Abstract
Purpose
Overexpression of the HER2 receptor is a biomarker for predicting those patients who may benefit from trastuzumab therapy. Radiolabelled Affibody molecules can be used to visualize HER2 expression in tumour xenografts with high sensitivity. However, previous studies demonstrated that the difference in uptake in xenografts with high and low HER2 expression levels is not proportional to the difference in expression levels. We hypothesized that discrimination between tumours with high and low HER2 expression may be improved by increasing the injected dose (reducing the specific activity) of the tracer.
Methods
The influence of injected dose of anti-HER2 111In-DOTA-ZHER2 342 Affibody molecule on uptake in SKOV-3 (high HER2 expression) and LS174T (low expression) xenografts was investigated. The optimal range of injected doses enabling discrimination between xenografts with high and low expression was determined. To verify this, tumour uptake was measured in mice carrying both SKOV-3 and LS174T xenografts after injection of either 1 or 15 μg 111In-DOTA-ZHER2:342.
Results
An increase in the injected dose caused a linear decrease in the radioactivity accumulation in the LS174T xenografts (low HER2 expression). For SKOV-3 xenografts, the dependence of the tumour uptake on the injected dose was less dramatic. The injection of 10–30 μg 111In-DOTA-ZHER2:342 per mouse led to the largest difference in uptake between the two types of tumour. Experiments in mice bearing two xenografts confirmed that the optimized injected dose enabled better discrimination of expression levels.
Conclusion
Careful optimization of the injected dose of Affibody molecules is required for maximum discrimination between xenografts with high and low levels of HER2 expression. This information has potential relevance for clinical imaging applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human epidermal growth factor receptor type 2 (HER2, also designated as ErbB-2) is a transmembrane tyrosine kinase, which is often overexpressed in carcinomas of the breast [1], ovary [2] and prostate [3], and in other carcinomas. It is believed that HER2 is an orphan receptor and that its signalling occurs by heterodimerization with other members of the HER receptor family [4], leading to increased cell proliferation and motility, and suppression of apoptosis. Overexpression of HER2 is considered to be a part of the malignant phenotype. HER2 is therefore the target of several types of specific molecular therapies. Administration of the humanized monoclonal anti-HER2 antibody trastuzumab significantly prolongs survival of breast cancer patients [5]. The overexpression of HER2 in malignant tumours is also utilized in the development of targeted delivery of cytotoxic drugs [6] and radionuclides [7] to cancer cells. In addition, therapeutic strategies have been pursued for quenching of HER2 signalling by inhibition of its tyrosine kinase domain [8] and by inhibition of the heat shock protein 90 (HSP90) chaperone machinery and the resulting degradation of HER2 [9]. However, only a fraction of tumours express HER2, and only patients having tumours with high expression levels (immunohistochemistry 3+) would benefit from anti-HER2 therapy [10].
In order to select patients who may benefit from trastuzumab therapy, both the American Association of Clinical Oncology [11] and the European Group on Tumour Markers [12] recommend determining HER2 expression in each new breast tumour or recurrence. It should be taken into account that the level of HER2 expression can change during the clinical course of breast cancer disease. Patients with initial HER2-negative primary tumours may later present with HER2-positive metastases, which can respond to HER2-targeted therapy [13, 14]. The standard methods for determining HER2 expression are immunohistochemistry and fluorescent in-situ hybridization analysis of tumour samples, but these are associated with a high percentage (up to 20%) of inaccurate results [10]. The use of radionuclide molecular imaging might permit the detection of HER2 overexpression in both the whole primary tumour volume and metastases, thus avoiding false-negative results arising from inter- and intratumour heterogeneity of HER2 expression. It would allow monitoring of changes in HER2 expression during the course of the disease by noninvasive procedures. Issues associated with anatomic inaccessibility of certain lesions, and possible biopsy-associated morbidity, could also be avoided by this approach.
Affibody molecules are a novel class of targeting proteins [15, 16]. They are based on the 58-amino-acid (7 kDa) scaffold of the Z domain and can be selected to bind with high affinity to various tumour-associated molecular targets. A number of imaging tracers have been based on the anti-HER2 ZHER2:342 Affibody molecule and its derivatives labelled with 18F, 99mTc, 124I, 111In and 68Ga [16]. These tracers demonstrated very specific targeting of HER2-expressing xenografts and provided high contrast imaging shortly (1–4 h) after injection [16]. Pilot clinical data have confirmed the utility of the synthetic Affibody molecule 111In/68Ga-DOTA-ZHER2:342 for imaging HER2-expressing metastases in breast cancer patients [17].
