Abstract
Purpose
4-[18F]-ADAM is a potent serotonin transport imaging agent. We studied its toxicity in rats and radiation dosimetry in monkeys before human studies are undertaken.
Methods
Single and multiple-dosage toxicity studies were conducted in Sprague-Dawley rats. Male and female rats were injected intravenously with 4-F-ADAM as a single dose of 1,023.7 μg/kg (1,000 times the human dose) or as five consecutive daily doses of 102.37 μg/kg (100 times the human dose). PET/CT scans were performed in seven Formosa Rock monkeys (four males and three females) using a Siemens Biograph scanner. After injection of 4-[18F]-ADAM (182±8 MBq), a low dose CT scan and a series of eight whole-body PET scans were performed. Whole-body images were acquired in 3-D mode. Time–activity data of source organs were used to calculate the residence times and estimate the absorbed radiation dose using OLINDA/EXM software.
Results
In the rats neither the single dose nor the five daily doses of 4-F-ADAM produced overt adverse effects clinically. In the monkeys the radiation doses received by most organs ranged between 7.1 and 35.7 μGy/MBq, and the urinary bladder was considered to be the critical organ. The effective doses extrapolated to male and female adult humans were 17.4 and 21.8 μSv/MBq, respectively.
Conclusion
Toxicity studies in Sprague-Dawley rats and radiation dosimetry studies in Formosa Rock monkeys suggested that 4-[18F]-ADAM is safe for use in human PET imaging studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abnormalities in the serotonin transporter (SERT) have been implicated in several neurological and psychiatric disorders [1, 2]. It is also the target for selective serotonin reuptake inhibitors [3, 4]. In vivo SERT imaging in humans would assist in the early diagnosis and follow-up of treatment in these diseases [5]. In the 1990s, [11C]-(+)-McN5652 was the most promising PET agent for studying SERT in humans [6–8]. However, this agent has high nonspecific binding and has only moderate signal contrast in human PET studies [9]. Its 18F analogues, [18F]FEt-McN [10] and [18F]FMe-McN [11] have shown some utility as SERT imaging agents in animals [12–14]. Recently, N,N-dimethyl-2-(arylthio)benzylamines (403U76) have been reported to possess very high selectivity and affinity for SERT binding sites [15]. Their analogues, [11C]N,N-dimethyl-2-(2-amino-4-cyano-phenylthio)benzylamine ([11C]DASB) [16], [11C]N,N-dimethyl-2-(2-amino-4-fluoromethylphenylthio)benzylamine ([11C]AFM) [17], [11C]N,N-dimethyl-2-(2-amino-4-methylphenylthio)benzylamine ([11C]MADAM) [18] and [11C]N,N-dimethyl-2-(2′-amino-4′-hydroxymethyl-phenylthio)benzylamine ([11C]HOMADAM) [19] were synthesized and found to be potential radioligands for studying SERT in humans [20–23]. Nevertheless, 18F has some advantages over 11C. Thus, 18F-labelled 403U76 analogues such as (2-[2-amino-4-chloro-5-[18F]fluorophenylthio)-N,N-dimethyl-benzenemethanamine ([18F]ACF) [24], N,N-dimethyl-2-(2-amino-4-[18F]fluorophenylthio)benzylamine (4-[18F]-ADAM) [25, 26], 2-[2-(dimethylaminomethyl)phenylthio]-5-[18F]fluoromethylphenylamine ([18F]AFM) [27], and 2-(2′-((dimethylamino)methyl)-4′-(3-[18F]fluoropropoxy)phenylthio)benzeneamine [28] have been synthesized and evaluated in animals [24, 27–30] as potent SERT imaging agents. Preliminary studies in rats and baboons have shown that 4-[18F]-ADAM is suitable for studying SERT in the living brain using PET [29]. Thus, we studied the toxicity and radiation dosimetry of 4-[18F]-ADAM in rats and monkeys before human studies are undertaken. We report here the acute toxicity and radiation dosimetry of 4-[18F]-ADAM in rats and monkeys.
