Abstract
Purpose
Neoadjuvant radiochemotherapy (RCT) is an accepted treatment for locally advanced rectal cancer (LARC) that improves surgical outcomes. If a pathological complete response is achieved, conservative surgery can be considered. The objective of our study was to assess the reliability of 18F-FDG PET/CT for evaluating the response to neoadjuvant RCT in LARC.
Methods
We prospectively studied 41 patients diagnosed with LARC and candidates for neoadjuvant RCT. PET/CT was performed before RCT and again 7 weeks later. A visual and semiquantitative analysis was carried out. The pathological response was classified according to the Mandard tumour regression grade (TRG). We analysed: (a) the relationship between TRG and the result of the posttreatment PET/CT scan, and (b) the correlation between the percentage of pathological response and the percentage decrease in SUVmax according to the response index (RI).
Results
The mean SUVmax of the rectal lesions at diagnosis was 13.6 and after RCT 3.96. The mean RI was 65.32 %. Sensitivity was 88.88 %, specificity 92.86 %, positive predictive value 96 %, negative predictive value 81 %. Of the 41 patients, 8 had TRG I (all negative PET/CT); 6 had TRG II (5 negative, 1 positive PET/CT); 16 had TRG III (13 positive, 3 negative PET/CT); 9 had TRG IV (all positive PET/CT); 2 had TRG V (all positive PET/CT). Of the 14 patients classified as responders (TRG I, II), 13 (92.86 %) had negative PET/CT. Of the 27 patients classified as nonresponders (TRG III–V), 24 (88.88 %) had positive PET/CT. Differences were statistically significant (p < 0.0001). The RI in responders was 79.9 % and in nonresponders was 60.3 %. Differences were statistically significant (p < 0.037).
Conclusion
PET/CT is a reliable technique for assessing response to neoadjuvant RCT in LARC, with a view to considering more conservative surgical treatment. The combination of the visual and semiquantitative analysis increases the diagnostic validity of PET/CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rectal carcinoma is a highly relevant neoplasia in daily clinical practice and as a consequence it is necessary to constantly develop diagnostic and therapeutic techniques to improve the survival and quality of life of patients with this disease.
Surgery is the fundamental curative approach for rectal carcinoma, so that in its early stage (stage I) a local approach using resection can be enough to avoid the morbidity associated with more extensive resections. In locally advanced stages (II/III) rectal carcinomas are treated using anterior resection with colorectal anastomosis, low anterior resection, or abdominoperineal amputation with resection of the sphincter apparatus and construction of a definitive colostomy.
In recent decades the use of neoadjuvant concomitant radiochemotherapy (RCT) has been encouraged in locally advanced stages [1, 2]. Its main advantages are the reduction in tumour size and stage [3, 4], increased resectability and sphincter conservation, and a lower rate of acute toxicity and local relapse [4–6]. Consequently, in a quite high number of patients, the tumour cells are completely eliminated (in as many as 30 % of patients in some series), a situation known as a pathological complete response [7, 8]. Furthermore, this treatment provides better long-term results with a very low or zero rate of local recurrence or distant relapse [9, 10].
Therefore, in the light of the good prognosis in patients with pathological complete response, new more conservative treatment strategies are being developed to avoid rectal resection. As a consequence, local resection with complete excision of the wall has been reported in patients in whom rectal examination and imaging provide sufficient information [11, 12] to carry out a thorough analysis of the tumour to ensure a pathological complete response. This provides many advantages with a consequent reduction in morbidity and mortality and the preservation of the sphincter apparatus [13, 14].
For these reasons, it is necessary to reliably establish tumour response to neoadjuvant therapy. However, conventional imaging techniques (endorectal ultrasonography, CT and MRI), which have been confirmed as indispensable tests for staging these patients, have not proven to be reliable predictors of response to neoadjuvant treatment, given that they tend to overestimate local tumour extension after treatment (fibrotic changes, oedema, etc.) [15, 16]. In contrast, functional imaging with 18F-FDG based on tumour glucose metabolism has proven to be capable of reliably predicting treatment response in showing greater certainty in detecting residual tumours [17–19], and in providing patients in full remission with the option of more conservative surgery. It also helps to identify the absence of neoadjuvant response, allowing the clinician to replace RCT protocols with more aggressive alternatives. In this regard, the degree of 18F-FDG uptake reduction after neoadjuvant treatment compared to its baseline value in the pretreatment stage has been proposed as an index for the early prediction of regression in tumours treated with RCT [20, 21].