Several different HER2-expressing cell lines were used in these preclinical studies. Interestingly, the difference in radioactivity uptake among tumour xenografts does not seem to be proportional to the difference in expression level of HER2 when 1 μg (0.14 nmol) of radiolabelled Affibody molecule is injected per mouse [18, 19]. This cannot be explained by nonspecific uptake, since the level of such uptake is negligible [18]. We hypothesized that this low peptide dose is not sufficient to saturate the HER2 receptors even in xenografts with low expression, and that the activity uptake at such low doses in xenografts showing both high and low expression is more affected by the delivery of Affibody molecules into the tumours (i.e. by perfusion restriction) than by the HER2 expression levels. If this hypothesis is correct, an increase in the injected dose of Affibody molecules (i.e. decreasing the specific radioactivity) would permit improved discrimination between tumours with high and low levels of HER2 expression. This would have direct implications for the optimal clinical use of this imaging agent, since discrimination between tumours with high and low HER2 expression levels is essential for both stratification of patients for HER2-targeting therapies and for monitoring response to such therapies.
In this study we experimentally investigated this prediction using BALB/c nu/nu mice bearing SKOV-3 (high HER2 expression) and LS174T (low HER2 expression) xenografts, using the synthetic Affibody molecule 111In-DOTA-ZHER2:342, which binds to HER2 with an apparent dissociation constant (K D) of 65 pM [18]. The influence of the injected peptide dose of the radiolabelled conjugate (i.e. its specific activity for a given level of injected radioactivity) on biodistribution and tumour uptake was studied.
Materials and methods
Materials
111In-indium(III) chloride was purchased from Tyco Healthcare Norden. Buffers were prepared using standard methods from chemicals supplied by Merck using high-quality Milli-Q water (resistance higher than 18 MΩ/cm). Any metal contamination from buffers used for labelling was eliminated using Chelex 100 resin (Bio-Rad Laboratories). Instant thin layer chromatography (ITLC) silica gel strips were from Gelman Sciences. A Ketalar-Rompun solution (20 μl of solution per gram of body weight), comprising 10 mg/ml ketamine (Ketalar, Pfizer) and 1 mg/ml xylazine (Rompun, Bayer), was used for anaesthesia.
In biodistribution studies, the radioactivity was measured using an automated gamma-counter with a 3-inch NaI(Tl) detector (1480 WIZARD, Wallac Oy). The distribution of radioactivity along the ITLC strips was measured on a Cyclone storage phosphor system and analysed using OptiQuant image analysis software (both from Perkin Elmer).
The data on tumour uptake and biodistribution were analysed by a two-tailed t-test using GraphPad Prism (version 4.00 for Windows, GraphPad Software) in order to determine the significance of differences (p < 0.05).
Cell lines
The cell lines used for inoculation of animals were purchased from American Type Tissue Culture Collection (ATCC) via LGC Promochem. In order to obtain xenografts with a high level of HER2 expression, the ovarian carcinoma cell line SKOV-3, with approximately 1.2×106 HER2 receptors per cell [7], was used. For inoculation of xenografts with a low-level of HER2 expression, the colon carcinoma cell line LS174T was used. According to the data of Lundberg et al. [20], this cell line expresses 22-fold less HER2 per cell than SKOV-3. The SKOV-3 cells were cultured in McCoy’s medium and the LS174T cells in Ham’s F10 medium (both from Flow Irvine). The media were supplemented with 10% fetal calf serum (Sigma), 2 mM l-glutamine and PEST (100 IU/ml penicillin, 100 μg/ml streptomycin), all from Biokrom.
Preparation of labelled conjugate
The DOTA peptide derivative of ZHER2:342 (DOTA-ZHER2:342) was custom-synthesized by Innovagen and delivered as a lyophilized powder. Labelling was performed according to the procedure described by Orlova et al. [18]. Briefly, the DOTA-ZHER2:342 powder was reconstituted in 0.2 M ammonium acetate buffer, pH 5.5, to a concentration of 1 mg/ml and aliquots containing 30 μg were prepared and stored frozen at −20°C. For labelling, an aliquot was mixed with 220 μl of 0.2 M ammonium acetate buffer, pH 5.5, and 80 μl of 111In-indium chloride solution containing 30 MBq. The mixture was incubated at 60°C for 30 min and then analysed using ITLC developed with 0.2 M citric acid, pH 2.0. In this system, radiolabelled Affibody molecules remain at the application point and free 111In migrates with the solvent front. The analytical system was verified using a blank experiment, where no Affibody molecules were added to the reaction mixture. The blank experiment showed that less than 0.5% of the radioactivity remained at the application point of the ITLC. Since the labelling efficiency was uniformly over 99%, no further purification was required. The labelling mixture was diluted with PBS. The formulation of injection solutions for each experiment is described below.
