Abstract
Purpose
To analyse different uptake patterns in 123I-MIBG scintigraphy/SPECT imaging and 18F-FDG PET in paediatric neuroblastoma patients.
Methods
We compared 23 123I-MIBG scintigraphy scans and 23 18F-FDG PET scans (mean interval 10 days) in 19 patients with a suspected neuroblastic tumour (16 neuroblastoma, 1 ganglioneuroblastoma, 1 ganglioneuroma and 1 opsomyoclonus syndrome). SPECT images of the abdomen or other tumour-affected regions were available in all patients. Indications for 18F-FDG PET were a 123I-MIBG-negative tumour, a discrepancy in 123I-MIBG uptake compared to the morphological imaging or imaging results inconsistent with clinical findings. A lesion was found by 123I-MIBG scintigraphy and/or 18F-FDG PET and/or morphological imaging.
Results
A total of 58 suspicious lesions (mean lesion diameter 3.8 cm) were evaluated and 18 were confirmed by histology and 40 by clinical follow-up. The sensitivities of 123I-MIBG scintigraphy and 18F-FDG PET were 50% and 78% and the specificities were 75% and 92%, respectively. False-positive results (three 123I-MIBG scintigraphy, one 18F-FDG PET) were due to physiological uptake or posttherapy changes. False-negative results (23 123I-MIBG scintigraphy, 10 18F-FDG PET) were due to low uptake and small lesion size. Combined 123I-MIBG scintigraphy/18F-FDG PET imaging showed the highest sensitivity of 85%. In 34 lesions the 123I-MIBG scintigraphy and morphological imaging findings were discrepant. 18F-FDG PET correctly identified 32 of the discrepant findings. Two bone/bone marrow metastases were missed by 18F-FDG PET.
Conclusion
123I-MIBG scintigraphy and 18F-FDG PET showed noticeable differences in their uptake patterns. 18F-FDG PET was more sensitive and specific for the detection of neuroblastoma lesions. Our findings suggest that a 18F-FDG PET scan may be useful in the event of discrepant or inconclusive findings on 123I-MIBG scintigraphy/SPECT and morphological imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neuroblastoma accounts for about 8% of paediatric malignancies and is responsible for approximately 15% of cancer deaths in children [1, 2]. At diagnosis, roughly 50% of the patients have distant metastases [3]. The 5-year event-free survival rate strongly depends upon age, molecular markers and the stage of disease [4]. It ranges from 99% in stage I to about 45% in stage IV disease [5, 6]. Therefore staging is crucial for appropriate treatment [7].
Due to its neuroendocrine origin, the malignancy takes up catecholamines and related substances. The catecholamine analogue 123I-meta-iodobenzylguanidine (123I-MIBG) is widely used to image tumours of the sympathetic nervous system and 123I-MIBG scintigraphy is well established for staging and evaluation of therapeutic response [8, 9]. However, the assessment of MIBG scans presents some difficulties: false-negative MIBG scans were reported as early as 1990 [10], and false-negative 123I-MIBG scintigraphy is still a problem, and may lead to incorrect down-staging [7]. In about 8% of neuroblastoma patients MIBG scintigraphy gives a false-negative result at diagnosis, even though there is clear evidence of disease [11]. In particular, MIBG scintigraphy gives a false result concerning bone marrow involvement [12]. The coexistence of hot and cold 123I-MIBG lesions still remains unclear.
Positron emission tomography with 18F-fluorodeoxyglucose (18F-FDG PET) depicts the metabolic state of cancer cells and provides information on the malignant potential of the disease [13]. 18F-FDG PET findings correlate well with the disease status [14]. Nevertheless, 18F-FDG PET has rarely been used in the context of neuroblastic tumours. Neuroblastomas concentrate 18F-FDG before cytoreductive therapy, whereas intra- and posttherapeutic uptake is variable [13]. Sharp et al. found 18F-FDG PET to be useful in low stage neuroblastoma in patients with tumours that weakly accumulate 123I-MIBG and at major decision points during therapy [15].
The aim of this retrospective study was to investigate the usefulness of 18F-FDG PET in a preselected population of patients with neuroblastoma and with inconclusive or inconsistent results on 123I-MIBG scintigraphy/SPECT imaging and morphological imaging.
