Abstract
Purpose
The aim of the study was to assess the role of CA 15.3, CT and positron emission tomography (PET)/CT in patients with breast cancer and suspected disease relapse after primary treatment.
Methods
We studied 111 consecutive patients (mean age 61 ± 12 years) with previous breast cancer, already treated and with clinical or biochemical suspicion of disease relapse. All patients underwent CT and 18F-fluorodeoxyglucose (FDG) PET/CT. In all patients, the value of CA 15.3 was compared to PET/CT and CT. The final diagnosis of relapse was established by invasive and noninvasive follow-up and was compared with CA 15.3, CT and PET/CT results. Univariate and multivariate analyses were used to identify the independent predictors of disease relapse and receiver-operating characteristic (ROC) curve for the identification of optimal CA 15.3 cutoff.
Results
Of all patients, 40 (36%) showed an increased CA 15.3 value, CT was positive in 73 (66%), whereas at PET/CT imaging 64 (58%) showed positive findings for disease relapse. Of 40 patients with increased marker levels, 22 patients had positive CT and 30 positive PET/CT (55 vs 75%, p < 0.001). At the end of follow-up, recurrence occurred in 32 (29%) patients, 16 (50%) of whom showed high levels of CA 15.3. PET/CT predicted relapse in 26 (81%) patients, whereas CT correctly identified 23 (72%). At univariate analysis, recurrence was significantly associated with high CA 15.3 values (p < 0.05) and positive PET/CT (p < 0.005). At multivariable analysis only positive PET/CT remained an independent predictor of disease relapse (p < 0.05). ROC analysis showed an optimal cutoff point for CA 15.3 of 19.1 U/ml (AUC 0.65, p < 0.01) to individuate positive PET/CT.
Conclusion
FDG PET/CT is more sensitive than CT and CA 15.3 in the evaluation of disease relapse. PET/CT might be considered a complementary imaging technique during follow-up in patients with breast cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is the most common malignant disease in Europe, with the highest incidence in northern countries. The early individuation of disease relapse could improve the prognosis and allow better management by starting a new treatment or changing ongoing therapy. Depending on the extent of the disease, up to 35% of the patients who receive full treatment (surgery and others) will ultimately develop local recurrence or secondary tumour dissemination to distant organs [1]. During follow-up of treated BC patients, disease relapse is suggested by symptom onset, ever-increasing marker levels or physical examination abnormalities. The American Society of Clinical Oncology (ASCO) 2007 recommendations for the use of tumour markers does not support the determination of CA 15.3 during follow-up of treated BC patients for monitoring the recurrence of disease [2].
In the past, some studies have shown that a CA 15.3 increase during follow-up may precede, in about two thirds of cases, the clinical and instrumental discovery of distant metastases with a lead time of several months [3]. Oncologists agree that a continuous progressive increase in circulating tumour markers can represent an early signal of tumour relapse, even if patients are asymptomatic and without any other clinical or instrumental signs of disease. Recent data suggest that 18F-fluorodeoxyglucose positron emission tomography (FDG PET) is a useful technique for detecting recurrent BC suspected on the basis of an asymptomatically elevated tumour marker level and negative conventional imaging results [4, 5]. In recent years PET/CT, as an integrated tool for the evaluation of suspected disease relapse for various tumours (e.g. lymphomas), has become routinely used and been shown to be superior to PET alone in restaging disease in patients previously treated, particularly when the only indicator of recurrence is a rise in serum tumour markers (such as CA 15.3) [6, 7]. As underlined by Zangheri et al. [8], PET/CT can be of value in restaging and especially for establishing the correct management of BC patients both early after the primary treatment and during follow-up. The aims of this study were (1) to assess the role of tumour markers, CT and 18F-FDG PET/CT in identification of disease relapse in patients with BC already treated and (2) to assess the impact of PET/CT findings on patient management.