Animal studies
The animal experiments were planned and performed in accordance with national regulations on protection of laboratory animals and were approved by the local Ethics Committee for Animal Research in Uppsala. Female outbred BALB/c nu/nu mice were used in all experiments. All mice were acclimatized for 1 week at the Rudbeck Laboratory animal facility before any experimental procedures were initiated.
For tumour implantation, 107 SKOV-3 cells or 106 LS174T cells were implanted subcutaneously on the right hind leg of the mice. The biodistribution experiments were performed 5 weeks after implantation of SKOV-3 cells and 2 weeks after implantation of LS174T cells. The biodistribution experiments were performed when the tumour size was approximately 0.5 cm3 (by visual inspection), and the exact tumour weight was determined at the time of the study. In the experiments where two separate xenografts were implanted in the same mouse, the LS174T cells were inoculated 3 weeks after the SKOV3 cells.
Influence of injected peptide dose on biodistribution of 111In-DOTA-ZHER2:342 in mice bearing either SKOV3 or LS174T xenografts
To evaluate the influence of the injected peptide dose (i.e. specific activity when radioactivity was kept constant) of Affibody molecules on tumour targeting, a series of 111In-DOTA-ZHER2:342 formulations with various predetermined specific radioactivities were prepared. An aliquot of 111In-DOTA-ZHER2:342 was diluted with a stock solution of unlabelled DOTA-ZHER2:342 in PBS to provide injection doses containing 0.1 μg (0.014 nmol), 1 μg (0.14 nmol), 5 μg (0.7 nmol, for mice bearing LS174T xenograft only), 10 μg (1.4 nmol), 30 μg (4.2 nmol) and 50 μg (7 nmol) DOTA-ZHER2:342 labelled with 50 kBq 111In per mouse. The preparations were diluted with PBS to provide an injection volume of 100 μl per mouse. Standards of each solution were prepared for ex vivo measurements. The preparations were injected subcutaneously into tumour-bearing mice. Four mice were used for each dose of conjugate. Four hours after injection, the animals were injected with a lethal dose of Ketalar-Rompun solution and sacrificed by heart puncture. The blood was withdrawn using a heparinized syringe and collected. In addition, heart, lung, liver, spleen, stomach, kidney, tumour and samples of the large intestine (void of its contents), muscle and bone were excised from the animals and collected. The samples were weighed and the radioactivity content was measured. The uptake in tumours and normal tissues was calculated as percent of injected dose per gram (% ID/g). This procedure was performed in mice bearing both SKOV-3 (high HER2 expression) and LS174T (low HER2 expression) xenografts.
The presence of shed HER2 (sHER2) in serum samples from five mice bearing SKOV-3 and five mice bearing LS174T xenografts was determined using a sHER2-matched antibody pair kit (Bender MedSystems, Vienna Austria) in a sandwich ELISA format. The assay was performed according to the manufacturer’s instructions. Briefly, as capture antibody a monoclonal anti-human sHER2 antibody was used and a horseradish peroxidase-conjugated monoclonal anti-human sHER2 antibody was used to detect sHER2. The absorbance was measured at 450 nm in a VICTOR3 multilabel counter (PerkinElmer). The software program GraphPad Prism (version 4.00 for Windows, GraphPad Software) was used to generate standard curves from the mean absorbance values of the HER2 standard protein samples using nonlinear regression. The relative amount of human sHER2 in the samples was determined by interpolation of the standard curve and corrected by the dilution factor to generate the concentrations of sHER2 in undiluted serum.
Gamma-camera imaging and tumour uptake in mice bearing both SKOV3 and LS174T xenografts
A separate experiment was performed in mice bearing both SKOV-3 and LS174T xenografts. Four mice were intravenously injected with 100 μl PBS solution containing 1 MBq and 1 μg of 111In-DOTA-ZHER2:342. Another four animals were injected with a solution containing 1 MBq and 15 μg of 111In-DOTA-ZHER2:342. Standards of each solution were prepared for ex vivo measurements. Four hours after injection, the animals were injected with a lethal dose of Ketalar-Rompun solution and sacrificed by cervical dislocation. In order to remove any radioactivity interfering with the imaging, the urinary bladders were excised. Two animals with LS174T and SKOV-3 xenografts of comparable sizes were taken from each group and simultaneously imaged using a Millennium GE gamma camera equipped with a medium-energy general purpose collimator. Static images (20 min), obtained with a zoom factor of 3, were digitally stored in a 256 × 256 matrix. The images were evaluated visually using a Hermes system (Nuclear Diagnostics, Stockholm). After imaging, the tumours of all eight mice were excised, weighed and placed in air-tight plastic containers. The radioactivity in the tumours and standards was measured simultaneously using an automated gamma counter. The uptake in each xenograft was expressed as per cent of injected dose per gram (% ID/g).