Materials and methods
Search algorithm
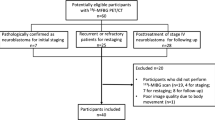
From July 2004 to July 2010, 245 123I-MIBG scintigraphy scans were performed in 108 patients with proven or suspected neuroblastic tumours, and 42 18F-FDG PET scans were performed in the same group during the same time period. 18F-FDG PET was performed if there was a 123I-MIBG-negative tumour, a discrepancy in the 123I-MIBG uptake compared to morphological imaging, or results inconsistent with the clinical findings. The inclusion criterion for the study was a maximum period of 30 days (mean 10 days, range 0–30 days) between 123I-MIBG scintigraphy, 18F-FDG PET and morphological imaging. If there was no coincident morphological study available, the patient was excluded. Patients with a proven diagnosis other than neuroblastoma, ganglioneuroblastoma, or ganglioneuroma were excluded. Examinations were performed either before or at least 6 weeks after chemotherapy. Patients were excluded if there had been any tumour-specific therapy between 123I-MIBG scintigraphy and 18F-FDG PET. Histopathology and/or clinical follow-up data for each lesion had to be available. Thus, 23 instances in 19 patients met the inclusion criteria.
Patients
Simultaneously 23 123I-MIBG scintigraphy scans and 23 18F-FDG PET scans were performed in 19 paediatric patients (ten male and nine female; mean age 5 years 11 months; age range 8 months to 19 years 1 month) and analysed retrospectively. Six combined 123I-MIBG scintigraphy scans and 18F-FDG PET scans were performed for initial staging and 17 during follow-up. Of the 19 patients, 16 had histologically proven neuroblastoma, 1 ganglioneuroblastoma, 1 ganglioneuroma and 1 opsomyoclonus syndrome. According to the International Neuroblastoma Staging System [16], patients were classified as stage I (3 patients), stage IIA (1), stage III (3) and stage IV (11). The patient with opsomyoclonus syndrome was not categorized. Concerning bone and bone marrow involvement, 123I-MIBG scintigraphy scans were ranked according to the SIOPEN classification system [17]. The skeleton was divided into 12 anatomical sections and a score from 0 to 6 was assigned to each section: score 0 (17 scans), 1 (2 scans), 2 (1 scan), 4 (1 scan), 5 (1 scan) and 19 (1 scan). In molecular testing, three neuroblastomas showed MYCN oncogene amplification and 1p deletion, 14 tumours were MYCN-negative and in one patient the MYCN amplification status was unknown.
Acquisition protocol
123I-MIBG scans were performed under thyroid blockade with perchlorate over 3 days, starting 30–60 min before administration of 123I-MIBG at a dose of 10 mg/kg per day. The administered dose of 123I-MIBG was adapted to body weight according to the dosage card of the EANM [18]. A dual-head gamma camera (Prism 2000 XP; Philips Medical Systems, Best, The Netherlands) was used with a medium-energy collimator. Imaging was performed 24 h after slow intravenous injection of 123I-labelled MIBG (spot images of the whole body, matrix 256 × 256 pixels, dorsal/ventral view, maximum acquisition time per image 10 min; above 120 cm body size whole-body scan with a table feed of 6 cm/min). In all patients, SPECT images of the abdomen or other tumour-affected regions were available (3° steps, 2 × 180°; 15 s per step; matrix 128 × 128 pixels).
18F-FDG PET scans were acquired with a Philips Allegro PET scanner. All patients fasted for at least 4 h and blood glucose levels were determined to monitor for deviation from normal levels prior to 18F-FDG PET imaging. Furosemide and butylscopolamine were administrated to minimize physiological activity in the bowel and bladder. 18F-FDG was injected intravenously 1 h before scanning at a dose according to body weight and the guidelines of the EANM [19], and the patient was instructed to rest until the beginning of the examination. PET was performed using three to ten bed positions (depending on the size of the patient) with a 2-min acquisition time per bed position. Images were attenuation-corrected and were acquired in 3-D mode using a row action maximum likelihood algorithm (3D RAMLA). The examination field-of-view was the whole body. To avoid motion artefacts, sedation was necessary in 6 of the 23 examinations.
Image analysis
Lesion-based image analysis was performed. A lesion was included in the analysis if it was considered positive for neuroblastoma involvement on 123I-MIBG scintigraphy, 18F-FDG PET or morphological imaging. Individual data analyses of both nuclear medicine modalities were performed by three independent observers, two for the 123I-MIBG imaging and one for the 18F-FDG PET. Images were analysed with knowledge of the clinical data, but without knowledge of the findings of the other imaging modality. To determine whether regression or disappearance of lesions was evident on follow-up examinations, observers needed to know the findings from the previous examination of the same modality. Combined assessment of both modalities was performed by the same three observers after the separate analyses.