Materials and methods
From June 2005 to December 2009, we selected 111 patients (110 women and 1 man) with a mean age of 61 ± 12 years (range 32–86 years) with previous BC, already treated with primary surgery and neoadjuvant therapy and/or adjuvant treatment (chemotherapy ± radiotherapy ± hormone therapy). Pathological TNM staging was evaluated according to the criteria of the American Joint Committee on Cancer (AJCC). In all patients with suspicious disease relapse, the interval between surgery and PET/CT was 4 ± 1 years. The reasons to perform the exams (CA 15.3, CT and PET/CT imaging) were (1) suspicion of disease relapse at clinical examination, (2) doubtful chest and bone radiograph and (3) equivocal whole-body imaging bone scans. Twenty-six (23%) patients were referred for symptoms of lung, liver or bone metastasis. In all patients, the value of CA 15.3 was available and a threshold higher than 31 U/ml was used to define the increased value. For further evaluations, all patients underwent both CT and subsequently 18F-FDG PET/CT within 5 ± 1 months. All PET/CT scans were performed at our single institution and a specialist nuclear medicine physician read all images. All patients gave their informed consent for the PET/CT and CT imaging. The present analysis has a retrospective character; thus, neither Institutional Review Board approval nor informed consent was required by the national institution.
Interpretation
The final diagnosis of disease recurrence was established by invasive and noninvasive (radiological or nuclear medicine) follow-up (mean time 7 ± 6 months). The diagnosis was verified by clinical follow-up in 39 (35%) patients and through consequent imaging studies (2 chest X-rays, 17 CT, 16 bone scans, 8 abdominal ultrasounds, 1 nuclear magnetic resonance, 10 PET/CT and 18 both PET/CT and bone scans) in the remaining population. The diagnosis of metastatic disease was made when the presence of new lesions at clinical evaluations and/or at imaging studies was demonstrated. In patients without disease relapse, neither change of ongoing treatment nor further treatment was performed and a close follow-up with imaging studies was started. The data obtained from the follow-up were compared with CA 15.3, CT and PET/CT findings and comparisons among their results were performed.
Image interpretation of CT data
Written clinical reports of conventional images were reviewed and classified as (a) negative, if all imaging tests were negative for disease; (b) equivocal, when abnormal findings were present on any imaging test but were not interpreted as suspicious for malignancy; (c) suspicious, if any test result was clearly described as suspicious for malignancy; or (d) positive, if findings were described as consistent with malignancy. To dichotomize the data, negative and equivocal findings were subclassified as negative, and suspicious and positive findings were categorized as positive.
PET imaging and analysis
Whole-body 18F-FDG PET/CT was performed using a dedicated PET/CT scanner (Biograph 16, Siemens Medical Solutions, Hoffman Estates, IL, USA). The PET component is a high-resolution scanner with a spatial resolution of 4.7 mm and has no septa, thus allowing three-dimensional-only acquisitions. The CT portion of the scanner is the 16-slice Somatom Sensation. Together with the PET system, the CT scanner is used both for attenuation correction of PET data and for localization of 18F-FDG uptake in PET images. All patients were advised to fast for at least 6 h before the integrated PET/CT examination. After injection of about 3 MBq of 18F-FDG per kilogram of body weight, patients rested for a period of about 60 min in a comfortable chair. Emission images ranging from the proximal femur and the base of the skull were acquired for 2–3 min per bed position. Acquired images were reconstructed using the attenuation-weighted ordered subset expectation maximization (OSEM) iterative reconstruction, with 2 iterations and 8 subsets. The Gaussian filter was applied to the image after reconstruction along the axial and transaxial directions. The data were reconstructed over a 128 × 128 matrix with 5.25-mm pixel size and 2-mm slice thickness. Processed images were displayed in coronal, transverse and sagittal planes.
Image analysis
At visual analysis, increased FDG uptake not corresponding to physiological uptake patterns and in any foci of increased uptake corresponding to a CT abnormality (tissue and/or lymph node) was recorded as positive for recurrent lesions. In contrast the absence of uptake was used to define a negative PET/CT finding. The interpretation of some doubtful areas of uptake was obtained by considering the standardized uptake value (SUV) that can orient the differential diagnosis between a benign and malignant lesion. Maximum SUV (SUVmax) was determined by drawing regions of interest (ROI) on the attenuation-corrected FDG PET/CT images around suspected lesion sites. The SUV calculation for the calibrated and decay-corrected series representing 3-D volume data is done using the following formula:
To assess FDG uptake more objectively, a cutoff value of 2.5 has frequently been used for the SUVmax.