Results
Influence of injected peptide dose on biodistribution of 111In-DOTA-ZHER2:342 in mice bearing SKOV3 or LS174T xenografts
The biodistribution data (4 h after injection) obtained by ex vivo measurements are presented in Table 1 (mice bearing LS174T xenografts with low HER2 expression) and Table 2 (mice bearing SKOV-3 xenografts with high HER2 expression). The average tumour weights were 0.51 ± 0.22 g and 0.36 ± 0.22 g for LS174T and SKOV-3 xenografts, respectively. The general biodistribution pattern of the conjugate was in a good agreement with previously reported data, i.e. rapid clearance from blood and normal tissues, high tumour uptake, and a high level of renal reabsorption [18]. There was also a very good agreement between the results of this experiment and of an experiment comparing subcutaneous and intravenous injections (Supplementary Table 1). Low levels of 111In uptake in bone were observed, indicative of its stable binding by the DOTA chelator.
There was an appreciable difference in the uptake of radioactivity in tumour xenografts depending on the amount of injected protein dose (Fig. 1a, note the logarithmic scale on the X-axis). An increase in the injected dose (in micrograms) caused a continuous decrease in the activity concentration in the low-expressing LS174T xenografts. However, in the case of SKOV-3 xenografts, with a high level of HER2 expression, the dependence of tumour uptake on the amount of injected protein was not as dramatic and showed a broad shoulder. There was no significant difference in the radioactivity uptake in SKOV-3 xenografts between 0.1, 1 or 10 μg of injected 111In-DOTA-ZHER2:342. However, an increase beyond 30 μg caused a significant (p < 0.05) decrease in tumour uptake in comparison with 0.1 μg of injected 111In-DOTA-ZHER2:342.
Influence of injected peptide dose on 111In-DOTA-ZHER2:342 uptake in xenografts with high expression of HER2 (SKOV-3) and low expression (LS174T) 4 h after injection. a Tumour uptake in LS174T and SKOV-3 xenografts as a function of injected dose. Each data point represents the mean value from four animals ± standard error. b Difference of tumour uptake in SKOV-3 and LS174T xenografts as a function of injected dose
There was no significant difference in 111In-DOTA-ZHER2:342 uptake between LS174T and SKOV-3 xenografts when 0.1 μg peptide was injected. However, at all other injected doses, the differences were significant (Fig. 1b). The largest difference in radioactivity uptake between the two tumour types was seen with 10 to 30 μg of injected peptide. In other words, the maximum discrimination between a high and low expressing tumour would be expected to depend on the specific activity (injected peptide dose) used. Importantly, the best discrimination was not observed in the case of maximal specific activity of the conjugate, but, on the contrary, at a relatively low specific activity.
Since the presence of a sHER2 extracellular domain from the SKOV-3 xenografts with high HER2 expression could have affected the biodistribution of the tracer, sHER2 blood levels were determined in a separate experiment. However, no detectable sHER2 was found in five mice with SKOV-3 tumours in the range of 0.11–0.55 g.
Gamma camera imaging and tumour uptake in mice bearing both SKOV3 and LS174T xenografts
The results of the initial biodistribution experiments led to the conclusion that it is difficult to discriminate between tumours with high and low levels of expression if the injected peptide dose is low (i.e. the specific activity is high) but that it is possible with a sufficiently high injected peptide dose. To test this hypothesis, animals bearing both low-expressing LS174T and high-expressing SKOV-3 xenografts were imaged on a clinical gamma camera following injection of different peptide doses of 111In-DOTA-ZHER2:342 (Fig. 2a) After injection of 1 μg per animal, both the LS174T and SKOV-3 xenografts were well-visualized (although the LS174T xenografts were smaller). However, when 15 μg was injected, more activity appeared to localize to the SKOV-3 xenografts than to the LS174T xenografts, leading to a clear discrimination between the two. Ex vivo measurements of the tumour uptake (in %ID/g) confirmed the imaging results (Fig. 2b). At a dose of 1 μg per animal, there was no statistically significant difference in tumour uptakes between the LS174T and SKOV-3 xenografts. At a dose of 15 μg, however, the difference was highly significant (p < 0.001). There was no difference in uptake by the SKOV-3 xenografts between the 1- and 15-μg doses.