The objective of the present study was to assess the reliability of 18F-FDG PET/CT for evaluating response to neoadjuvant therapy in locally advanced rectal cancer, comparing tumour glucose metabolism in pretreatment and posttreatment scans and its correlation with the level of pathological response.
Material and methods
Patients
We carried out a prospective longitudinal study in 41 patients (25 men and 16 women, mean age 66 years) diagnosed with locally advanced rectal adenocarcinoma (stages II/III) in our hospital between January 2009 and September 2011, and who were candidates for neoadjuvant therapy with RCT. The following exclusion criteria were applied: pregnancy, neoadjuvant therapy contraindicated due to comorbidity, presence of another synchronic tumour, suspicion of an inherited condition (familial adenomatous polyposis), and inflammatory bowel disease.
All the patients followed conventional diagnostic/staging procedures for characterizing the rectal lesion (location and size, distance from the sphincter apparatus, circumferential resection margin, its relationship with neighbouring organs, infiltration of the mesorectum, and the existence of adenopathies) through the usual techniques of rectal examination, endorectal ultrasound-guided biopsy, complete colonoscopy and pelvic MRI. In addition, staging of distant disease was performed by 18F-FDG PET/CT and a thoracoabdominopelvic CT scan.
Neoadjuvant radiochemotherapy
Neoadjuvant radiotherapy (RT) consisted of three-dimensional conformal RT following CT planning in the prone decubitus position, using three fields (one anterior and two lateral). The RT dose was 46–50 Gy to the whole risk volume, followed by an overdose of 4–8 Gy to the macroscopic tumour volume with a margin of 1–1.5 cm. Approximate treatment duration was five and a half weeks. Chemotherapy (capecitabine, 825 mg/m2 twice daily) was administered concomitantly.
18F-FDG PET/CT scan and image analysis
Two PET/CT scans were carried out, one after the initial diagnosis to complete disease staging, and another 7 weeks after completion of neoadjuvant treatment to evaluate the metabolic response. The patients were asked to fast for at least 4 h before the 18F-FDG PET/CT scan. Their blood glucose levels were within the normal range (70–120 mg/dL) prior to intravenous injection of 370 MBq (10 mCi) of 18F-FDG. Data were acquired on an integrated PET/CT system (Gemini GXL-Philips) within 60–90 min of injection. The procedure for data acquisition was as follows: CT scanning was performed first without administration of oral or intravenous contrast agent from the head to the pelvic floor with 120 kV, 100 mA and a 5-mm section thickness. Immediately after CT scanning, a PET emission scan covering the identical transverse field of view was obtained. The acquisition time was 3 min per table position. PET image datasets were reconstructed iteratively by applying the CT data for attenuation correction, and coregistered images were displayed on a workstation.
Studies were interpreted by qualitative and semiquantitative analysis. According to the qualitative visual analysis and on the basis of the normal biodistribution of 18F-FDG, lesions were identified as foci with increased tracer accumulation relative to that in comparable normal contralateral structures and surrounding soft tissues. Tumour metabolic activity was quantified in terms of the standardized uptake value (SUV) normalized to the injected dose and to body weight. The maximum single-pixel SUV (SUVmax, mean ± SD) of the lesions was calculated drawing manually the regions of interest around the tumour and on all the consecutive transaxial slices that contained the tumour so that the whole tumour was included in the regions of interest. We considered SUVmax greater than 2.5 to be positive.