Statistical methods
Continuous data are presented as mean and standard deviation and categorical data as percentages. The end-points were (1) to compare the value of tumour marker CA 15.3 to PET/CT and CT findings, (2) to establish the relationship between diagnosis of relapse and CA 15.3, CT and PET/CT findings and (3) to assess the impact of PET/CT on the therapeutic decision. Associations for paired samples were assessed using the t test or Mann-Whitney test depending on the normality of the variable, verified with the Shapiro-Wilk test. Comparisons between dichotomized variables were performed by the chi-square test, or Fischer’s exact test, as appropriate; p < 0.05 was considered statistically significant. The sensitivity, specificity, and positive and negative predictive values (PPV and NPV) were determined by person-based analysis. Univariate and multivariate logistic regression analyses were performed to identify the independent predictors of disease recurrence. A receiver-operating characteristic (ROC) analysis was performed for identification of both the CA 15.3 cutoff to better predict the positive PET/CT and the area under the ROC curve (AUC). Statistical analysis was performed with SPSS software (SPSS Inc., Advanced Models 15.0, Chicago, IL, USA).
Results
Separate evaluation of tumour markers and imaging findings
Of all patients (n = 111), 71 (64%) had a normal value of CA 15.3 and 40 (36%) an increased level. CT was negative in 38 (34%) patients and positive in 73 (66%), whereas at PET/CT imaging, 47 (42%) patients showed negative findings and 64 (58%) positive findings for disease (Fig. 1). Demographic and clinical data of patients for CA 15.3 and PET/CT findings are depicted in Tables 1 and 2, respectively. As shown, stage III was significantly associated with high levels of CA 15.3 (p < 0.05) and positive PET/CT (p < 0.005). Of 40 patients with an increase in marker values, 22 had positive CT while 30 had positive PET/CT (55 vs 75%, p < 0.001) (Fig. 2). The mean value of CA 15.3 was significantly higher in patients with positive PET/CT than in patients with negative PET/CT (67.5 ± 16.4 vs 22.4 ± 2.7, p < 0.05), while the mean tumour marker value was not significantly different among patients with negative and positive CT (39.8 ± 7.1 vs 52.9 ± 14.3, respectively, p = NS)
Predictive value of tumour markers, CT and PET/CT
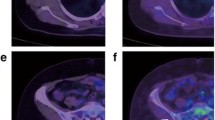
At the end of follow-up (mean time 7 ± 6 months), disease relapse occurred in 32 of 111 (29%) patients; of these only 9 (28%) had symptoms related to metastasis. The interval between surgery and disease relapse was 5 ± 1 years. The CA 15.3 value was increased in only 16 of 32 (50%) of these patients, showing a false-positive rate of 30% and a false-negative rate of 50%. CT alone revealed recurrence of disease in 23 of 32 (true-positive rate 72%) patients; otherwise in 9 of 32 (28%) patients, CT imaging yielded false-negative results, although 5 of them had an increased value of CA 15.3. PET/CT predicted the relapse of disease in 26 of 32 patients, with a true-positive rate of 81% and a true-negative rate of 52%. The false-positive rate from FDG PET/CT was 48%. The main reason for this high false-positive rate was due to abnormal FDG uptake at adrenal glands or bone or lung and/or liver that was not subsequently confirmed by other investigations during follow-up. Two examples of patients with a false-positive PET/CT scan are depicted in Fig. 3. As shown, the first patient (Fig. 3a), a woman with left BC, treated with radical mastectomy, demonstrated an FDG uptake in correspondence to a supraclavicular lymph node; after 4 months without starting any therapy she underwent another PET/CT exam with a negative test result. Figure 3b depicts the case of a woman with suspected lung recurrence at the first PET/CT scan; 3 months later without initiating any therapy a new PET/CT exam demonstrated a completely negative result. Among six patients with disease relapse not detected by PET/CT, only two had high levels of CA 15.3. A high level of tumour marker and positive PET/CT were shown in only 14 (44%) patients with disease relapse. The diagnostic accuracies of disease relapse for tumour markers, imaging findings and in cases of elevated/normal CA 15.3 values are depicted in Table 3. The sensitivity and NPV of PET/CT imaging were significantly higher in the entire population as well as in patients with a high level of CA 15.3 (81 and 87%, 88 and 80%, respectively). CT imaging showed a very high specificity (93%) in patients with relapse and with an increased value of tumour marker. Table 4 depicts clinical, histopathological and exam findings and related features statistically associated with disease relapse. At univariate analysis, recurrence of disease was significantly associated with high levels of CA 15.3 [odds ratio (OR) 0.43, 95% confidence interval (CI) 0.18–1.01, p < 0.05] and positive PET/CT (OR 0.21, 95% CI 0.08–0.57, p < 0.005). At multivariable analysis only positive PET/CT (OR 0.24, 95% CI 0.08–0.67, p < 0.005) remained an independent predictor of disease relapse. At ROC analysis, the value of 19.1 U/ml for CA 15.3 demonstrated the optimal cutoff point for positive PET/CT findings with a sensitivity of 65% and specificity of 60% (AUC 0.65, p < 0.01; 95% CI 0.55–0.75) (Fig. 4). However, by reducing the cutoff of CA 15.3 from 31.0 to 19.1 U/ml, the recovery of patients with disease relapse was only 6%.