Gamma camera imaging and tumour uptake in mice bearing both SKOV3 and LS174T xenografts 4 h after injection of 111In-DOTA-ZHER2:342 with different specific radioactivities. a Planar images collected from all mice simultaneously. The animal contours were derived from a digital photograph. b Tumour radioactivity uptake in Balb/c nude mice bearing both SKOV-3 and LS174T xenografts after injection of different 111In-DOTA-ZHER2:342 protein doses. Four animals were used for each injected dose. Data are presented as the mean values from four mice ± standard error. The average tumour weights were 0.4 ± 0.2 g and 0.9 ± 0.3 g, for LS174T and SKOV3 xenografts, respectively
Discussion
High specific activity is usually considered as a major advantage of a targeting conjugate [21, 22] or labelling method [23–25]. Often, such an approach is well justified by a limited number of binding sites for targeting conjugates or by the necessity to avoid inducing a pharmacological effect from the use of a radiolabelled receptor ligand or its analogue as an imaging probe. At the same time, there is growing evidence that high specific activity is not always optimal from an imaging point of view. For example, it does not provide the highest possible tumour accumulation or the highest possible tumour-to-organ radioactivity concentration ratios. Preclinical data show that the tumour uptake of somatostatin [26–28] and bombesin [29] analogues is dependent on the dose injected in a bell-shaped manner. Recently, we have demonstrated that an increase in the peptide dose improves the imaging of EGFR expression in tumour xenografts using Affibody molecules, presumably due to saturation of binding sites in normal tissues [30]. Moreover, clinical data suggest that a high specific activity (low injected protein dose) may be suboptimal for radionuclide imaging using somatostatin analogues [31, 32] or monoclonal antibodies [33–35]. Thus, the optimal rather than the maximum specific activity of an imaging probe should be pursued for imaging applications.
A number of Affibody-based conjugates labelled with positron emitting nuclides such as 124I, 68Ga and 18F have recently been described [16]. It has been emphasized that PET would enable better quantification of HER2 expression in tumours [36–38]. It is true that PET can quantify the radioactivity concentration in a volume of interest with a quite high accuracy. However, the radioactivity concentration of a tracer measured by a single scan [39] does not always reflect the concentration of the corresponding molecular target. More accurate in vivo measurement of cancer-associated molecular target concentration is possible by implementation of methods developed for quantification of neuroreceptors [39]. One approach is the use of several injections of a tracer with different specific activity. Another is based on dynamic scans with subsequent nonlinear regression analysis using compartmental models or graphical analysis methods. Unfortunately, both approaches have limitations for introduction into clinical routine. Multiple injections are associated with logistical issues. In the case of dynamic scans, only one bed position can be imaged (axial field of view about 15 cm), and often only one tumour lesion can be assessed [40]. In addition, a second scan might be required later to evaluate irreversible binding due to internalization of the imaging probe. It is considered to be clinically more advantageous to obtain relevant data concerning molecular target expression without dynamic scans [39, 41].
This study demonstrated that optimization of the peptide dose, i.e. specific activity for a given level of injected radioactivity, is essential for discrimination between tumours with different levels of molecular target expression by a single scan. At a high specific activity (injected peptide dose of 0.1 μg), the radioactivity uptake in xenografts with high and low HER2 expression is the same. The blood clearance of Affibody molecules is rapid, and the concentration gradient between blood and tumour should decrease rapidly, reversing at a certain time point. After reversing the gradient, the diffusion should change direction. Only a limited fraction of the injected Affibody molecules would be able to penetrate into the tumour interstitium and bind to receptors. In the case of a low peptide dose, that fraction might not be able to saturate all receptors even in the case of low expression levels. Thus, the tumour uptake would be limited only by the rate of extravasation and the rate of diffusion in the tumour interstitium. Evidently, a high affinity (fast on-rate) of the tracer is a precondition for efficient binding at low receptor density, low concentrations of the tracer and slow internalization. Increasing the injected peptide dose should increase the amount of delivered imaging probe, which would cause a progressive saturation of receptors on the diffusion path. This effect should be most pronounced in the case of low expressing tumours. Indeed, the level of 111In-DOTA-ZHER2:342 uptake in LS174T xenografts (low HER2 expression) decreased steadily with increases in injected dose in the range studied here, most likely due to competition from its nonlabelled counterpart. In the case of high expression levels of the target, the saturation of receptors would occur more slowly when increasing the dose of injected peptide. This was reflected by a shoulder in the uptake in SKOV-3 xenografts between 0.1 and 10 μg of injected 111In-DOTA-ZHER2:342. A further increase in the injected peptide dose also decreased the uptake in SKOV-3 xenografts, which would be expected for a conjugate with specific targeting. These differences in dose-dependent tumour uptake suggest that an optimal peptide dose range exists over which the signal for high target-expressing tumours remains high while that from low-expressing tumours is suppressed.