123I-MIBG scans and 18F-FDG PET images were reviewed on a HERMES workstation (Nuclear Diagnostics, Haegersten, Sweden). After combined 123I-MIBG scintigraphy/18F-FDG PET evaluation, the same three observers compared the findings with morphological imaging (18 MRI scans, 5 CT scans).
A total of 58 lesions were evaluated. Two patients had several examination pairs; hence 12 lesions were reanalysed in at least one (up to three) follow-up examinations. A lesion was classified a “primary tumour” if it occurred in the adrenal gland, the sympathetic trunk or local recurrence of the original tumour was found. All other lesions were considered “metastases”. In both modalities, each lesion was judged either positive or negative with regard to tumour involvement. On the 123I-MIBG scintigraphy scans, a lesion was judged positive if nonphysiological focal uptake was seen. For semiquantitative analysis, the maximum count rate of each lesion was noted, and divided by the maximum count rate of the patient’s right liver lobe. This count rate ratio (CRR) was used to establish a diagnostic confidence score. On the 18F-FDG PET scans, a lesion was judged positive if it showed nonphysiological glucose metabolism. For the diagnostic confidence score, the maximum standardized uptake value (SUVmax) of each lesion was determined.
To reach a decision on lesions with discrepant results in the combined image analysis, a diagnostic confidence score with three levels was established for each modality:
-
1.
123I-MIBG scintigraphy (CRR <1), 18F-FDG PET (SUVmax <1)
-
2.
123I-MIBG scintigraphy (CRR 1–1.5), 18F-FDG PET (SUVmax 1–2)
-
3.
123I-MIBG scintigraphy (CRR >1.5), 18F-FDG PET (SUVmax >2)
This diagnostic confidence score was assigned to each suspicious lesion on 123I-MIBG scintigraphy and 18F-FDG PET scans separately. If there was a discrepancy between the two modalities, the finding of the modality with the higher diagnostic confidence level was used. If both modalities were discrepant and revealed the same score, the lesion was judged positive.
Standard of reference
The standard of reference was the histopathological findings of the lesion (18 lesions) or by follow-up evaluation (40 lesions). In particular, for patients with stage IV neuroblastoma, histopathological verification of all metastases is impossible. Therefore, follow-up examinations after a minimum period of 6 months were used for verification of the lesions: MRI (20 lesions) or CT (3 lesions) and/or 123I-MIBG scintigraphy (17 lesions) and/or 18F-FDG PET (12 lesions). A lesion was classified as either “false-positive” or “true-negative” if it disappeared without tumour therapy during the observation period. A lesion was classified as either “true-positive” or “false-negative” if it persisted or progressed during follow-up, or showed objective regression with specific therapy.
Results
A total of 58 lesions were evaluated, of which 46 (79%) proved to be vital tumour tissue. The anatomical lesion sites are shown in Table 1, and the morphological diameters (mean lesion diameter 3.8 cm) and the lesion distribution according to the stage of disease are presented in Table 2. The lesion-based results are summarized in Table 3.
123I-MIBG scintigraphy
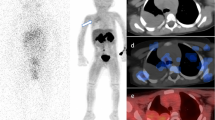
123I-MIBG scintigraphy showed false-positive findings in three patients due to physiological activity in a normal adrenal gland (two lesions; Fig. 1) and physiological bowel uptake (one lesion). They were found in patients with stage III disease (lesion diameter in the central abdomen >5 cm) and stage IV disease (lesion diameter in the adrenal gland <1 cm) and in the patient with opsomyoclonus syndrome (lesion diameter in the adrenal gland 1–2 cm).
A 1½-year old girl with neuroblastoma at the follow-up evaluation 3 months after the end of chemotherapy with a false-positive finding on 123I-MIBG scintigraphy. 123I-MIBG scintigraphy images show a suspect lesion in the region of the left adrenal gland (a–c). The corresponding 18F-FDG PET images are true-negative with no increased uptake (d–f), indicating that the changes seen on 123I-MIBG scintigraphy are physiological
False-negative results (23 lesions; mean lesion diameter 1.7 cm) occurred in bone/bone marrow metastases in the vertebral column (five lesions), pelvis (four lesions), femur (seven lesions), paravertebral region (three lesions), and adrenal region (four lesions; Fig. 2). The 23 false-negative findings are shown in relation to disease stage in Table 4.
