Abstract
Purpose
In studies where [11C]raclopride (RAC) positron emission tomography (PET) is used to assess changes in striatal dopamine, it is important to control for cognitive states, such as drug craving, that could alter dopamine levels. In cigarette smokers, transdermal nicotine patches (TNP) can control nicotine craving, but the effects of nicotine patches on RAC binding are unknown. Thus, we sought to determine the test-retest reliability of RAC binding in the presence of nicotine patches.
Methods
Eleven male smokers were scanned twice with RAC on separate days while wearing TNP.
Results
Across the striatum, test-retest variability was 7.63 ± 5.88; percent change in binding potential was 1.11 ± 9.83; and the intraclass correlation coefficient was 0.91 (p < 0.0001).
Conclusion
Baseline RAC binding is highly reproducible in smokers wearing nicotine patches. This suggests that TNP are an acceptable method for controlling cigarette craving during studies that utilize RAC to examine changes in dopamine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is possible to assess in vivo changes in relative dopamine levels with positron emission tomography (PET) and dopaminergic radioligands that are sensitive to alterations in endogenous dopamine concentration [1–4] (e.g., [11C]raclopride, [18F]fallypride, and [11C]FLB). The goal of such studies is the comparison of dopamine D2 receptor availability during a test condition relative to a baseline or resting condition, with the difference in D2 signal between these two states attributed to changes in dopamine levels. There are many types of challenge paradigms (cognitive, motor, pharmacologic), methods of PET acquisition (single bolus, bolus plus infusion), and study designs (one or two scans). Regardless of the scientific question being asked, or the particulars of the methodological approach, all experiments share a critical assumption: within subjects, the baseline state represents a stable comparator for the challenge condition [2, 5]. Unfortunately, this assumption can be easily violated by transient changes in cognitive states that may alter dopamine [1, 2, 5]. When possible, it would be desirable to control internal states that may contribute to confounding measurements of D2 availability.
Several studies indicate that the internal state of drug craving is related to striatal dopamine levels [6–10]. For example, both Volkow et al. [6] and Wong et al. [7] presented cocaine users with cocaine-related cues and found that cue-induced striatal dopamine release correlated with self-reported cue-induced craving. This evidence strongly supports the concept that increases in striatal dopamine levels are related to drug craving. In 2004, Heinz and colleagues [10] reported decreased D2 availability in the ventral striatum was negatively correlated with alcohol craving severity. It is reasonable to view lower D2 availability as a function of higher dopamine levels, and therefore this report is consistent with the idea that higher dopamine concentration may be associated with drug craving. On the other hand, Brody et al. [8, 9] reported that smoking-induced reductions in nondisplaceable binding potential (BPND) were associated with decreased craving for cigarettes and proposed that increases in dopamine alleviate cigarette craving. In this case, however, the presence of chemosensory sensations and habitual motor routines of cigarette smoking may have increased dopamine independently of craving. Thus, in studies of populations with high rates of cigarette smoking, differences in nicotine withdrawal, and hence craving between scan states, present a serious challenge for data interpretation. Although transdermal nicotine patches could be used to control nicotine craving in smokers, it is not known whether stable baseline measurements of D2 availability are possible with nicotine patches. Therefore, the purpose of this study was to determine the reliability of striatal [11C]raclopride (RAC) binding in the presence of transdermal nicotine patches in nicotine-dependent cigarette smokers.
Materials and methods
All procedures were approved by the Indiana University Institutional Review Board and performed in accordance with the ethical standards of the Belmont Report (US Department of Health and Human Services, 1979). All subjects signed informed consent statements agreeing to participate in the study. Subjects were right-handed, social drinking male smokers who were in otherwise good physical and mental health. The absence of either alcohol abuse or dependence was confirmed by the Semi-Structured Assessment for the Genetics of Alcoholism [11]. Subjects were excluded from participation if they endorsed recreational use of legal or illicit stimulants, painkillers, or sedatives, and/or consumption of > 1 marijuana cigarette (or equivalent) per week. Subject demographics are presented in Table 1. Nicotine dependence was assessed with the Fagerström Test for Nicotine Dependence [12].