Material and methods
Acute toxicity studies of 4-F-ADAM in rats
Synthesis of 4-F-ADAM
The 4-F-ADAM was synthesized by Chemistry and Life Sciences Division, Research Triangle Institute, Research Triangle Park, NC, using a method reported previously [25] under contract with the National Institute of Mental Health (NIMH, contract number N01-MH-32005). The identity and chemical purity of this compound was confirmed by 1H NMR, mass spectral elemental analysis, HPLC and TLC.
Acute toxicity studies
The toxicity studies were carried out in male and female Sprague-Dawley rats by SRI International Toxicology Laboratory, Menlo Park, CA, and were fully supported by NIMH (M422-05) under the Molecular Pharmacology Research Program.
Animal procedures conducted at SRI were approved by SRI’s Institutional Animal Care and Use Committees (IACUC). SRI’s animal facilities have been accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC). SRI’s facilities are registered with the US Department of Agriculture as a Research Facility. SRI files assurances with the Public Health Service (PHS) Office of Laboratory Animal Welfare (OLAW) and adheres to PHS standards and practices.
Animals
Thirty male (6–8 weeks old, weighing 175–225 g) and 30 female (7–9 weeks old, weighing 150–200 g) Sprague-Dawley rats (Charles River Laboratories) were used in this study. Upon arrival, the rats were placed in quarantine for 3 days. The general appearance of the animals was evaluated by the attending veterinarian, who documented that the animals were healthy before releasing them from quarantine. The rats were fed with Purina Certified Rodent Chow (#5002; Richmond, IN) ad libitum. Water (purified, reverse osmosis) was provided ad libitum during quarantine and study periods. The rats were housed (five per cage) in stainless steel cages in an animal room that was monitored for temperature (65–79°F, 18–26°C) and humidity (30–70%) with a light cycle of 12 h light/12 h dark.
Preparation of dose formulation
The test article, 4-F-ADAM, was formulated in sterile phosphate-buffered saline (Gibco Invitrogen, Grand Island, NY). Dose formulations were prepared by dissolving the appropriate amount of test article in the vehicle to achieve target concentrations using a sterile stir bar and sonication. Dose formulations were stored refrigerated at 2–8°C until the day of use. Formulations were brought to room temperature prior to administration to the animals. Drugs were administered via intravenous (i.v.) injection into a lateral tail vein. The i.v. route is intended for human clinical trials. Therefore, i.v. injection was selected to model the intended route of administration to humans.
Verification of formulation
All 4-F-ADAM formulations used for the toxicity studies were analysed by SRI staff using reversed-phase HPLC methods. The conditions were as follows: Luna C-18 analytical column (4.6×250 mm, 5 μm particle size); mobile phase linear gradient (solvent A/solvent B 85/15 for 10 min followed by 50/50 for 1 min and then 85/15 for 4 min); flow rate 1.0 ml/min; UV detector 245 nm; ambient temperature. Solvent A comprised 0.1% TFA in water, solvent B 0.1% TFA in CH3CN. The HPLC system used was a Hewlett-Packard Model 1100 Series liquid chromatography system. Data were analysed using HP Chemstation Software; version A.06. Concentration, homogeneity, and stability of the formulation were confirmed before testing and were found to be within 10% of target concentrations for all treatment groups.
Dosage calculation
The procedures used to determine the 4-F-ADAM dosage to be administered to rats required that all dosages were converted from units of milligrams per kilogram body weight to units of milligrams per square metre body surface area. Basing the dosage on body surface area allows determination of an equivalent dosage in another species. The equation used was:
where F is a constant based on the species of animal being tested (a value of 37 for humans). Dosages were based on the body weight recorded on day 1 before dosing (and converted to milligrams per square metre), and preset multiples of the maximum proposed dose of 8.3 μg to be administered to a 50-kg person were administered to the test dosage groups (Table 1). The dosages used in these studies, expressed in both milligrams per kilogram and milligrams per square metre, as well as individual species F values are presented in Table 1
.