18F-FDG PET
On 18F-FDG PET, there was one false-positive finding in a patient with stage IV disease. This was seen in the adrenal region and was due to posttherapy uptake (lesion diameter <1 cm). With regard to false-negative findings (ten lesions; mean lesion diameter 1.6 cm), the following neuroblastoma lesions were missed: adrenal gland tumour (two lesions), bone/bone marrow metastases in the tibia/femur (three lesions), vertebral column (one lesion), retrocrural region (one lesion), presacral region (one lesion), paravertebral region (one lesion), and thymus (one lesion). These lesions could not be differentiated from physiological uptake and occurred in one patient with stage IIA disease (thymus, lesion diameter 2–5 cm), in one patient with stage III disease (presacral region, 2–5 cm) and in patients with stage IV disease (two lesions, adrenal gland, 2–5 cm; two lesions, tibia/femur, <1 cm; one lesion, tibia/femur, 1–2 cm; one lesion, vertebral column, 1–2 cm; one lesion, retrocrural region, 1–2 cm; one lesion, paravertebral region, 2–5 cm).
MRI/CT
All 58 lesions were correlated morphologically with either MRI (52 lesions) or CT (6 lesions) scans. The nine following false-positive findings were obtained in patients with stage IV disease: region of the primary tumour in the adrenal gland (two lesions, lesion diameter <1 cm and 2–5 cm) due to posttherapy changes; bone/bone marrow involvement in the femur and pelvis (three lesions, lesion diameter <1 cm; one lesion, 1–2 cm; one lesion, 2–5 cm) and the vertebral column (two lesions, lesion diameter <1 cm and 2–5 cm) due to oedematous nonviable residual tumour. Three false-negative findings occurred in patients with stage IV disease. These were located in the femur (one) and the vertebral column (two) because of small tumour size (Fig. 3).
A 4-year-old boy at primary diagnosis of a left-sided neuroblastoma with a false-negative finding on MRI. On the MRI image (T2-weighted STIR sequence) suspect bone/bone marrow lesions were found in vertebral bodies L3 and L5. L1 (arrow) does not show pathological changes (a), whereas on the 18F-FDG PET images (b, c) and on the 123I-MIBG scintigraphy image (d) a suspicious lesion is apparent in L1. In addition, the primary tumour mass is demonstrated by all three imaging modalities
Comparison of 123I-MIBG scintigraphy and 18F-FDG PET
In 33 lesions, discrepancies between 123I-MIBG scintigraphy and 18F-FDG PET were found. In 29 of these 33 lesions, vital tumour lesions could only be detected by one modality (8 by 123I-MIBG scintigraphy, 21 by 18F-FDG PET; Fig. 4). Four of these 33 lesions were classified as true-negative by only one modality (one by 123I-MIBG scintigraphy, three by 18F-FDG PET).
A 2-year-old girl with a left-sided persistent neuroblastoma after surgery and chemotherapy and a false-negative finding on 18F-FDG PET. In the 18F-FDG PET images no suspicious lesion is seen in the region of the left adrenal gland (a–c). On the MRI image it is not possible to differentiate between posttherapy changes and viable tumour (d); however, on the 123I-MIBG scintigraphy image pathological uptake is seen (e–g) indicating persistent tumour. The lesion was confirmed histopathologically
Combining the two modalities, one false-positive and seven false-negative results remained. Physiological adrenal 123I-MIBG uptake was misinterpreted as residual vital tumour tissue after surgery in one patient. Seven false-negative findings were either due to a misinterpretation of viable tumour lesions as postoperative changes in the adrenal gland region (two lesions, lesion diameter 2–5 cm and >5 cm) or due to small size and/or low metabolic activity in bone marrow metastases in the vertebral column (one lesion, lesion diameter <1 cm), in the femur (two lesions, lesion diameter <1 cm). Two metastases were misinterpreted as physiological uptake: a retrocrural lymph node metastasis (lesion diameter 1–2 cm) and a metastasis in the thymus (lesion diameter 2–5 cm).
Comparison of 123I-MIBG scintigraphy and morphological imaging
The comparison of 123I-MIBG scintigraphy and morphological imaging modalities resulted in 34 discrepant findings (Table 5).
In the primary diagnosis, 12 discrepant results were found. In all 12 lesions, 18F-FDG PET was the modality that provided the correct evaluation (Fig. 5). In the follow-up, 123I-MIBG scintigraphy and morphology showed 22 discrepant findings. In 20 lesions 18F-FDG PET was the modality that provided the correct evaluation of the lesion.