Two examples of false-positive findings at FDG PET/CT. a A woman with left BC, treated with radical mastectomy, who demonstrated an FDG uptake in correspondence to a supraclavicular lymph node; after 4 months without starting any therapy she underwent another PET/CT exam with a negative test result. b A woman with suspected lung recurrence at the first PET/CT scan; 3 months later without initiating therapy a new PET/CT exam demonstrated a completely negative result
Impact of CA 15.3 and PET/CT on management
In patients with a diagnosis of disease relapse (n = 32), chemotherapy was performed in 21 (65%), surgery in 1 (3%), continuing hormone therapy in 6 (19%) and no therapy in 4 (13%) patients. Therapeutic management was changed in 11 patients with increased CA 15.3, starting chemotherapy, whereas positive PET/CT modified the therapeutic regimen in 18 patients; in particular 17 patients started chemotherapy for BC and 1 patient for lymphoma. The change in management was significantly important after PET/CT evaluation (change in 56 vs 34%, respectively, for PET/CT and CA 15.3).
Discussion
There is general agreement that a progressive increase of a circulating tumour marker observed in a patient previously treated can represent an early signal of tumour relapse, even if described in asymptomatic patients without any other clinical or instrumental signs of cancer. The “biochemical evidence” of a possible cancer relapse, suggested by increased tumour markers, led oncologists to discover the sites of the cancer lesions through conventional radiological imaging techniques or nuclear medicine modalities [9, 10]. During follow-up, in clinical practice an increase and in particular a persistent growth in the time of CA 15.3 alerts the clinician; unfortunately for the retrospective nature of the study, a trend of tumour markers is missing.
Symptomatic patients or with clinical suspicion of disease relapse, although with negative markers or both negative markers and CT, may anyway present disease recurrence. In fact, in our study 16 of 32 patients had normal values of CA 15.3, 9 showed normal CT, while only 6 patients had negative PET/CT. At present, as described by Siggelkow et al. [4], PET/CT should be performed only in cases where the tumour marker is increasing and conventional imaging is doubtful. In our study population, PET/CT recognized the majority of patients with recurrent disease, irrespective of the value of CA 15.3 and CT findings, identifying 81% of cancer recurrence and losing only 19%, with a gain of 30% toward tumour markers and 10% toward CT. Considering patients with high levels of CA 15.3 and positive PET/CT, only 14 relapses were correctly diagnosed, decreasing the predictive value of PET/CT to about 40%. Despite reducing the cutoff point of CA 15.3 from 31.0 to 19.1 U/ml, no advantage was obtained, increasing recurrence detection by only 6%. In BC women with elevated tumour marker levels, Haug et al. [11] reported a high diagnostic power of PET/CT in detecting metastases or local recurrence, showing a sensitivity of 96%, specificity of 89%, PPV of 96% and NPV of 89%. In contrast, in our study PET/CT findings showed a high sensitivity (88%) and high NPV (89%), but low specificity (33%) and low PPV (47%) in the same subset of patients. The low specificity of PET/CT (high false-positive rate) in patients with high levels of tumour marker was probably due to ongoing hormone therapy in some patients, degenerative vertebral disease, atelectasis with pneumonitis, reconstruction artefacts in particular in patients with breast expanders and finally the inflammations that are the common cause of false-positive findings with FDG. However, in patients with elevated CA 15.3, 75% had positive PET; the increase of markers should not be the only element to perform a PET/CT examination, because if we had followed this way we would have lost 25% of relapses in our population. Kamel et al. [12] compared FDG PET findings to tumour marker level (CA 15.3) in 60 patients. In a lesion-based analysis, a total of 50 lesions were validated by histology, conventional imaging and follow-up. PET was true-positive in 28 lesions, true-negative in 16, false-positive in 3 and false-negative in 3. Interestingly, some of the patients with true-positive PET results did not have elevated tumour marker levels. This study as well as our experience raises some doubts on the propriety of performing PET/CT based only on a single increase of CA 15.3, unless a very sensible tumour marker could allow detection of minimal diseases and quantitatively reflect tumour burden. Probably the combination of constant CA 15.3 growth during and after therapy and suspicion of clinical progression might prompt one to perform PET/CT, avoiding false-positives or false-negatives.