Dosing procedure
Male and female rats (ten males and ten females per group) were given 4-F-ADAM i.v. as a single dose of 1,023.7 μg/kg (1,000 times the human dose) on day 1 or as five daily doses of 102.37 μg/kg per day (100 times the human dose) on days 1–5. Control rats (ten males, ten females) were given a single i.v. dose of vehicle (sterile phosphate-buffered saline) at an equivalent volume (5 ml/kg) on day 1. Dosing of the 5-day dosing group was initiated 4 days prior to dosing of the single-dose animals.
Observation protocol
All animals in this study were observed twice-daily on weekdays and once daily at weekends for signs of mortality, morbidity, injury, and availability of food and water. Detailed clinical observations were recorded daily throughout the study. Individual body weights were measured and recorded for each animal on day 1 prior to dosing and at necropsy (days 3 and 15 for groups 1 and 2, and days 7 and 19 for group 3). Food consumption was measured for a period of about 24 hours twice weekly throughout the study. Blood for clinical pathology evaluation was collected on days 3, 7, 15 and 19. Animals were killed on days 3 and 15 (interim and terminal necropsy of groups 1 and 2) or days 7 and 19 (interim and terminal necropsy of group 3) by overdose of sodium pentobarbital (150 mg/kg) administered by i.p. injection.
Clinical pathology
After collection from SRI, the clinical pathology samples were sent to Quality Clinical Labs (haematology and clinical chemistry parameters; Mountain View, CA) and AniLytics (coagulation parameters; Gaithersburg, MD). Blood samples were collected into tubes containing EDTA (haematology samples), sodium citrate (coagulation samples) or no anticoagulant (clinical chemistry samples).
Gross necropsy
On days 3, 7, 15 and 19, after recording body weight and collection of clinical pathology blood samples, animals were killed and given a complete post-mortem examination, which included a thorough inspection of all body orifices and surfaces and an examination of all cranial, thoracic and abdominal organs. The weights of the following organs were recorded: adrenal glands, brain, heart, kidneys, liver, spleen, thymus, testes and ovaries. All of the organs and tissues were retained and fixed in phosphate-buffered 10% formalin.
Histopathologic examination
Sections of the retained tissues were embedded in paraffin, cut at a thickness of approximately 5 μm, and stained with haematoxylin and eosin by Environmental Pathology Laboratories (Herndon, VA). All tissues from animals in the control and high dosage groups were examined by a board-certified veterinary pathologist. If a tissue had gross findings at the time of necropsy, the same tissue from the low and mid dosage groups was also examined.
Statistical analyses
One-way ANOVA was performed for body weight, food consumption, organ weights and clinical pathology data (LABCAT In-Life v. 6.2). When appropriate, a post-hoc analysis (Dunnett’s t-test) was carried out. In all cases, statistical significance was defined as p≤0.05.
Dosimetry studies of 4-[18F]-ADAM in monkeys
Synthesis of 4-[18F]-ADAM
The radioligand 4-[18F]-ADAM was synthesized in an automated synthesizer by fluorination of the corresponding nitro precursor with K[18F]/K2.2.2 followed by reduction with NaBH4/Cu(OAc)2 and purification by HPLC and solid phase extraction [25, 26]. The radiochemical yield of 4-[18F]-ADAM was 1.5±0.3% (n=13, EOS) and the mean specific radioactivity was 64.8±28.5 GBq/μmol (n=13, EOS) at the time of injection. The radiochemical and chemical purities were >95% and 98%, respectively, as determined by HPLC and TLC.
Animals
The animal study protocol used for this study was approved by the IACUC of the National Defense Medical College. Seven fasted Formosan Rock monkeys (Macaca cyclopis) comprising three females (5.3±1.5 kg) and four males (5.6±0.8 kg) were immobilized with ketamine (2 mg/kg), anaesthetized with 2% isoflurane via an endotracheal tube, and given atropine sulphate (2 mg i.m.) to minimize secretions during the course of the experiment. Body temperature was kept constant at 37°C with an additional blanket. The heart rate, pO2 and pCO2 were checked every 10 min and kept in the normal range throughout the imaging sessions.