A 3½-year-old boy at primary diagnosis of a stage IV neuroblastoma and discrepant findings on 123I-MIBG scan and morphological imaging. 123I-MIBG scintigraphy (a SPECT, b registered SPECT/MRI) only shows a slightly increased tracer uptake at the lower and lateral border of the tumour (arrows). However, 18F-FDG PET (c maximum intensity projection) and MRI (d) images demonstrate extended bulky disease and 18F-FDG hypermetabolism in the entire primary tumour
Two bone/bone marrow lesions in the proximal femur were incorrectly classified by both functional imaging modalities; there was no discrepancy between 123I-MIBG scintigraphy and 18F-FDG PET. These lesions were seen on the MRI images (lesion diameter <1 cm).
Discussion
The question as to whether the detection of neuroblastoma lesions that are weakly or not affine to 123I-MIBG can be improved by simultaneous 18F-FDG PET was investigated by this retrospective study. The utility of the simultaneous use of 18F-FDG PET and 123I-MIBG scintigraphy in children with neuroblastoma had already been assessed by Sharp et al. [15]. 18F-FDG PET was found to be superior to 123I-MIBG scintigraphy in children with stage I or II neuroblastoma, but inferior in patients with stage IV neuroblastoma, based on the International Neuroblastoma Staging System. 18F-FDG PET provided additional information in the chest, abdomen and pelvis, and depicted neuroblastomas which did not accumulate or only weakly accumulated 123I-MIBG. However, in patients with stage IV disease, 123I-MIBG scintigraphy was superior to 18F-FDG PET especially during initial chemotherapy [15]. In our study, false-positive and false-negative 18F-FDG PET and 123I-MIBG scintigraphy findings were distributed almost equally throughout all tumour stages. This may be due to the fact that patients were included in our study only if 123I-MIBG scintigraphy and 18F-FDG PET examinations had been performed before or at least 6 weeks after cytotoxic therapy. Shulkin et al. suggested that 18F-FDG PET was inferior to 123I-MIBG scintigraphy when used shortly after or during systematic therapy due to a lower tumour-to-nontumour uptake ratio and physiological 18F-FDG accumulation [13].
Lesion-based analysis
123I-MIBG scintigraphy showed an overall sensitivity of 50% and a specificity of 75% for the detection of neuroblastoma involvement. This is markedly lower than in a recently published multicentre trial and meta-analysis that confirmed a generally high sensitivity and specificity, mostly over 80% [20, 21]. These low values in our retrospective study are probably due to the fact that the patient population was preselected with a high number of negative or inconclusive findings on 123I-MIBG scintigraphy scans. In this patient population, 18F-FDG PET exhibited a notably higher sensitivity and specificity (78% and 92%, respectively) than 123I-MIBG scintigraphy.
Most false-positive 123I-MIBG scintigraphy findings are caused by nonspecific radioactive accumulation in the urinary tract and in gastrointestinal structures [22]. This was also the case in our study with false-positive uptake in the adrenal region and in the gastrointestinal tract. 18F-FDG PET helped to correctly categorize these wrongly classified lesions.
False-negative 123I-MIBG scintigraphy findings were mostly seen in bone and bone marrow, confirming the well-known fact that the sensitivity of 123I-MIBG scans is limited in the detection of single bone and bone marrow metastases [22]. The SIOPEN classification system for bone or bone marrow involvement [17] enables the evaluation and standardized comparison of metastatic disease detected on 123I-MIBG scans. Due to our patient selection with negative or inconclusive 123I-MIBG scans, only low score values were expected in the SIOPEN classification. This does not reflect a particular weakness of the classification system. However, in our setting with many bone/bone marrow lesions that were false-negative on123I-MIBG scintigraphy, the SIOPEN classification led to incorrect downstaging. Further false-negative lesions on 123I-MIBG scintigraphy were located in the adrenal gland and in the sympathetic trunk. Possible reasons for these false-negative findings have been discussed in the literature, and include the absence of a mechanism by which 123I-MIBG is transported into the tumour cells, small tumour size and the coexistence of various neoplastic clones with different uptake behaviour [11, 23].
18F-FDG PET has been reported to be equal or superior to 123I-MIBG scintigraphy for identifying neuroblastoma lesions in soft tissue and extracranial skeletal structures [24]. In our series, 18F-FDG PET correctly identified almost all false-negative lesions as vital tumour tissue and proved its utility especially in lesions negative on 123I-MIBG scintigraphy. Due to surrounding oedema, the two remaining false-negative lesions were shown on morphological imaging.
