut-off percentages as determined using Pearson bivariate correlation (r = 0.735 and 0.766, respectively).
Tumour volume and subvolume comparison between PET and DW imaging
The mean DW imaging volume was 30.48 ± 22.41 cm3. There was no significant difference (P > 0.05) between FDG PET (at SUVmax cut-off percentages of 35 % and 40 %), T2-W and DW imaging volumes (Fig. 3). As detailed in Table 3, there was no significant correlation between SUVmax and ADCmin (r = −0.074, P = 0.501) or between SUVmean and ADCmean (r = −0.505, P = 0.201) across all 35 primary tumours. For the 28 squamous cell carcinomas, there was also no significant correlation between SUVmax and ADCmin (r = −0.363, P = 0.342) or between SUVmean and ADCmean (r = −0.354, P = 0.150) as determined using Pearson correlation analysis. As shown in Fig. 4, PET subvolumes with increasing cut-off percentages were inversely related to changes in ADCmean value on DW imaging (P < 0.001, ANOVA), which indicates an intratumoral concordance between increased metabolic activity on FDG PET and more restricted diffusion on DW imaging.
Comparison between PET tumour volumes (35 % SUVmax and 40 % SUVmax), and T2-W and DW volumes (paired t test). The only significant difference was between mean volumes at 35 % SUVmax and 40 % SUVmax (P = 0.000). There were no significant differences between mean FDG PET volumes (at 35 % SUVmax or 40 % SUVmax), and T2-W volume and DW volume (P > 0.05). *P < 0.001
Discussion
Hybrid PET/MR imaging is advantageous in the delineation and localization of tissue masses. T2-W imaging provides high tissue contrast and has been proven to be superior to CT for lesion detection and staging of primary cervical cancer [16]. The maintenance of constant volume concordance between the PET and MR components of hybrid PET/MR is important in defining PET gross tumour volumes and fusing multimodality images, since hybrid PET/MR should provide essentially perfect temporal and spatial correlation between the two modalities. In this study, T2-W imaging was used as the gold standard for the assessment of tumour volume. Our results indicated that both anatomical and functional volume concordance could be maintained among PET, and T2-W and DW imaging in primary cervical cancer. This evidence will throw light on past conflicting ideas in SUV-based tumour volume estimation, on current practice in PET/MR fusion imaging, and on future plans in molecular imaging involving PET and DW imaging.
Discrepancies exist in the determination of the volume of primary cervical tumours among FDG PET, CT and MRI. Our results showed that SUVmax cut-off percentages of 35 % and 40 % are satisfactory values for the assessment of cervical cancers with pelvic hybrid PET/MR. However, previous studies have provided some inconsistent conclusions. For example, Miller and Grigsby [5] and Ma et al. [17] found that CT volume is correlated with PET volume at a SUVmax cut-off percentage of 40 % comparing PET data with CT images, and adopted this cut-off value in their subsequent studies concerning FDG PET and MRI. Upasani et al. suggested that the mean difference between MRI and PET volumes was lowest with a SUVmax cut-off value of 30 % and they used values of 30 % and 35 % in their study [6]. Tumour delineation from separate MRI and PET imaging data results in major inaccuracies in anatomical coregistration, possibly caused by differences in scanning time and position between the two modalities. This might be a main reason for inconsistencies in determining FDG PET cut-off values. FDG PET and T2-W volumes may vary due to different tumour metabolic volumes. PET SUVmax thresholds of about 35 % to 40 can be recommended based on our results for general analysis, although the optimal threshold value should be adopted on an individual basis [9, 17]. Good anatomical coregistration between the two modalities is essential for whole-body PET/MR. During this study, good coregistration was observed in all scanned patients, in agreement with other published studies using the same scanner [12, 18]. Furthermore, PET SUV quantification is essential for contouring pelvic malignancies. Lesion size is one of the factors that may influence SUV (especially a size <2 cm due to the partial volume effect) [11, 19]. For this reason, a tumour diameter of more than 2 cm was used as an inclusion criterion in the present study. Different threshold values the volume of disease delineated on the PET scan. Although there are various autocontouring methodologies in literature, there is still no consensus, and therefore effectively no standardization, regarding their use [20]. As in related studies on primary cervical tumour volume calculation and comparison, SUVmax percentage was also adopted in our study to minimize interobserver variability [6, 17].