PET imaging protocol
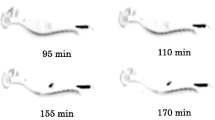
Whole-body transmission and emission scans were acquired with a Biograph PET/CT scanner (Biograph Duo, Siemens, USA) which has a 58.5-cm transverse and a 15.5-cm axial field of view, and 4.8 mm FWHM spatial resolution. After injection of 4-[18F]-ADAM (182±8 MBq), a low-dose CT scan (130 kVp, 50 mAs) and a series of eight whole-body PET scans were performed (15, 55, 95, 110, 155, 170, 215 and 230 min, respectively, after injection). Total acquisition time was 240 min. Each scan covered the monkey’s body from the head to the thigh and consisted of five or six bed positions depending on the size of the monkey. In each bed position, data were acquired for 2 min in 3-D mode. The data were then reconstructed by OS-EM (ordered subsets expectation maximization) on a 128×128 matrix (slice thickness 5 mm), two iterations eight subsets, 3-mm FWHM gaussian filter, and corrected by photon attenuation using the CT scan.
Image data analysis
The image data analysis and residence time calculation were performed by methods reported previously, with some modifications [31–33]. Each CT and PET whole-body image was successively loaded to the PMOD 2.5 software (PMOD Technologies, Switzerland; www.pmod.com) to generate the fusion images. Organs with high uptake of 4-[18F]-ADAM, such as brain, lungs, heart, liver, gallbladder, kidneys, spleen and bladder were easily identified and outlined. Organs with low uptake of 4-[18F]-ADAM or complicated anatomy, such as stomach, intestine and thyroid were processed manually. The regions of interest of these organs were manually drawn as precisely as possible on the organ itself on each horizontal slice. The activity in each organ was non-decay-corrected and expressed as percent injected dose per organ (n=7, mean±SD).
Residence times and absorbed dose calculations
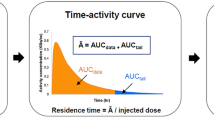
To calculate the absorbed radiation dose of each organ as well as the effective dose, the injected dose of 4-[18F]-ADAM and the non-decay-corrected time–activity curves (TACs) of the brain, lungs, heart, liver, gallbladder, spleen, kidneys, and bladder of seven monkeys were individually entered into an Excel spreadsheet and the data processed individually for each animal. The difference between the injected dose and the sum of the whole-body radioactivity (assuming that there was no excretion) plus the radioactivities of the above-mentioned organs was taken as the TAC of the remainder.
The residence time (hours) of the selected organ was calculated as the area under the TAC of the source organ from time zero to infinity over the initial total body activity (about five half-lives of 18F, i.e. about 550 min). The area under the TAC of each organ (brain, lungs, heart, liver, kidneys , spleen and bladder) was generated using the following strategy including trapezoidal integration of the first four TAC data points through the origin and exponential decline of the four remaining TAC data points to infinity such that the residence times of these organs were obtained [31].
The human dosimetry was estimated from both the male and female monkey biodistribution data. The organ weight and body mass were used for allometric scaling [33]. That is, the residence time in each organ was converted to the corresponding human value by multiplication by a factor to scale organ and body weights (in kilograms) as (wm,b/wm,o)(wh,o/wh,b), where wm,b is the monkey body weight, wm,o is the monkey organ weight, wh,b is the human body weight and wh,o is the human organ weight. However, the wm,o values for the Formosan Rock monkey were unavailable, so instead we used the wm,o values for the rhesus monkey (Macaca mulatta) in our calculations. Both monkey organ weights [34, 35] and human organ weights [36] were obtained from the literature. The residence time of 4-[18F]-ADAM in the rest of the body was obtained by subtracting the total organ residence time from the reciprocal of the 18F decay constant.
Absorbed radiation doses were calculated from the residence times in all source organs for each monkey by entering the information into the Java-based OLINDA 1.0/EXM computer program [37] using the model for a 70-kg adult male and female phantom.