Study procedures
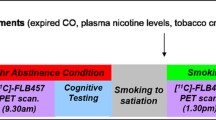
Procedures in this study are similar to those described previously [13]. Subjects underwent identical procedures on 2 separate days. Figure 1 illustrates the general time line. Briefly, subjects presented to the Indiana Clinical Research Center at approximately 8 a.m. Shortly after arrival, an IV catheter was placed in an antecubital vein, and a transdermal nicotine patch was placed on the upper arm of each subject. Patch dose was based on subjects’ self-report of cigarettes smoked per day (14 mg dose with 10 < 20 cigarettes per day; 21 mg dose with > 20 cigarettes). Two subjects were given a 14-mg patch; all others received a 21-mg patch. Subjects were given a full breakfast. As part of another study protocol, the morning of each study day, subjects received an IV alcohol infusion to a target breath alcohol concentration (BrAC) of 60 mg% using the alcohol clamp method. The rationale and implementation of the alcohol clamping technique have been described in detail elsewhere [14, 15]. The BrAC of all subjects returned to 0 mg% prior to scanning. The Cigarette Withdrawal Scale (CWS) [16], a self-report Likert rating scale, was given periodically throughout each study day. Nicotine craving was measured with the second dimension on the CWS, which specifically captures the individual’s current subjective state of cigarette craving. There are four questions on this dimension, each with a 5-point scale; possible scores for cigarette craving range from 4 to 20. Ratings were taken upon arrival for the study (time 1), and before and after the resting (baseline) scan (times 2 and 3). Eleven subjects completed both baseline RAC scans. A 12th subject voluntarily withdrew from the study after the first RAC scan.
Scanning and reconstruction procedures
A magnetization prepared rapid acquisition gradient echo (MP-RAGE) magnetic resonance image (MRI) was acquired on all subjects using a Siemens 3T Trio for anatomic coregistration of PET data (see “Image processing procedures”).
Subjects received two baseline RAC scans in the early afternoon on 2 separate days. Time of injection was typically between 14:00 and 15:00. Breath alcohol concentration was 0 mg% prior to scanning. The time between the end of the morning alcohol infusion and the baseline RAC scan was typically ∼ 4 h. RAC synthesis was completed as described previously [17]. RAC PET scans were acquired on a Siemens EXACT HR+ (3-D mode; septa retracted). Prior to each PET scan, a 10-min transmission scan using three internal rod sources was acquired for attenuation correction. RAC PET scans were initiated with the IV infusion of 535 ± 45.4 MBq RAC (mass dose 0.13 ± 0.06 nmol/kg) over 1.5 min. Dynamic acquisition occurred for 50 min (time frames: 10 × 30 s, 45 × 60 s). Dynamic PET images were generated using Siemens Fourier rebinning (FORE) and filtered backprojection algorithms including corrections for attenuation, randoms, scatter, and dead time.
Image processing procedures
Image processing is similar to that described previously [13, 18]. MRI Digital Imaging and Communications in Medicine (DICOM) and RAC PET images were converted to Neuroimaging Informatics Technology Initiative (NIfTI) format (http://nifti.nimh.nih.gov/) using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). For each subject, dynamic PET data were coregistered to an early-time mean image to facilitate motion correction. The early mean PET image was coregistered to the MRI scan using the normalized mutual information algorithm in SPM5, with the transformation matrix from this coregistration subsequently applied to the motion-corrected dynamic PET data. Each subject’s MRI was spatially normalized to Montreal Neurological Institute (MNI) space. The transformation matrix obtained from the spatial normalization step was then applied to the motion-corrected, MRI-registered PET data from each subject.
All regions of interest (ROIs) were drawn on an average normalized MRI from all subjects, using MRIcron (http://www.sph.sc.edu/comd/rorden/mricron/). Striatal ROIs consisted of the left and right ventral striatum, precommissural dorsal caudate, precommissural dorsal putamen, postcommissural caudate, and postcommissural putamen and were drawn according to specific anatomic landmarks [19, 20]. For the reference region (tissue that contains little to no D2/D3 receptor density), an ROI was created that contained all cerebellar gray matter except for the vermis. Time-activity curves for each ROI were generated from the dynamic RAC data using the MarsBaR toolbox for SPM5 (http://marsbar.sourceforge.net/). For each striatal ROI, D2/D3 receptor availability was indexed with BPND, the binding potential of RAC calculated as bound tracer concentration relative to nondisplaceable tracer concentration [21]. Estimations of BPND were conducted using the multilinear reference tissue model (MRTM) [22].