In false-negative findings, there was no significant difference between 123I-MIBG scintigraphy and 18F-FDG PET with regard to the morphological mean lesion diameter: 1.7 cm on 123I-MIBG scintigraphy, 1.6 cm on 18F-FDG PET. However, when looking at all 58 lesions, the mean morphological lesion diameter was 3.8 cm.
Primary diagnosis
At primary diagnosis, 18F-FDG PET missed one lesion in the bone marrow of the vertebral column. It correctly identified all lesions that were false-negative on 123I-MIBG scintigraphy. As initial staging requires highly sensitive imaging modalities, 18F-FDG PET proved to be a valuable additional staging examination in our study. Nevertheless, in a nonselected population of neuroblastoma patients, the sensitivity of 123I-MIBG scintigraphy in primary diagnosis is around 90% [25]. Therefore, 123I-MIBG scintigraphy remains a crucial initial staging modality. In primary diagnosis, morphological imaging is needed to evaluate operability and to detect bone marrow metastases with a high sensitivity [26]. In our study, however, three bone/bone marrow lesions were missed by MRI. All of them were correctly detected by 18F-FDG PET; 123I-MIBG scintigraphy showed only two of these bone/bone marrow lesions and missed one.
Follow-up
During follow-up, our lesion-based analysis showed a sensitivity of 48% vs. 64% and a specificity of 82% vs. 91% for 123I-MIBG scintigraphy and 18F-FDG PET. Difficulties assessing neuroblastoma lesions at follow-up have been discussed. Lesions positive on 123I-MIBG scintigraphy at primary diagnosis that were negative on the 123I-MIBG scan at relapse, although recurrence was proved, have been reported [27–29]. Possible reasons are primary negative lesions on 123I-MIBG scintigraphy or tumour cells which survive chemotherapy and afterwards fail to accumulate 123I-MIBG [30]. In disagreement with our results, Taggart et al. found 123I-MIBG scintigraphy to be more sensitive than 18F-FDG PET for individual anatomical lesion detection in relapsed neuroblastoma [31]. This difference may be due to our preselected patient population with many neuroblastoma lesions that were weakly affine to 123I-MIBG. Consequently, in our series, 18F-FDG PET was superior to 123I-MIBG scintigraphy in the follow-up. Morphological imaging in the follow-up evaluation lacks specificity because vital residual disease is difficult to differentiate from necrotic tissue [32]. With a specificity of 18%, MRI/CT was not appropriate as a single imaging modality in the follow-up in our study.
123I-MIBG scintigraphy and 18F-FDG PET imaging combined
Combined imaging with 123I-MIBG scintigraphy and 18F-FDG PET was more sensitive than 18F-FDG PET alone. However, on combining the two functional imaging modalities, eight incorrectly classified lesions remained. Postoperative changes and limited lesion size and/or low metabolic activity in the modality with the higher diagnostic confidence level were the main reasons for the false lesion classification. Of these eight lesions, six showed discrepant findings on 123I-MIBG scintigraphy and 18F-FDG PET. In discrepant 123I-MIBG scintigraphy/18F-FDG PET findings, comparison with morphological imaging should be performed in order to increase diagnostic safety in verifying subtle findings on the functional imaging [32]. In our study, only two falsely classified lesions in bone/bone marrow remained with concordant false-negative results on 123I-MIBG scintigraphy and 18F-FDG PET.
123I-MIBG scintigraphy and MRI/CT imaging combined
In a clinical setting, morphological imaging is added to functional imaging to assess a patient’s disease. Of 34 discrepant findings on 123I-MIBG scintigraphy and morphological imaging, all but two were correctly identified by 18F-FDG PET. They were false-negative bone/bone marrow lesions of small size in the femur that were shown on MRI due to tumoral oedema. A possible reason for negative 18F-FDG PET findings is a nonmetabolic state during examination. In follow-up examinations both lesions showed a clear progression on 18F-FDG PET.
On the other hand, in two lesions both 123I-MIBG scintigraphy and morphological imaging produced equivalent false findings: false-negative in a bone/bone marrow lesion in the vertebral column and false-positive in the adrenal gland. The diagnostic confidence scores on 123I-MIBG scintigraphy and morphological imaging for those two lesions were low, indicating equivocal findings. In both cases 18F-FDG PET indicated the true finding.
On the basis of our results, 18F-FDG PET is recommended as an additional imaging modality in the event of discrepancies or equivocal findings on 123I-MIBG scintigraphy and morphological imaging.