During follow-up, the advantages of performing PET/CT for the detection of disease relapse are the combined evaluation of metabolic and morphological changes, the early identification of metastatic sites, the possibility to have referral images for monitoring the response to therapy in the interim and ultimately reducing the psychological stress due to sequential negative imaging procedures in patients with persistent tumour marker elevation and finally to let the early and appropriate use of hormone and cytotoxic drugs reduce or delay tumour-related symptoms. According to our results, the main disadvantages of PET/CT are low specificity and low PPV, which perhaps could be improved by increasing the sample size. The right diagnoses of disease relapse and metastasis locations are needed to decide therapeutic management, improving the quality of life of cancer patients. The GIVIO investigators [13] reported that it is mandatory to choose the right follow-up protocols both in disease-free patients and in those with symptomatic progression and metastatic disease. For example, the early detection of bone metastasis may benefit patients, since they could receive early treatment with bisphosphonates to reduce bone fractures and further tumour dissemination in the skeleton [14]. The impact of PET/CT on management modification in BC patients with rising tumour markers has already been reported by Buck et al. [15], showing a change in 36% of patients. In our study, PET/CT alone determined therapeutic management change in 56% of patients. The possible advantage of FDG PET in this clinical situation and the influence on therapeutic strategy have been investigated only in a few studies so far [16–23]. In our study, PET/CT also identified a patient with second cancer (lymphoma), allowing suitable therapy. The main limitation of this study is that the number of patients and of disease relapses in our collective is quite small; thus, for the confirmation of our results further larger studies will be necessary. Furthermore, for the calculation of sensitivity, specificity and other parameters a person-based approach was used instead of one that was lesion-based. This procedure reflects that treatment decisions are generally made based on the presence of recurrent or metastatic disease, rather than the number of lesions involved. Consequently, it is clinically more relevant to consider the patient-based data rather than the lesion-based analysis. A general problem is also to establish standard criteria for follow-up. We used, where it was possible, the histological findings, otherwise a comparison with other modalities of imaging. Finally, a low specificity demonstrated by PET/CT could be explained by criteria used for the definition of a positive scan.
Future prospective
Grunfeld et al. [24] concluded that irrespective of the nature of the follow-up examinations up to 69% of recurrence and metastases occur as interval events, becoming obvious within different periods of time. PET/CT might be useful to reduce this phenomenon, considering its peculiarity to assess early metabolic abnormalities. Clearly, further studies are mandatory to evaluate its diagnostic power. The first step could be to design prospective studies in which the role of PET/CT in the detection of disease relapse will be considered in high-risk patients. In our series, the number of triple negative cancer patients (oestrogen receptor, progesterone receptor and c-erbB2, all negative) with disease relapse was significantly higher than in patients with hormone receptor-positive/c-erbB2-positive (50 vs 25%, p < 0.05). Moreover, the next studies could address the impact on patient management as well as the cost-effectiveness of FDG PET/CT.
Conclusions
FDG PET/CT appears to be more sensitive than CT and CA 15.3 in the evaluation of disease relapse. The metabolic information provided by hybrid imaging PET/CT might be considered as a complement to other common techniques during long-term follow-up, increasing the sensitivity in the evaluation of potential disease sites. Nevertheless, both CA 15.3 and PET/CT are based on metabolic changes due to tumour activity. They provide information on disease progression in a different way than conventional imaging, but PET/CT seems to better predict the presence of disease relapse than tumour marker values in patients with BC.
References
van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000;92:1143–50.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007;25:5287–312.
Grassetto G, Fornasiero A, Otello D, Bonciarelli G, Rossi E, Nashimben O, Minicozzi AM, Crepaldi G, Pasini F, Facci E, Mandoliti G, Marzola MC, Al-Nahhas A, Rubello D. (18)F-FDG-PET/CT in patients with breast cancer and rising Ca 15-3 with negative conventional imaging: a multicentre study. Eur J Radiol 2010 May 21 [Epub ahead of print]
Siggelkow W, Rath W, Buell U, Zimny M. FDG PET and tumour markers in the diagnosis of recurrent and metastatic breast cancer. Eur J Nucl Med Mol Imaging 2004;31:S118–24.
Liu CS, Shen YY, Lin CC, Yen RF, Kao CH. Clinical impact of [(18)F]FDG-PET in patients with suspected recurrent breast cancer based on asymptomatically elevated tumor marker serum levels: a preliminary report. Jpn J Clin Oncol 2002;32:244–7.