We also found good functional volume concordance between PET and DW imaging. The mean DW imaging volume was similar to the FDG PET volume (at a SUVmax cut-off percentage of 35 % or 40 %) and the T2-W volume. Thus, FDG PET and DW imaging were comparable and in agreement for the tumour as a whole. To further elucidate the relationship between FDG PET and DW imaging, subvolume analysis was also performed to investigate the concordance between the functional subvolumes. Hybrid PET/MR imaging of primary cervical cancer provides good and quick image fusion automatically, and thus shows advantages in subvolume segmentation. Subvolume segmentation showed that PET subvolumes with increasing SUVmax cut-off percentages were inversely associated with the ADCmean value on DW imaging. This suggests that increased glucose metabolism may be caused by high intratumoral cellular density, resulting in lower ADC values measured on DW imaging [10]. Although not as well-established as FDG PET, DW imaging may also be used to characterize the tumour type, detect cancer masses, and monitor and evaluate tumour treatment response [21, 22]. DW imaging and FDG PET might have similar applications in the field of clinical oncology, and combined analysis might be an interesting topic. However, evidence clarifying the similarities between DW imaging and FDG PET is needed. Cervical cancer in our study could be selected as an example tumour, since it is a typical tumour with both hypermetabolism on FDG PET and obvious diffusion restriction on DW imaging [10].
Functional volume and subvolume concordance between PET and DW imaging, as addressed in primary cervical cancer in this study, provides further clarification as to whether there is a correlation between SUV and ADC. SUVmax and SUVmean reflect the highest and average tumour metabolism, while ADCmin and ADCmean reflect the highest and average tissue cellularity, respectively. Both SUVmax and ADCmin have been used for tumour grading and prediction of prognosis [23]. SUVmean and ADCmean correlate with tissue glucose metabolism and cellular density on a tumour-wide basis, although the use of these might neglect intratumour heterogeneity. However, as shown in Table 3, this study as well as previous studies on primary cervical cancer showed that there is no significant correlation either between SUVmax and ADCmin or between SUVmean and ADCmean in squamous cell cervical carcinomas [17]. Functional volume and subvolume concordance between FDG PET and DW imaging does not prove an obvious causal relationship between glucose metabolism and tissue cellularity, i.e. SUV and ADC, respectively. An intratumoral correlation may be applicable within the same tumour mass, although it does not always lend itself to straightforward generalization across different masses. This is supported by the conclusion of Ho et al. that an inverse correlation only exists between SUV (SUVmax/SUVmean) and ADC (ADCmin/ADCmean) ratios [9]. These SUV and ADC ratios may minimize the effects of individualization. SUV and ADC are closely related to tissue cell type [24, 25]. The study by Ho et al. showed only an inverse correlation between the SUV and ADC ratios in adenocarcinoma and adenosquamous carcinoma, but no significant correlation in squamous cell carcinoma [9]. A recent hybrid PET/MR study regarding non-small-cell lung cancer showed a significant inverse correlation between ADCmean and not only SUVmean but also SUVmax [26]. Thus, hybrid PET/MR imaging may play an important role in evaluating the possible complementary role of PET and DW imaging based on subvolume analysis, regardless of the tumour cell type. The clinical significance of this relationship between PET and DW imaging should be further addressed in larger studies with various clinical settings.
This preliminary hybrid PET/MR study in cervical cancer had some limitations. First, the patient sample was not large and patients with tumours of different cell type might be enrolled in future studies. Second, individualized volume concordance among PET, T2-W and DW imaging might also be investigated based on individual PET thresholds according to different tumour sizes, which we are investigating in a future study. Finally, tumour volumes were drawn manually (especially for DW imaging volumes on ADC maps), which might have led to errors in MRI volume estimation. However, we sought to minimize this bias by consensus interpretation by two competent radiologists, and the image spatial resolution was satisfactory for tumour delineation on both three-planar T2-W images and axial ADC maps.
Conclusion
The current study demonstrated that hybrid PET/MR is feasible for the evaluation of primary cervical cancer. Anatomical volume concordance between PET and T2-W imaging and functional volume concordance between PET and DW imaging could be achieved using SUVmax cut-off percentages of 35 % and 40 % in primary cervical cancers. Intratumoral functional volume concordance was also observed between PET and DW imaging by subvolume segmentation. Tumour subvolumes with increased metabolic activity on FDG PET also have more restricted diffusion on DW imaging, indicating greater cell density. With the development of PET/MR scanning systems, a combined analysis of these two imaging techniques might hold promise for future development of molecular imaging and individualized therapy of cancer.
References
Gaertner FC, Furst S, Schwaiger M. PET/MR: a paradigm shift. Cancer Imaging. 2013;13:36–52.
Kidd EA, Grigsby PW. Intratumoral metabolic heterogeneity of cervical cancer. Clin Cancer Res. 2008;14:5236–41.
Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. The standardized uptake value for F-18 fluorodeoxyglucose is a sensitive predictive biomarker for cervical cancer treatment response and survival. Cancer. 2007;110:1738–44.
Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology. 2013;266:717–40.