Results
Acute toxicity of 4-F-ADAM in rats
The acute toxicity of 4-F-ADAM was studied in rats using single dosing and multiple dosing paradigms. The following parameters were evaluated: mortality/morbidity, clinical observations, body weight, food consumption, clinical pathology (haematology and serum chemistry), organ weights, necropsy macroscopic observation and histopathology. All animals survived until their scheduled necropsy. Soft stool was observed in four of ten male animals in the repeat-dosing group versus one of ten males in the control group. No other drug-related clinical adverse effects were observed, such as hyperactive, agitated or otherwise changed behaviour and myoclonus were observed. In addition, no adverse clinical pathological effects were observed, such as hyponatraemia and increased bleeding time, body weight, food consumption or organ weights, and necropsy findings (Table 1)
Protocol-specified tissues from all animals were evaluated microscopically. Minimal to mild inflammation and myofibre necrosis/degeneration were present in skeletal muscle from two of five single-dosing males and one of five repeat-dosing males at the interim time of death, and from one of five single-dosing males at the terminal time of death. Minimal inflammation was also present in skeletal muscle from one of five control group females at the terminal time of death.
Biodistribution of 4-[18F]-ADAM in monkeys
Typical whole-body biodistribution of 4-[18F]-ADAM in male and female monkeys are shown in Figs. 1 and 2, respectively. The uptake and the TACs of 4-[18F]-ADAM in selected organs are shown in Table 2 and Fig. 3, and are expressed as percent injected dose per organ (non-decay-corrected). The uptake of 4-[18F]-ADAM in the liver, brain, lungs and kidneys were high in the early time points and declined slowly, whereas the uptake in the gallbladder and bladder were low in the beginning and gradually increased throughout the experiments.
Absorbed radiation dose estimates
The residence times of selected organs in monkeys are shown in Table 2. Among these organs, the bladder, liver and lungs had long residence times. Using the above residence times, the individual organ doses in humans extrapolated from the male and female monkeys were calculated with OLINDA/EXM software and the results are shown in Table 3. The absorbed radiation dose data showed that the bladder would receive the highest dose followed by the lungs, kidneys and liver. The effective doses in male and female humans, according to ICRP 60 [38], extrapolated from the data in male and female monkeys were 17.4 and 21.8 μSv/MBq, respectively.
Discussion
4-[18F]-ADAM has been demonstrated to be a potent serotonin transport imaging agent in rats and baboon [29, 30]. In order to evaluate it as a promising SERT imaging agent in human, it is necessary to estimate its mass dosage associated with no observable adverse effect level (NOAEL) and the organ radiation dose burden in animal models before human PET studies are undertaken.
The acute toxicity studies of 4-F-ADAM were carried out in rats to determine potential toxic effects, identify potential target organs of toxicity, and determine its NOAEL. Since some patients may need to receive 4-[18F]-ADAM twice within 1 or 2 weeks, we used both single dosing and multiple dosing paradigms for the rat toxicity studies. The dosages we administered to the rats were 100 to 1,000 times more than will be administered to humans. Intravenous administration of 4-F-ADAM to male and female rats for a single day at 1,023.7 μg/kg (6,142.2 μg/m2) or for 5 days at 102.37 μg/kg per day (614.22 μg/m2 per day) did not produce overt adverse effects clinically. Additionally, we found no clear evidence that skeletal muscle lesions were related to the treatment with 4-F-ADAM. Given the fact that this microscopic finding in the skeletal muscle of the treated males occurred with a low incidence and with minor severity and that a similar lesion was observed in a control female, it is considered to be of minimal toxicological significance. Soft stool observed in four out of ten repeat-dosing animals may have reflected a serotonergic effect and is also considered to be of minor toxicological significance and is not expected to be a dose-limiting effect in clinical trials. Thus, the NOAEL is considered to be greater than 1,023.7 μg/kg for a single i.v. dose and greater than 102.37 μg/kg per day for a 5-day i.v. dosage regimen.