Metrics of test-retest reproducibility of baseline RAC binding
The relative reproducibility of striatal BPND between day 1 and day 2 was examined with three calculations, test-retest variability (TRV) to assess relative variation in BPND between days as a function of the overall average BPND across days, percent change in BPND (∆BPND) as a qualitative descriptor of differences in BPND between day 1 and day 2, and the intraclass correlation coefficient (ICC, one-way random effects model; as implemented in the PASW statistical package [23, 24]), a metric of similarity between measurements on each day. TRV was calculated as: |BPday1−BPday2|/[(BPday1 + BPday2)/2] [25, 26]. ∆BP between day 1 and day 2 was calculated as: [(BPday1−BPday2)/BPday1] × 100. Paired t tests were used to determine if striatal BPND was significantly different between scan days.
Other statistical tests
Independent t tests were used to test for differences in injected radioactivity and injected mass dose between scan days. To examine the stability of cigarette craving (CWS dimension 2 score), repeated-measures analysis of variance (ANOVA) (2 days × 3 time points) was used to test for effects of scan day, time point, and day × time point.
Results
RAC scan parameters
Average number of days between scans was 11.7 ± 25.2 (range 1–83 days). Injected radioactivity of RAC on day 1 and day 2 was 537 ± 54.7 and 533 ± 36.5 MBq, respectively. Corresponding mass doses were 0.13 ± 0.05 and 0.13 ± 0.06 nmol/kg. Injected radioactivity and mass doses were not significantly different between scan days.
Subject data
The demographic characteristics of the subjects are shown in Table 1. Eight subjects reported smoking a full pack of cigarettes per day; two reported a half-pack, and one reported two packs per day. Three subjects tested positive for marijuana on both scan days; these subjects endorsed sporadic recreational use of marijuana within the previous month. One subject tested positive for opiates on day 2 (subject had undergone a dental procedure and reported taking a single tablet of acetaminophen and No. 3 codeine phosphate 2 days prior). Two of these subjects had TRV for striatal RAC binding well within 1 SD of the sample average (7.6 ± 5.9%; Table 2); the other was within 1.5 SD of the mean TRV.
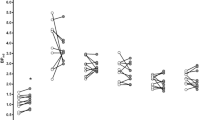
Exact timing of patch placement was not available for one subject on day 1. Across the remaining data points (n = 21), the interval between patch placement and resting scan was 5.9 ± 0.9 h. Ratings for the CWS were unavailable for one subject at both day 1-time 2 and day 2-time 3; for one subject at day 2- time 2; and for one subject at day 2-time 3. Cigarette craving scores are presented in Fig. 2. Repeated-measures ANOVA indicated that the CWS rating was stable within subjects, i.e., there were no main effects of day or time point, and no day × time point interaction.
Mean ± SD cigarette craving ratings from day 1 (filled squares) and day 2 (open circles), and times 1, 2, and 3 (please see Fig. 1 for approximate timing of ratings). The x-axis crosses the y-axis at the value of 4 to denote that 4 is the lowest possible score on the index; for reference, 20 is the highest possible score. Craving ratings did not vary within subjects across either day or time point (see text for details)
Test-retest reproducibility of resting RAC signal
BPND values for both scan days, percent change of BPND between days (%∆), the TRV, and ICC are presented in Table 2.
Discussion
In the current sample of 11 otherwise healthy cigarette smokers, there was good reproducibility of striatal RAC binding in the presence of transdermal nicotine patches. Overall, our test-retest metrics (Table 2) comport well with literature values for single-bolus RAC studies in healthy control subjects [25, 27–29]. The average striatal data were exceptionally stable, with slight variations in reliability between subregions. We chose to demonstrate test-retest reliability with three methods. The percent change in BPND between scans is the most commonly used parameter of effect size and corresponds to the calculation most commonly used to describe relative changes in dopamine levels between conditions. TRV quantifies the size of absolute variability across measurements. Both indices provide useful estimates of the effect sizes needed to achieve statistical significance with a two-scan dopamine challenge paradigm (although statistical significance is possible with smaller effect sizes). In this study, these assessments of RAC test-retest reliability suggest a range of variability consistent with what others have reported when scanning the same individuals across days. Overall, the ICC coefficient r values in this study were also quite good and indicate that estimations of BPND on day 1 correlated well with measurements on day 2. Lower ICC values were found in small regions that tend to have slightly noisier time-activity curves and hence more variable estimates of BPND.