Other factors could contribute to a low uptake in tumours with high HER2 expression at low injected peptide doses. For example, such tumours might release an appreciable amount of sHER2 into the blood circulation [42]. This shed antigen could intercept a tracer in the blood before it reached the tumour. It has been suggested that interactions with sHER2 was the reason for suboptimal targeting at low doses of 89Zr-trastuzumab in a clinical study [35]. However, in the present study, any influence from shed antigen in the blood could be excluded since our data demonstrated that the blood of mice bearing SKOV-3 xenografts did not contain measurable amounts of sHER2. In the absence of sHER2, the higher blood radioactivity levels in mice with SKOV-3 xenografts in comparison with mice with LS174T xenografts was an intriguing finding. A possible explanation might be the “binding site barrier” [43], i.e. the localization of high-affinity conjugates predominantly on the rim of the tumours with high antigen expression. Internalization of 111In-DOTA-ZHER2:342 is slow [44], and a fraction of the conjugate might dissociate from receptors in tumours and re-enter the blood circulation. This effect should be more pronounced in the case of tracer localization in the tumour periphery.
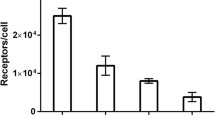
Thus, the results of this study suggest that the use of radiotracers with moderate specific activity may allow more reliable detection of differences in the levels of molecular targets during a single scan. This would be of practical value in at least two different clinical situations. First, when developing clinical dosing protocols that are optimized to discriminate between tumours with high and low expression during determination of HER2 status for newly diagnosed tumours. Treatment of breast cancer with trastuzumab is recommended if the HER2 expression level is 3+ or else 2+ with subsequent positive FISH test, while patients with HER2-negative status (immunohistochemistry 0 –1+ or FISH negative) should not receive trastuzumab [11]. According to Ross et al. [45], cells containing less than 20,000 HER2 receptors would show no staining (0), and cells containing about 100,000 receptors would show membrane staining of level 1+. Thus, LS174T cells, expressing 60,000 receptors per cell would be classed as borderline between 0 and 1+ according to immunohistochemistry. Still, the LS174T xenografts were imaged as HER2-positive at high specific radioactivity (low peptide dose) of 111In-DOTA-ZHER2:342. In clinics, such high sensitivity of HER2 imaging using 111In-DOTA-ZHER2:342 with high specific activity might indicate trastuzumab treatment in patients suffering from tumours with low HER2 expression, leading to overtreatment. Such situations would be avoided by the use of an optimal dose of the imaging probe. Second, optimization of the injected dose is important for monitoring of therapy directed towards downregulation/degradation of HER2, such as inhibition of HSP90 [9]. Preclinical data [38] suggest that treatment with HSP90 inhibitors reduces HER2 expression by two- to threefold, rather than removing it completely. Thus, better discrimination between different expression levels is required in this case to detect response to therapy.
Conclusion
This study demonstrated for the first time that the optimal, not the highest possible, specific activity is required for maximal discrimination of the relative levels of HER2 expression in tumour models using Affibody molecules. This information might be clinically useful both for the selection of patients for anti-HER2 therapy and for monitoring the efficacy of such therapy using molecular imaging. In principle, this information should also be helpful for detection of the expression levels of different targets using other molecular imaging agents.
References
Carlsson J, Nordgren H, Sjostrom J, Wester K, Villman K, Bengtsson NO, et al. HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review. Br J Cancer 2004;90:2344–8.
Verri E, Guglielmini P, Puntoni M, Perdelli L, Papadia A, Lorenzi P, et al. HER2/neu oncoprotein overexpression in epithelial ovarian cancer: evaluation of its prevalence and prognostic significance. Clin Study Oncol 2005;68:154–61.
Morote J, de Torres I, Caceres C, Vallejo C, Schwartz S Jr, Reventos J. Prognostic value of immunohistochemical expression of the c-erbB-2 oncoprotein in metastatic prostate cancer. Int J Cancer 1999;84:421–5.
Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 2006;7:505–16.
Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene 2007;26:3637–43.