Limitations
In our retrospective study, the number of included patients was small due to the maximum period of 30 days between imaging modalities and due to the pre-selection criterion of negative or inconclusive 123I-MIBG scintigraphy. The majority of our patients suffered from stage IV disease. A multicentre approach investigating the possible use of 18F-FDG PET in neuroblastomas negative on 123I-MIBG scintigraphy could obtain a higher number of patients with a more homogeneous distribution of disease.
Moreover, the preselected patient group in our study does not represent the general population of patients with neuroblastoma. The sensitivity and specificity of 123I-MIBG scintigraphy obtained in our study is not valid for the general population of patients with neuroblastoma. This makes it difficult to compare our results with studies already published in the literature.
The specificity of an imaging modality includes information on the detection of true-negative findings. The number of true-negative findings was very low because all but one of our patients suffered from vital tumour disease at the time of the primary diagnosis. In order to avoid distorting the specificity, we did not calculate it for the 123I-MIBG scintigraphy in this context.
Conclusion
In this study with a preselected patient population, 123I-MIBG scintigraphy and 18F-FDG PET showed noticeable differences in their uptake patterns. 18F-FDG PET was more sensitive and specific for the detection of neuroblastoma lesions that were weakly affine to 123I-MIBG. Our results suggest that an 18F-FDG PET scan can be recommended if there are discrepant or inconclusive findings on 123I-MIBG scintigraphy/SPECT imaging and morphological imaging.
References
Spix C, Aareleid T, Stiller C, Magnani C, Kaatsch P, Michaelis J. Survival of children with neuroblastoma. time trends and regional differences in Europe, 1978–1992. Eur J Cancer. 2001;37:722–9.
Taggart D, Dubois S, Matthay KK. Radiolabeled metaiodobenzylguanidine for imaging and therapy of neuroblastoma. Q J Nucl Med Mol Imaging. 2008;52:403–18.
DuBois SG, Kalika Y, Lukens JN, Brodeur GM, Seeger RC, Atkinson JB, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–9.
Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O’Leary M, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–34.
Balwierz W, Wieczorek A, Klekawka T, Garus K, Bolek-Marzec K, Perek D, et al. Treatment results of children with neuroblastoma: report of Polish Pediatric Solid Tumor Group. Przegl Lek. 2010;67:387–92.
Perwein T, Lackner H, Sovinz P, Benesch M, Schmidt S, Schwinger W, et al. Survival and late effects in children with stage 4 neuroblastoma. Pediatr Blood Cancer. 2011; doi:10.1002/pbc.23036.
Boubaker A, Bischof Delaloye A. Nuclear medicine procedures and neuroblastoma in childhood. Their value in the diagnosis, staging and assessment of response to therapy. Q J Nucl Med. 2003;47:31–40.
Schmidt M, Simon T, Hero B, Schicha H, Berthold F. The prognostic impact of functional imaging with (123)I-mIBG in patients with stage 4 neuroblastoma >1 year of age on a high-risk treatment protocol: results of the German Neuroblastoma Trial NB97. Eur J Cancer. 2008;44:1552–8.
Boubaker A, Bischof Delaloye A. MIBG scintigraphy for the diagnosis and follow-up of children with neuroblastoma. Q J Nucl Med Mol Imaging. 2008;52:388–402.
Gordon I, Peters AM, Gutman A, Morony S, Dicks-Mireaux C, Pritchard J. Skeletal assessment in neuroblastoma – the pitfalls of iodine-123-MIBG scans. J Nucl Med. 1990;31:129–34.
Biasotti S, Garaventa A, Villavecchia GP, Cabria M, Nantron M, De Bernardi B. False-negative metaiodobenzylguanidine scintigraphy at diagnosis of neuroblastoma. Med Pediatr Oncol. 2000;35:153–5.
Kushner BH, Yeh SD, Kramer K, Larson SM, Cheung NK. Impact of metaiodobenzylguanidine scintigraphy on assessing response of high-risk neuroblastoma to dose-intensive induction chemotherapy. J Clin Oncol. 2003;21:1082–6.
Shulkin BL, Hutchinson RJ, Castle VP, Yanik GA, Shapiro B, Sisson JC. Neuroblastoma: positron emission tomography with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose compared with metaiodobenzylguanidine scintigraphy. Radiology. 1996;199:743–50.
Kushner BH. Neuroblastoma: a disease requiring a multitude of imaging studies. J Nucl Med. 2004;45:1172–88.