The metabolic response shown on the PET/CT scan after treatment was assessed visually and in terms of the reduction in SUVmax compared to the baseline scan, and the percentage difference in SUVmax or the response index (RI) between the initial PET/CT scan and the scan after treatment calculated according to the formula:
RI = [(pretreatment SUVmax − posttreatment SUVmax)/(pretreatment SUVmax)] × 100
Surgery and histopathological study
Surgical treatment was carried out during the 8th week after neoadjuvant treatment. The postsurgical histopathological stage and the percentage of pathological response were determined by analysis of a surgical specimen using the Mandard tumour regression grade (TRG) [22, 23], which classifies the tumour into five histological grades: TRG I is the absence of cancer cells/complete regression (100 % pathological response); TRG II is the presence of isolated tumour cells scattered throughout the fibrosis (90 %); TRG III is an increase in the number of cancer cells but with fibrosis still predominating (50–89 %); TRG IV is residual cancer outgrowing the fibrosis (10–49 %); TRG V is the absence of regression (<10 %). According to TRG the patients were divided into two groups: responders (TRG I and II) and nonresponders (TRG III to V).
Neoadjuvant response was analysed from two perspectives:
-
1.
The relationship between TRG and the result of the posttreatment PET/CT scan.
-
2.
The correlation between the percentage pathological response and the percentage decrease in SUVmax.
Statistical analysis
The numerical data are reported as means ± standard deviation, and the qualitative variables with frequencies and percentages. For the comparative study of the means we used nonparametric tests (Kruskal-Wallis). To contrast the qualitative variables we used the chi-squared test. All results with a confidence level of 0.05 were considered positive. The statistical analysis was carried out using the SPSS program v. 18.0.
Results
18F-FDG PET/CT detected the primary tumour in all patients. The mean SUVmax of the rectal lesions at diagnosis was 13.6. After neoadjuvant treatment the mean SUVmax was 3.96. The mean RI was 65.32 %. The mean percentage fibrosis in the histopathological samples after neoadjuvant treatment was 70.65 % (Table 1).
Of the 41 patients studied, in 25 (60.97 %) the PET/CT scan was positive after neoadjuvant treatment (24 true-positive and 1 false-positive), and in 16 (39.03 %) the scan was negative (13 true-negative and 3 false-negative). The test had high diagnostic effectiveness (p < 0.0001), with a sensitivity of 88.88 %, a specificity of 92.86 %, a positive predictive value of 96 % and a negative predictive value of 81 % (Table 2).
Relationship between TRG and result of posttreatment PET/CT scan
Of eight patients with complete regression (TRG I), all had a negative PET/CT scan. Of six patients with isolated tumour cells (TRG II), five had a negative PET/CT scan and one a positive scan. Of 16 patients with more residual cancer cells but with fibrosis predominating (TRG III), 13 had a positive PET/CT scan and 3 a negative scan. Of nine patients with residual cancer outgrowing fibrosis (TRG IV), all had a positive PET/CT scan. Finally, of two patients with absence of regression (TRG V), all had a positive PET/CT scan. Overall, of the 14 patients classified as responders (TRG I and II), 13 (92.86 %) had a negative PET/CT scan and 1 (7.14 %) a positive scan. Of the 27 patients classified as nonresponders (TRG III, IV and V), 24 (88.88 %) had a positive PET/CT scan and 3 (11.11 %) a negative scan. The differences between the level of pathological response and the result of the PET/CT scan were significant (p < 0.0001; Table 3).
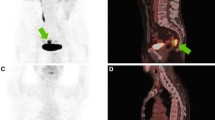
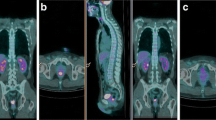
Figures 1 and 2 show two representative cases of a nonresponder and a false-negative patient, respectively.