Suárez M, Pérez-Castejón MJ, Jiménez A, Domper M, Ruiz G, Montz R, et al. Early diagnosis of recurrent breast cancer with FDG-PET in patients with progressive elevation of serum tumor markers. Q J Nucl Med 2002;46:113–21.
Flamen P, Hoekstra OS, Homans F, Van Custem E, Maes A, Stroobants S, et al. Unexplained rising carcinoembryonic antigen (CEA) in the postoperative surveillance of colorectal cancer: the utility of positron emission tomography (PET). Eur J Cancer 2001;37:862–9.
Zangheri B, Messa C, Picchio M, Gianolli L, Landoni C, Fazio F. PET/CT and breast cancer. Eur J Nucl Med Mol Imaging 2004;31:S135–42.
Strauss LG, Conti PS. The application of PET in clinical oncology. J Nucl Med 1991;32:623–48.
Brown RS, Leung JY, Fisher SJ, Frey KA, Ethier SP, Wahl RL. Intratumoral distribution of tritiated-FDG in breast carcinoma: correlation between Glut-1 expression and FDG uptake. J Nucl Med 1996;37:1042–47.
Haug AR, Schmidt GP, Klingenstein A, Heinemann V, Stieber P, Priebe M, et al. F-18-Fluoro-2-deoxyglucose positron emission tomography/computed tomography in the follow-up of breast cancer with elevated levels of tumor markers. J Comput Assist Tomogr 2007;31:629–34.
Kamel EM, Wyss MT, Fehr MK, von Schulthess GK, Goerres GW. [18F]-Fluorodeoxyglucose positron emission tomography in patients with suspected recurrence of breast cancer. J Cancer Res Clin Oncol 2003;129:147–53.
The GIVIO Investigators. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. A multicenter randomized controlled trial. JAMA 1994;271:1587–92.
Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med 1998;339:357–63.
Buck A, Schirrmeister H, Kühn T, Shen C, Kalker T, Kotzerke J, et al. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging 2002;29:1317–23.
Eubank WB, Mankoff DA, Vesselle HJ, Eary JF, Schubert EK, Dunnwald LK, et al. Detection of locoregional and distant recurrences in breast cancer patients by using FDG PET. Radiographics 2002;22:5–17.
Eubank WB, Mankoff DA, Takasugi J, Vesselle H, Eary JF, Shanley TJ, et al. 18Fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol 2001;19:3516–23.
Hathaway PB, Mankoff DA, Maravilla KR, Austin-Seymour MM, Ellis GK, Gralow JR, et al. Value of combined FDG PET and MR imaging in the evaluation of suspected recurrent local-regional breast cancer: preliminary experience. Radiology 1999;210:807–14.
Moon DH, Maddahi J, Silverman DH, Glaspy JA, Phelps ME, Hoh CK. Accuracy of whole-body fluorine-18-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med 1998;39:431–5.
Bender H, Kirst J, Palmedo H, Schomburg A, Wagner U, Ruhlmann J, et al. Value of 18fluoro-deoxyglucose positron emission tomography in the staging of recurrent breast carcinoma. Anticancer Res 1997;17:1687–92.
Lonneux M, Borbath II, Berlière M, Kirkove C, Pauwels S. The place of whole-body PET FDG for the diagnosis of distant recurrence of breast cancer. Clin Positron Imaging 2000;3:45–9.
Minn H, Soini I. [18F]Fluorodeoxyglucose scintigraphy in diagnosis and follow up of treatment in advanced breast cancer. Eur J Nucl Med 1989;15:61–6.
Yap CS, Seltzer MA, Schiepers C, Gambhir SS, Rao J, Phelps ME, et al. Impact of whole-body 18F-FDG PET on staging and managing patients with breast cancer: the referring physician’s perspective. J Nucl Med 2001;42:1334–7.
Grunfeld E, Mant D, Yudkin P, Adewuyi-Dalton R, Cole D, Stewart J, et al. Routine follow up of breast cancer in primary care: randomised trial. BMJ 1996;13:665–9.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Evangelista, L., Baretta, Z., Vinante, L. et al. Tumour markers and FDG PET/CT for prediction of disease relapse in patients with breast cancer. Eur J Nucl Med Mol Imaging 38, 293–301 (2011). https://doi.org/10.1007/s00259-010-1626-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1626-7