One potential concern about the use of nicotine patches is the possibility that nicotine itself might cause measurable dopamine release [30, 31]. Two human RAC studies by Brody et al. support this view [8, 9]. However, those designs included the actual physical act of smoking cigarettes. When nicotine is delivered intranasally to humans, or intravenously to unanesthetized monkeys, there is no evidence of significant decreases in RAC binding [32, 33]. Taken together, these latter studies strongly suggest that nicotine itself does not release dopamine to levels measurable by RAC PET. It is also possible that the physical and sensory properties associated with cigarette smoking are the key components of the smoking-induced dopamine release reported by Brody et al.
In this study, the administration of IV alcohol to subjects the morning of the RAC PET study may be an unintended source of variance in baseline RAC BPND. In our previous work, we found no evidence of alcohol-induced dopamine release in social drinkers [34, 35]. However, healthy social drinkers do not typically expose themselves to alcohol shortly after breakfast. We cannot exclude the possibility that alcohol exposure early in the morning may have caused unpredictable changes in the dopamine tone of healthy social drinkers.
Another limitation of this study was the absence of test-retest values for striatal RAC binding in smokers without nicotine patches, which could have assessed any variability in baseline striatal BPND attributable to nicotine withdrawal. However, the most important assumption in a typical dopaminergic RAC PET challenge paradigm is that the within-subject state of basal dopamine is stable. The present data demonstrate that it is possible to control nicotine craving (which is highly likely to alter endogenous dopamine) while keeping estimates of baseline D2 availability stable.
Conclusion
The presence of transdermal nicotine patches does not appear to affect the stability of baseline RAC binding potential. We suggest that, in RAC challenge studies, the use of nicotine patches in smoking subjects is feasible to eliminate unwanted variance in D2 availability caused by nicotine craving and concomitant alterations in striatal dopamine levels.
References
Egerton A, Mehta MA, Montgomery AJ, Lappin JM, Howes OD, Reeves SJ, et al. The dopaminergic basis of human behaviors: a review of molecular imaging studies. Neurosci Biobehav Rev 2009;33:1109–32. doi:10.1016/j.neubiorev.2009.05.005.
Yoder KK, Kareken DA, Morris ED. Assessing dopaminergic neurotransmission with PET: basic theory and applications in alcohol research. Curr Med Imaging Rev 2011;7:118–24.
Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 2000;20:423–51.
Morris ED, Yoder KK. Positron emission tomography displacement sensitivity: predicting binding potential change for positron emission tomography tracers based on their kinetic characteristics. J Cereb Blood Flow Metab 2007;27:606–17. doi:10.1038/sj.jcbfm.9600359.
Yoder KK, Kareken DA, Morris ED. What were they thinking? Cognitive states may influence [(11)C]raclopride binding potential in the striatum. Neurosci Lett 2008;430:38–42.
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 2006;26:6583–8.
Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 2006;31:2716–27.
Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, et al. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry 2006;63:808–16.
Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, et al. Smoking-induced ventral striatum dopamine release. Am J Psychiatry 2004;161:1211–8.
Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser-Sinopoli SM, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry 2004;161:1783–9.
Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger Jr JI, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol 1994;55:149–58.
Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerstrom Tolerance Questionnaire and the Fagerstrom Test for Nicotine Dependence. Addict Behav 1994;19:33–9.
Yoder KK, Albrecht DS, Kareken DA, Federici LM, Perry KM, Patton EA, et al. Test-retest variability of [(11)C]raclopride-binding potential in nontreatment-seeking alcoholics. Synapse 2011;65:553–61. doi:10.1002/syn.20874.
Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res 1999;23:617–23.