The organ radiation absorbed dose studies of 4-[18F]-ADAM were carried out in Formosan Rock monkeys. Following 4-[18F]-ADAM injection, the uptakes of radioactivity in the brain and liver were high in the beginning and then declined gradually thereafter. The radioactivity in the gallbladder and bladder increased with time suggesting the 4-[18F]-ADAM may be eliminated via the hepatobiliary and renal systems as are [11C]DASB and [123I]ADAM [31, 33, 39–43]. The uptake of 4-[18F]-ADAM in the lungs was high (6.1 %ID/organ at 15 min after injection) which has also been observed for other monoamine transporter radioligands, such as [11C]cyanoimipramine, [11C]-(+)-McN5652, [11C]DASB, (S,S)-[18F]FMeNER-D2, [18F]FECNT, [123I]ADAM and [123I]β-CIT [33, 40, 41, 44–47]. The exact reason(s) for the high uptake of these radioligands in the lungs is unclear. However, it has been suggested that this may be due to the specific binding of the circulating 5-HT on pulmonary membranes, nonspecific binding of amine by macrophages, and the large blood volume in the lungs [41, 46, 48–51].
The guidelines on radiation exposure for human subjects involved in research studies varies internationally. Radiation risk estimates were recently issued by the National Institutes of Health (NIH) in the United States and the effective dose estimates are considered to be the most accurate measure of radiation risks. Based on the NIH guidelines, the maximum exposure is 50 mSv of effective dose per year for a research subject [52]. On the other hand, under the guidelines of the European Commission, the intermediate risk levels in adults is equivalent to an effective dose range of 1–10 mSv per annum [53]. To estimate the human dosimetry from the Formosan Rock monkey whole-body PET image data, the organ weight and body mass were used for allometric scaling after obtaining the residence time of each organ for each subject. However, the organ weights of Formosan Rock monkey are unavailable and the rhesus monkeys (Macaca mulatta) are genetically similar to the Formosan Rock monkeys (Macaca cyclopis) [54, 55]. We therefore used organ weights of the rhesus monkey to calculate the human dosimetry. The results (Table 3) showed that the human estimated dosimetry of 4-[18F]-ADAM in most organs ranged between 7.1 and 35.7 μGy/MBq, and were comparable with those of other SERT [31, 33, 39–43], DAT or NET imaging agents [44–46, 56] (Table 4). The critical organ of 4-[18F]-ADAM was the urinary bladder that has also been reported for other monoamine transporter radioligands [33, 40, 43, 45, 46, 56]. However, compared to other widely used 18F radiopharmaceuticals, such as [18F]FDG (119 μGy/MBq) [57] and [18F]FDOPA (159 μGy/MBq) [58], the radiation dose of 4-[18F]-ADAM in the urinary bladder was relatively low. Increasing both the bladder volume and frequency of voiding will reduce the radiation dose in the urinary bladder [59–61].
In addition, in this study almost all organs, except the urinary bladder, had higher 4-[18F]-ADAM uptakes in females (n=3) than in males (n=4), particularly the liver, lungs and kidneys. The gender differences in radiation dosimetry of this SERT imaging agent have also been observed in a small group of adult females (n=4) and adult males (n=3) using 123I-ADAM as an imaging agent [42]. However, a recent study in humans has shown that, compared to males, females have significantly higher 5-HT1A receptor and lower SERT binding potentials in a wide array of cortical and subcortical brain regions [62]. The exact reason(s) for this discrepancy and gender differences are unclear. A systematic study with a large population may be warranted.
Conclusion
The toxicity studies in rats showed that i.v. administration of 4-F-ADAM as a single dose of 1,023.7 μg/kg (1,000 times the human dose) or as five daily doses of 102.37 μg/kg per day (100 times the human dose) did not produce overt adverse effects clinically. The radiation dosimetry estimates obtained in Formosa Rock monkeys extrapolated to humans suggested that the received doses in most organs in humans would range between 7.1 and 35.7 μGy/MBq. The urinary bladder was considered to be the critical organ. It received around 96.6 and 90.4 μGy/MBq for male and female human adults, respectively, which are below NIH guidelines, These results suggest that 4-[18F]-ADAM is safe for human studies from both pharmacological and radiation perspectives.
References
Mann JJ. Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior. Neuropsychopharmacology 1999;21:99S–105S.
Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, Moore RY, et al. Serotonin in aging, late-life depression, and Alzheimer’s disease: the emerging role of functional imaging. Neuropsychopharmacology 1998;18:407–30.