Miller TR, Grigsby PW. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced cervical cancer treated by radiation therapy. Int J Radiat Oncol Biol Phys. 2002;53:353–9.
Upasani MN, Mahantshetty UM, Rangarajan V, Purandare N, Merchant N, Thakur M, et al. 18-fluoro-deoxy-glucose positron emission tomography with computed tomography-based gross tumor volume estimation and validation with magnetic resonance imaging for locally advanced cervical cancers. Int J Gynecol Cancer. 2012;22:1031–6.
Herneth AM, Guccione S, Bednarski M. Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol. 2003;45:208–13.
McVeigh PZ, Syed AM, Milosevic M, Fyles A, Haider MA. Diffusion-weighted MRI in cervical cancer. Eur Radiol. 2008;18:1058–64.
Ho KC, Lin G, Wang JJ, Lai CH, Chang CJ, Yen TC. Correlation of apparent diffusion coefficients measured by 3 T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. Eur J Nucl Med Mol Imaging. 2009;36:200–8.
Olsen JR, Esthappan J, DeWees T, Narra VR, Dehdashti F, Siegel BA, et al. Tumor volume and subvolume concordance between FDG-PET/CT and diffusion-weighted MRI for squamous cell carcinoma of the cervix. J Magn Reson Imaging. 2013;37:431–4.
Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48:932–45.
Zaidi H, Ojha N, Morich M, Griesmer J, Hu Z, Maniawski P, et al. Design and performance evaluation of a whole-body Ingenuity TF PET-MRI system. Phys Med Biol. 2011;56:3091–106.
Jung DC, Ju W, Choi HJ, Kang S, Park S, Yoo CW, et al. The validity of tumour diameter assessed by magnetic resonance imaging and gross specimen with regard to tumour volume in cervical cancer patients. Eur J Cancer. 2008;44:1524–8.
Mayr NA, Taoka T, Yuh WT, Denning LM, Zhen WK, Paulino AC, et al. Method and timing of tumor volume measurement for outcome prediction in cervical cancer using magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2002;52:14–22.
King AD, Ahuja AT, Yeung DK, Fong DK, Lee YY, Lei KI, et al. Malignant cervical lymphadenopathy: diagnostic accuracy of diffusion-weighted MR imaging. Radiology. 2007;245:806–13.
Pui MH, Wang QY, Xu B, Fan GP. MRI of gynecological neoplasm. Clin Imaging. 2004;28:143–52.
Ma DJ, Zhu JM, Grigsby PW. Tumor volume discrepancies between FDG-PET and MRI for cervical cancer. Radiother Oncol. 2011;98:139–42.
Schramm G, Langner J, Hofheinz F, Petr J, Beuthien-Baumann B, Platzek I, et al. Quantitative accuracy of attenuation correction in the Philips Ingenuity TF whole-body PET/MR system: a direct comparison with transmission-based attenuation correction. MAGMA. 2013;26:115–26.
Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med. 2004;45:1431–4.
Lucignani G. SUV and segmentation: pressing challenges in tumour assessment and treatment. Eur J Nucl Med Mol Imaging. 2009;36:715–20.
Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI – a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol. 2008;5:220–33.
Hamstra DA, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J Clin Oncol. 2007;25:4104–9.
Nakamura K, Joja I, Kodama J, Hongo A, Hiramatsu Y. Measurement of SUVmax plus ADCmin of the primary tumour is a predictor of prognosis in patients with cervical cancer. Eur J Nucl Med Mol Imaging. 2012;39:283–90.
Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379–87.
Higano S, Yun X, Kumabe T, Watanabe M, Mugikura S, Umetsu A, et al. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241:839–46.
Heusch P, Buchbender C, Kohler J, Nensa F, Beiderwellen K, Kuhl H, et al. Correlation of the apparent diffusion coefficient (ADC) with the standardized uptake value (SUV) in hybrid 18F-FDG PET/MRI in non-small cell lung cancer (NSCLC) lesions: initial results. Rofo. 2013;185:1056–62.
Acknowledgments
We sincerely thank Antonis Kelemis for his constructive advice on the writing of the paper and his great help with language correction. We thank our colleagues for their devoted work throughout the study. We thank all the patients who participated in the study. We also thank Xuna Zhao and Hong Chen of Philips Healthcare for their technical assistance.
Conflicts of interest
The authors declare no conflicts of interest other than a research collaboration established between The Department of Radiology, Shengjing Hospital of China Medical University, and Philips Healthcare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, H., Xin, J., Zhang, S. et al. Anatomical and functional volume concordance between FDG PET, and T2 and diffusion-weighted MRI for cervical cancer: a hybrid PET/MR study. Eur J Nucl Med Mol Imaging 41, 898–905 (2014). https://doi.org/10.1007/s00259-013-2668-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2668-4