Mandler R, Wu C, Sausville EA, Roettinger AJ, Newman DJ, Ho DK, et al. Immunoconjugates of geldanamycin and anti-HER2 monoclonal antibodies: antiproliferative activity on human breast carcinoma cell lines. J Natl Cancer Inst 2000;92:1573–81.
Persson M, Tolmachev V, Andersson K, Gedda L, Sandström M, Carlsson J. [177Lu]pertuzumab: experimental studies on targeting of HER-2 positive tumour cells. Eur J Nucl Med Mol Imaging 2005;32:1457–62.
Cameron DA, Stein S. Drug insight: intracellular inhibitors of HER2-clinical development of lapatinib in breast cancer. Nat Clin Pract Oncol 2008;5:512–20.
Citri A, Kochupurakkal BS, Yarden Y. The achilles heel of ErbB-2/HER2: regulation by the Hsp90 chaperone machine and potential for pharmacological intervention. Cell Cycle 2004;3:51–60.
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al.; American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25:118–45.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al.; American Society of Clinical Oncology. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007;25:5287–312.
Molina R, Barak V, van Dalen A, Duffy MJ, Einarsson R, Gion M, et al. Tumor markers in breast cancer – European Group on Tumor Markers recommendations. Tumour Biol 2005;26:281–93.
Zidan J, Dashkovsky I, Stayerman C, Basher W, Cozacov C, Hadary A. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer 2005;93:552–6.
Lower EE, Glass E, Blau R, Harman S. HER-2/neu expression in primary and metastatic breast cancer. Breast Cancer Res Treat 2009;113:301–6.
Nygren PA. Alternative binding proteins: affibody binding proteins developed from a small three-helix bundle scaffold. FEBS J 2008;275:2668–76.
Löfblom J, Feldwisch J, Tolmachev V, Carlsson J, Ståhl S, Frejd FY. Affibody molecules: engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett 2010;584:2670–80.
Baum RP, Prasad V, Müller D, Schuchardt C, Orlova A, Wennborg A, et al. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nucl Med 2010;51:892–7.
Orlova A, Tolmachev V, Pehrson R, Lindborg M, Tran T, Sandström M, et al. Synthetic affibody molecules: a novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer Res 2007;67:2178–89.
Ahlgren S, Wållberg H, Tran TA, Widström C, Hjertman M, Abrahmsén L, et al. Targeting of HER2-expressing tumors with a site-specifically 99mTc-labeled recombinant affibody molecule, ZHER2:2395, with C-terminally engineered cysteine. J Nucl Med 2009;50:781–9.
Lundberg E, Höidén-Guthenberg I, Larsson B, Uhlén M, Gräslund T. Site-specifically conjugated anti-HER2 Affibody molecules as one-step reagents for target expression analyses on cells and xenograft samples. J Immunol Methods 2007;319:53–63.
Breeman WA, de Jong M, de Blois E, Bernard BF, Konijnenberg M, Krenning EP. Radiolabelling DOTA-peptides with 68Ga. Eur J Nucl Med Mol Imaging 2005;32:478–85.
Breeman WA, De Jong M, Visser TJ, Erion JL, Krenning EP. Optimising conditions for radiolabelling of DOTA-peptides with 90Y, 111In and 177Lu at high specific activities. Eur J Nucl Med Mol Imaging 2003;30:917–20.
Ballinger JR, Cooper MS, Mather SJ. Re: controversies – [Tc(CO)3]+ chemistry: a promising new concept for SPET? Eur J Nucl Med Mol Imaging 2004;31:304–5.
Lewis MR, Raubitschek A, Shively JE. A facile, water-soluble method for modification of proteins with DOTA. Use of elevated temperature and optimized pH to achieve high specific activity and high chelate stability in radiolabeled immunoconjugates. Bioconjug Chem 1994;5:565–76.
Velikyan I, Beyer GJ, Bergström-Pettermann E, Johansen P, Bergström M, Långström B. The importance of high specific radioactivity in the performance of 68Ga-labeled peptide. Nucl Med Biol 2008;35:529–36.
Breeman WA, Kwekkeboom DJ, Kooij PP, et al. Effect of dose and specific activity on tissue distribution of indium-111-pentetreotide in rats. J Nucl Med 1995;36:623–7.
de Jong M, Breeman WA, Bernard BF, van Gameren A, de Bruin E, Bakker WH, et al. Tumour uptake of the radiolabelled somatostatin analogue [DOTA0, TYR3]octreotide is dependent on the peptide amount. Eur J Nucl Med 1999;26:693–8.