18F-FDG PET/CT scan in a nonresponder. In the baseline scan (upper row) there is increased 18F-FDG uptake in the middle third of the rectum (SUVmax 12.8), and in the scan after neoadjuvant treatment (lower row), tumour uptake is still present (SUVmax 6.8) revealing residual vital tumour tissue. The postsurgical TRG was IV (20 % pathological response), and the patient was classified as true-positive
18F-FDG PET/CT scan in a false-negative patient. In the baseline scan (upper row) there is increased 18F-FDG uptake in the middle third of the rectum (SUVmax 15), and normal distribution in the scan after neoadjuvant treatment with a SUVmax of 2.3 (lower row). The histopathological examination revealed residual cancer cells with 85 % pathological response (TRG III)
Relationship between pathological response and decrease in SUVmax according to the RI
In the TRG I patients, the mean RI was 71.7 %, in the TRG II patients, the mean RI was 79.16 %, and in the TRG III, IV and V patients, the mean RIs were 72.97 %, 47.59 % and 30.8 %, respectively. The differences between the level of pathological response and the RI were significant (p = 0.013). Overall, the mean RI in the responders was 79.9 ± 4.69 %, and the mean RI in the nonresponders was 60.3 ± 4.6 % (p < 0.037; Table 4).
Discussion
We consider several aspects of the characteristics of the sample, methodology and results of our study, and confirm that the population under study did not differ from those in other similar studies. All the patients were in the same evolutionary stage, and by including only patients with stages II/III and not those with stage IV, we ensured that the final results were unaffected by the worse prognosis that the presence of distant metastases would have conferred. There is unanimity [24] over the better results obtained with the concomitant use of RCT (in terms of toxicity, control of local disease, local recurrence and survival) rather than the exclusive use of RT. This has made it possible to standardize a common neoadjuvant treatment protocol in different institutions allowing a certain amount of variability for optimizing treatment in an individual patient. The treatment used in our patients was no different from the commonly accepted method. Similarly, the interval between the adopted procedures was that recommended by the World Health Organization [25]: a late PET/CT scan 7 weeks after neoadjuvant therapy, and early surgery 1 week later.
Although the validity of 18F-FDG PET/CT for monitoring the effects of neoadjuvant therapy is recognized, its capacity to predict TRG according to differences in uptake intensity between before and after treatment is not generally accepted. Therefore, we investigated this aspect in our sample as one of the objectives of the study. 18F-FDG PET/CT detected the primary tumour in all patients. In addition, statistical significance was found between the PET/CT and histopathological results in terms of TRG, grouping the patients into responders (TRG I and II) and nonresponders (TRG III, IV and V; p < 0.0001), and also between the PET/CT results and the five categories of pathological response (p < 0.0001). The diagnostic validity found in our study was high (sensitivity 88.88 %, specificity 92.86 %, positive predictive value 96 % and negative predictive value 81 %) and greater than that obtained in previous studies [26, 27], regardless of whether the authors used visual analysis or a semiquantitative method. We used a combined analysis that was not mutually exclusive involving visual and semiquantitative analyses through the SUVmax. This combined analysis was especially useful in those patients in whom the posttreatment SUVmax was around the strict cut-off point of 2.5, because the visual analysis helped define a positive or negative result. In this regard, 11 patients had a SUVmax of 2.5 or very close to 2.5, and the two methods provided consistent findings (PET positive or negative) in nine patients and discrepant findings in two, who were ultimately classified according to the visual evaluation of the PET scan. Thus, either the PET/CT result or the pathological tumour response was true-negative and the other true-positive, a situation that would not have occurred if only SUVmax had been used for the evaluation, in which case they would have been false-positive and false-negative, respectively.
In this study we used the TRG after histopathological examination of the total residual tumour mass, thus eliminating the possibility of an incomplete histological analysis which could have led to a wrong interpretation such as finding a patient to be false positive who was really true positive. One patient had a SUVmax of 2.5 (a negative value in strict terms), but a positive visual evaluation, so was classified as false-positive because the patient was TRG II (isolated tumour cells). It is unlikely that there were sufficient tumour cells to account for the uptake (uptake by these cells would have been below the level of detection by PET), or that discrete inflammatory activity could have accounted for the uptake (we tried to avoid this by carrying out a late posttreatment PET/CT scan when the inflammatory tissue caused by the RT should have disappeared). However, the false-positive finding in this patient did not affect the statistical significance of the results. In contrast, we found three false-negative TRG III patients (tumour with fibrosis predominating) with SUVmax values of 1, 1.6 and 2.3, respectively, and with a negative visual evaluation. This finding could have been due to the residual tumour being less than 10 mm in these patients, because it is widely believed that the detection capacity of PET is limited in this regard. Alternatively, the lesion could have been larger but with a low metabolic rate due to cell disruption induced by treatment, and therefore was not shown by PET. Disease progression as a cause of these false-negative findings is ruled out in our study because of the short time between the posttreatment PET/CT scan and surgery.