O’Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res 1998;22:202–10.
Etter JF. A self-administered questionnaire to measure cigarette withdrawal symptoms: the Cigarette Withdrawal Scale. Nicotine Tob Res 2005;7:47–57. doi:10.1080/14622200412331328501.
Fei X, Mock BH, DeGrado TR, Wang JQ, Glick-Wilson BE, Sullivan ML, et al. An improved synthesis of PET dopamine D2 receptors radioligand [11C]raclopride. Synth Commun 2004;34:1897–907.
Yoder KK, Morris ED, Constantinescu CC, Cheng TE, Normandin MD, O’Connor SJ, et al. When what you see isn’t what you get: alcohol cues, alcohol administration, prediction error, and human striatal dopamine. Alcohol Clin Exp Res 2009;33:139–49. doi:10.1111/j.1530-0277.2008.00821.x.
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 2003;23:285–300.
Mai JK, Assheuer J, Paxinos G. Atlas of the human brain. San Diego: Academic; 1997.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007;27:1533–9. doi:10.1038/sj.jcbfm.9600493.
Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, et al. Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 2003;23:1096–112. doi:10.1097/01.WCB.0000085441.37552.CA.
McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods 1996;1:30–46.
Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86:420–8.
Hirvonen J, Aalto S, Lumme V, Någren K, Kajander J, Vilkman H, et al. Measurement of striatal and thalamic dopamine D2 receptor binding with 11C-raclopride. Nucl Med Commun 2003;24:1207–14. doi:10.1097/01.mnm.0000104642.79626.e8.
Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 2001;21:1034–57.
Volkow ND, Fowler JS, Wang GJ, Dewey SL, Schlyer D, MacGregor R, et al. Reproducibility of repeated measures of carbon-11-raclopride binding in the human brain. J Nucl Med 1993;34:609–13.
Hietala J, Någren K, Lehikoinen P, Ruotsalainen U, Syvälahti E. Measurement of striatal D2 dopamine receptor density and affinity with [11C]-raclopride in vivo: a test-retest analysis. J Cereb Blood Flow Metab 1999;19:210–7.
Schlösser R, Brodie JD, Dewey SL, Alexoff D, Wang GJ, Fowler JS, et al. Long-term stability of neurotransmitter activity investigated with 11C-raclopride PET. Synapse 1998;28:66–70.
Nisell M, Nomikos GG, Svensson TH. Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol 1994;75:348–52.
Schiffer WK, Gerasimov MR, Marsteller DA, Geiger J, Barnett C, Alexoff DL, et al. Topiramate selectively attenuates nicotine-induced increases in monoamine release. Synapse 2001;42:196–8. doi:10.1002/syn.10000.
Montgomery AJ, Lingford-Hughes AR, Egerton A, Nutt DJ, Grasby PM. The effect of nicotine on striatal dopamine release in man: a [11C]raclopride PET study. Synapse 2007;61:637–45.
Tsukada H, Miyasato K, Kakiuchi T, Nishiyama S, Harada N, Domino EF. Comparative effects of methamphetamine and nicotine on the striatal [(11)C]raclopride binding in unanesthetized monkeys. Synapse 2002;45:207–12.
Yoder KK, Kareken DA, Seyoum RA, O’Connor SJ, Wang C, Zheng QH, et al. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res 2005;29:965–70.
Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O’Connor SJ, et al. Heterogeneous effects of alcohol on dopamine release in the striatum: a PET study. Alcohol Clin Exp Res 2007;31:965–73.
Acknowledgments
This work was supported by: ABMRF/The Foundation for Alcohol Research (KKY), National Institute on Alcoholism and Alcohol Abuse P60 AA007611 Pilot 50 (KKY), and the Indiana Clinical and Translational Sciences Institute (from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award, grant #UL RR025761). The authors would like to thank Barbara Glick and Brandon Steele for assistance with radiochemistry.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoder, K.K., Albrecht, D.S., Kareken, D.A. et al. Reliability of striatal [11C]raclopride binding in smokers wearing transdermal nicotine patches. Eur J Nucl Med Mol Imaging 39, 220–225 (2012). https://doi.org/10.1007/s00259-011-1965-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1965-z