Sharp SE, Shulkin BL, Gelfand MJ, Salisbury S, Furman WL. 123I-MIBG scintigraphy and 18F-FDG PET in neuroblastoma. J Nucl Med. 2009;50:1237–43.
Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–77.
Matthay KK, Shulkin B, Ladenstein R, Michon J, Giammarile F, Lewington V, et al. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: a report for the International Neuroblastoma Risk Group (INRG) Task Force. Br J Cancer. 2010;102:1319–26.
Olivier P, Colarinha P, Fettich J, Fischer S, Frokier J, Giammarile F, et al. Guidelines for radioiodinated MIBG scintigraphy in children. Eur J Nucl Med Mol Imaging. 2003;30:B45–50.
Stauss J, Franzius C, Pfluger T, Juergens KU, Biassoni L, Begent J, et al. Guidelines for 18F-FDG PET and PET-CT imaging in paediatric oncology. Eur J Nucl Med Mol Imaging. 2008;35:1581–8.
Vik TA, Pfluger T, Kadota R, Castel V, Tulchinsky M, Farto JC, et al. (123)I-mIBG scintigraphy in patients with known or suspected neuroblastoma: Results from a prospective multicenter trial. Pediatr Blood Cancer. 2009;52:784–90.
Jacobson AF, Deng H, Lombard J, Lessig HJ, Black RR. 123I-meta-iodobenzylguanidine scintigraphy for the detection of neuroblastoma and pheochromocytoma: results of a meta-analysis. J Clin Endocrinol Metab. 2010;95:2596–606.
Pfluger T, Schmied C, Porn U, Leinsinger G, Vollmar C, Dresel S, et al. Integrated imaging using MRI and 123I metaiodobenzylguanidine scintigraphy to improve sensitivity and specificity in the diagnosis of pediatric neuroblastoma. AJR Am J Roentgenol. 2003;181:1115–24.
Connolly LP, Drubach LA, Ted Treves S. Applications of nuclear medicine in pediatric oncology. Clin Nucl Med. 2002;27:117–25.
Kushner BH, Yeung HW, Larson SM, Kramer K, Cheung NK. Extending positron emission tomography scan utility to high-risk neuroblastoma: fluorine-18 fluorodeoxyglucose positron emission tomography as sole imaging modality in follow-up of patients. J Clin Oncol. 2001;19:3397–405.
Shulkin BL, Shapiro B. Current concepts on the diagnostic use of MIBG in children. J Nucl Med. 1998;39:679–88.
Pfluger T, Schmid I, Coppenrath E, Weiss M. Modern nuclear medicine evaluation of neuroblastoma. Q J Nucl Med Mol Imaging. 2010;54:389–400.
McDowell H, Losty P, Barnes N, Kokai G. Utility of FDG-PET/CT in the follow-up of neuroblastoma which became MIBG-negative. Pediatr Blood Cancer. 2009;52:552.
Kushner BH, Kramer K, Modak S, Cheung NK. Sensitivity of surveillance studies for detecting asymptomatic and unsuspected relapse of high-risk neuroblastoma. J Clin Oncol. 2009;27:1041–6.
Colavolpe C, Guedj E, Cammilleri S, Taieb D, Mundler O, Coze C. Utility of FDG-PET/CT in the follow-up of neuroblastoma which became MIBG-negative. Pediatr Blood Cancer. 2008;51:828–31.
Frappaz D, Bonneu A, Chauvot P, Edeline V, Giammarile F, Siles S, et al. Metaiodobenzylguanidine assessment of metastatic neuroblastoma: observer dependency and chemosensitivity evaluation. The SFOP Group. Med Pediatr Oncol. 2000;34:237–41.
Taggart DR, Han MM, Quach A, Groshen S, Ye W, Villablanca JG, et al. Comparison of iodine-123 metaiodobenzylguanidine (MIBG) scan and [18F]fluorodeoxyglucose positron emission tomography to evaluate response after iodine-131 MIBG therapy for relapsed neuroblastoma. J Clin Oncol. 2009;27:5343–9.
Goo HW. Whole-body MRI of neuroblastoma. Eur J Radiol. 2010;75:306–14.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melzer, H.I., Coppenrath, E., Schmid, I. et al. 123I-MIBG scintigraphy/SPECT versus 18F-FDG PET in paediatric neuroblastoma. Eur J Nucl Med Mol Imaging 38, 1648–1658 (2011). https://doi.org/10.1007/s00259-011-1843-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1843-8