Bernhardt P, Kölby L, Johanson V, Nilsson O, Ahlman H, Forssell-Aronsson E. Biodistribution of 111In-DTPA-D-Phe1-octreotide in tumour-bearing nude mice: influence of amount injected and route of administration. Nucl Med Biol 2003;20:253–60.
Schuhmacher J, Zhang H, Doll J, Mäcke HR, Matys R, Hauser H, et al. GRP receptor-targeted PET of a rat pancreas carcinoma xenograft in nude mice with a 68Ga-labeled bombesin(6-14) analog. J Nucl Med 2005;46:691–9.
Tolmachev V, Rosik D, Wållberg H, Sjöberg A, Sandström M, Hansson M, et al. Imaging of EGFR expression in murine xenografts using site-specifically labelled anti-EGFR (111)In-DOTA-Z (EGFR:2377) Affibody molecule: aspect of the injected tracer amount. Eur J Nucl Med Mol Imaging 2010;37:613–22.
Kwekkeboom D, Krenning EP, de Jong M. Peptide receptor imaging and therapy. J Nucl Med 2000;41:1704–13.
Velikyan I, Sundin A, Eriksson B, Lundqvist H, Sörensen J, Bergström M, et al. In vivo binding of [68Ga]-DOTATOC to somatostatin receptors in neuroendocrine tumours – impact of peptide mass. Nucl Med Biol 2010;37:265–75.
Divgi CR, Welt S, Kris M, Real FX, Yeh SD, Gralla R, et al. Phase I and imaging trial of indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J Natl Cancer Inst 1991;83:97–104.
Pandit-Taskar N, O'Donoghue JA, Morris MJ, Wills EA, Schwartz LH, Gonen M, et al. Antibody mass escalation study in patients with castration-resistant prostate cancer using 111In-J591: lesion detectability and dosimetric projections for 90Y radioimmunotherapy. J Nucl Med 2008;49:1066–74.
Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, et al. Biodistribution of (89)Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 2010;87:586–92.
Cheng Z, De Jesus OP, Namavari M, De A, Levi J, Webster JM, et al. Small-animal PET imaging of human epidermal growth factor receptor type 2 expression with site-specific 18F-labeled protein scaffold molecules. J Nucl Med 2008;49:804–13.
Kramer-Marek G, Kiesewetter DO, Martiniova L, Jagoda E, Lee SB, Capala J. [(18F]FBEM-ZHER2:342-Affibody molecule – a new molecular tracer for in vivo monitoring of HER2 expression by positron emission tomography. Eur J Nucl Med Mol Imaging 2008;35:1008–18.
Kramer-Marek G, Kiesewetter DO, Capala J. Changes in HER2 expression in breast cancer xenografts after therapy can be quantified using PET and (18)F-labeled affibody molecules. J Nucl Med 2009;50:1131–9.
Laruelle M. The role of model-based methods in the development of single scan techniques. Nucl Med Biol 2000;27:637–42.
Weber WA. Quantitative analysis of PET studies. Radiother Oncol 2010;96:308–10.
Zhang X, Xiong Z, Wu Y, Cai W, Tseng JR, Gambhir SS, et al. Quantitative PET imaging of tumor integrin alphavbeta3 expression with 18F-FRGD2. J Nucl Med 2006;47:113–21.
Kath R, Höffken K, Otte C, Metz K, Scheulen ME, Hülskamp F, et al. The neu-oncogene product in serum and tissue of patients with breast carcinoma. Ann Oncol 1993;4:585–90.
Adams GP, Schier R, McCall AM, et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res 2001;61:4750–5.
Wållberg H, Orlova A. Slow internalisation of anti-HER2 Affibody monomer: implications for development of labeled tracers. Cancer Biother Radiopharm 2008;23:435–42.
Ross JS, Fletcher JA, Bloom KJ, Linette GP, Stec J, Symmans WF, et al. Targeted therapy in breast cancer: the HER-2/neu gene and protein. Mol Cell Proteomics 2004;3:379–98.
Acknowledgments
This study was supported by grants from the Swedish Cancer Society (Cancerfonden) and the Swedish Research Council (Vetenskapsrådet).
Conflicts of interest
AW has an affiliation (employment) with Affibody AB, Stockholm, Sweden, which holds the intellectual property rights and trademarks for Affibody molecules.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM1
(DOC 58 kb)
Rights and permissions
About this article
Cite this article
Tolmachev, V., Wållberg, H., Sandström, M. et al. Optimal specific radioactivity of anti-HER2 Affibody molecules enables discrimination between xenografts with high and low HER2 expression levels. Eur J Nucl Med Mol Imaging 38, 531–539 (2011). https://doi.org/10.1007/s00259-010-1646-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1646-3