All patients except one, in whom there was an increase in posttreatment SUVmax, showed a decrease in SUVmax after neoadjuvant treatment. The mean pretreatment SUVmax was 13.6 and the mean posttreatment SUVmax was 3.96, representing a decrease of 71 %. Regarding our second objective (to compare the level of pathological response with the RI), there were statistically significant differences (p < 0.037) between responders with a mean RI of 79.9 ± 4.69 % and nonresponders with a mean RI of 60.3 ± 4.6 % (the mean RI of the whole population was 65.32 %). These values are somewhat higher than those found in previous studies in responders and nonresponders (approximately 60 %) [25, 28–30], and the previous studies also showed a good overall response to neoadjuvant treatment.
We conclude that 18F-FDG PET/CT is a reliable technique for evaluating response to neoadjuvant therapy in locally advanced rectal cancer, with a view to considering more conservative surgical treatment, although more precise studies are needed to support these results. Furthermore, the combination of the visual and semiquantitative analysis of the PET data increases the diagnostic validity of the examination. Our patients also responded very satisfactorily to neoadjuvant treatment, according to the mean RI.
References
Booset JF, Collette L, Calais G, Minuer L, Maigon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23.
De Paoli A, Chiara S, Luppi G, Friso ML, Beretta GD, Del Prete S, et al. Capecitabine in combination with preoperative radiation therapy in locally advanced, resectable, rectal cancer: a multicentric phase II study. Ann Oncol. 2006;17:246–51.
Graf W, Dahlberg M, Osman MM, Holberg L, Palhlman L, Glimelius B. Short-term preoperative radiotherapy results in down-staging of rectal cancer: a study of 1316 patients. Radiother Oncol. 1997;43:133–7.
Janjan NA, Khoo VS, Rich TA, Evetts PA, Goswitz MS, Allen PK, et al. Locally advanced rectal cancer: surgical complications after infusional chemotherapy and radiation therapy. Radiology. 1998;206:131–6.
Peeters KC, Marijnen CA, Nagtegaal ID, Kranenborg EK, Putter H, Wiggers T, et al. The TNM trial after a median follow-up of 6 years: increased local control and survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701.
Read TE, McNevin MS, Gross EKM, Whiteford HM, Lewis JL, Ratkin G, et al. Neoadjuvant therapy for adenocarcinoma of the rectum: tumor response and acute toxicity. Dis Colon Rectum. 2001;44:513–22.
Crane CH, Skibber JM, Feig BW, Vauthey JN, Thames HD, Curley SA, et al. Response to preoperative chemoradiation increases the use of sphincter-preserving surgery in patients with locally advanced carcinoma. Cancer. 2003;97:517–24.
Moore HG, Gittleman AE, Minsky BD, Wong D, Paty PB, Weiser M, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47:279–86.
Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, et al. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:752–60.
Bujko K, Michalski W, Kepka L, Nowacki MP, Nasierowxka-Guttmejer A, Tokar P, et al. Association between pathologic response in metastatic lymph nodes after preoperative chemoradiotherapy and risk of distant metastases in rectal cancer: an analysis of outcomes in randomized trial. Int J Radiat Oncol Biol Phys. 2007;67:369–77.
Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro Jr U, Silva e Sousa Jr AH, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–7. discussion 717–8.
Habr-Gama A, Perez RO. Non-operative management of rectal cancer after neoadjuvant chemoradiation. Br J Surg. 2009;96:125–7.
Bujko K, Sopylot R, Kepka L. Local excision after radiochemotherapy for rectal cancer: is it safe? Clin Oncol. 2007;19:693–700.
Lezoche E, Guerrieri M, Paganini AM, et al. Long term results in patients with T2-3 N0 distal rectal cancer undergoing radiotherapy before transanal endoscopic microsurgery. Br J Surg. 2005;92:1546–52.
Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum. 2005;48:722–8.
Rau B, Hunerbein M, Barth C, et al. Accuracy of endorectal ultrasound after preoperative radiochemotherapy in locally advanced rectal cancer. Surg Endosc. 1999;13:980–4.
Guillem JG, Puig-La Calle Jr J, Akhurst T, Tickoo S, Ruo L, Minsky BD, et al. Prospective assessment of primary rectal cancer response to preoperative radiation and chemotherapy using 18-fluorodeoxyglucose positron emission tomography. Dis Colon Rectum. 2000;43:18–24.
Capirci C, Rubello D, Chierichetti F, Crepaldi G, Carpi A, Nicolini A, et al. Restaging after neoadjuvant chemoradiotherapy for rectal adenocarcinoma: role of F18-FDG PET. Biomed Pharmacother. 2004;58:451–7.
Kristiansen C, Loft A, Berthelsen AK, Graff J, Lindebjerg J, Bisgaard C, et al. PET/CT and histopathologic response to preoperative chemoradiation therapy in locally advanced rectal cancer. Dis Colon Rectum. 2008;51:21–5.
Koike I, Ohmura M, Hata M, Takahashi N, Oka T, Ogino I, et al. FDG-PET scanning after radiation can predict tumor regrowth three months later. Int J Radiat Oncol Biol Phys. 2003;57:1231–8.
Maas M, Rutten JG, Nelemans PJ, Lambregts D, Cappendijk V, Beets G, et al. What is the most accurate whole-body imaging modality for assessment of local and distant recurrent disease in colorectal cancer? A meta-analysis. Eur J Nucl Med Mol Imaging. 2011;38:1560–71.
Mandard AM, Dalibard F, Madard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiation therapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–6.
Dhadda AS, Dickinson P, Zaitoun AM, Gandhi N, Bessell EM. Prognostic importance of Mandard tumour regression grade following pre-operative chemo/radiotherapy for locally advanced rectal cancer. Eur J Cancer. 2011;47:1138–45.
Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–7.
Capirci C, Rampin L, Erba PA, Galeotti F, Crepaldi G, Banti E, et al. Sequential FDG-PET/CT reliability predicts response of locally advanced rectal cancer to neoadjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging. 2007;34:1583–93.
Kalff V, Duong C, Drummond EG, Matthews JP, Hicks RJ. Findings on 18F-FDG PET scans after neoadjuvant chemoradiation provides prognostic stratification in patients with locally advanced rectal carcinoma subsequently treated by radical surgery. J Nucl Med. 2006;47:14–22.
Cascini GL, Avallone A, Delrio P, Guida C, Tatangelo F, Marone P, et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med. 2006;47:1241–8.
Capirci C, Rubello D, Pasini F, Galeotti F, Bianchini E, Del Favero G, et al. The role of dual-time combined 18-fluorodeoxyglucose positron emission tomography and computed tomography in the staging and restaging workup of locally advanced rectal cancer, treated with preoperative chemoradiation therapy and radical surgery. Int J Radiat Oncol Biol Phys. 2009;74:1461–9.
Sánchez R. Utilidad del estudio 18F-FDG PET/TAC en la valoración de la terapia neoadyuvante en el cáncer de recto. Tesis Doctoral. Departamento de Radiología y Medicina Física. Universidad de Granada. 2009.
Melton GB, Lavely WC, Jacene HA, Schulick RD, Choti MA, Wahl RL, et al. Efficacy of preoperative combined 18-fluorodeoxyglucose positron emission tomography and computed tomography for assessing primary rectal cancer response to neoadjuvant therapy. J Gastrointest Surg. 2007;11:961–9.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murcia Duréndez, M.J., Frutos Esteban, L., Luján, J. et al. The value of 18F-FDG PET/CT for assessing the response to neoadjuvant therapy in locally advanced rectal cancer. Eur J Nucl Med Mol Imaging 40, 91–97 (2013). https://doi.org/10.1007/s00259-012-2257-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2257-y