Frazer A. Antidepressants. J Clin Psychiatry 1997;58:9–25.
Horschitz S, Hummerich R, Schloss P. Down-regulation of the rat serotonin transporter upon exposure to a selective serotonin reuptake inhibitor. Neuroreport 2001;12:2181–4.
Brust P, Hesse S, Mueller U, Szabo Z. Neuroimaging of the serotonin transporter: possibilities and pitfalls. Curr Psychiatry Rev 2006;2:111–49.
Szabo Z, Kao PF, Scheffel U, Suehiro M, Mathews WB, Ravert HT, et al. Positron emission tomography imaging of serotonin transporters in the human brain using [11C](+)McN5652. Synapse 1995;20:37–43.
Parsey RV, Kegeles LS, Hwang DR, Simpson N, Abi-Dargham A, Mawlawi O, et al. In vivo quantification of brain serotonin transporters in humans using [11C]McN 5652. J Nucl Med 2000;41:1465–77.
Parsey RV, Hastings RS, Oquendo MA, Huang YY, Simpson N, Arcement J, et al. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry 2006;163:52–8.
Frankle WG, Huang Y, Hwang DR, Talbot PS, Slifstein M, Van Heertum R, et al. Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J Nucl Med 2004;45:682–94.
Suehiro M, Greenberg JH, Shiue CY, Gonzalez C, Dembowski B, Reivich M. Radiosynthesis and biodistribution of the S-[18F]fluoroethyl analog of McN5652. Nucl Med Biol 1996;23:407–12.
Zessin J, Eskola O, Brust P, Bergman J, Steinbach J, Lehikoinen P, et al. Synthesis of S-([18F]fluoromethyl)-(+)-McN5652 as a potential PET radioligand for the serotonin transporter. Nucl Med Biol 2001;28:857–63.
Brust P, Zessin J, Kuwabara H, Pawelke B, Kretzschmar M, Hinz R, et al. Positron emission tomography imaging of the serotonin transporter in the pig brain using [11C](+)-McN5652 and S-([18F]fluoromethyl)-(+)-McN5652. Synapse 2003;47:143–51.
Marjamäki P, Zessin J, Eskola O, Grönroos T, Haaparanta M, Bergman J, et al. S-[18F] fluoromethyl-(+)-McN5652, a PET tracer for the serotonin transporter: evaluation in rats. Synapse 2003;47:45–53.
Kretzschmar M, Brust P, Zessin J, Cumming P, Bergmann R, Johannsen B. Autoradiographic imaging of the serotonin transporter in the brain of rats and pigs using S-([18F]fluoromethyl)-(+)-McN5652. Eur Neuropsychopharmacol 2003;13:387–97.
Ferris RM, Brieaddy L, Mehta N, Hollingsworth E, Rigdon G, Wang C, et al. Pharmacological properties of 403U76, a new chemical class of 5-hydroxytryptamine- and noradrenaline-reuptake inhibitor. J Pharm Pharmacol 1995;47:775–81.
Wilson AA, Ginovart N, Schmidt M, Meyer JH, Threlkeld PG, Houle S. Novel radiotracers for imaging the serotonin transporter by positron emission tomography: synthesis, radiosynthesis, and in vitro and ex vivo evaluation of 11C-labeled 2-(phenylthio)araalkylamines. J Med Chem 2000;43:3103–10.
Huang Y, Hwang DR, Bae SA, Sudo Y, Guo N, Zhu Z, et al. A new positron emission tomography imaging agent for the serotonin transporter: synthesis, pharmacological characterization, and kinetic analysis of [11C]2-[2-(dimethylaminomethyl)phenylthio]-5-fluoromethylphenylamine ([11C]AFM). Nucl Med Biol 2004;31:543–56.
Tarkiainen J, Vercouillie J, Emond P, Sandell J, Hiltunen J, Frangin Y, et al. Carbon-11 labelling of MADAM in two different positions: a highly selective PET radioligand for the serotonin transporter. J Labelled Cpd Radiopharm 2001;44:1013–23.
Jarkas N, Votaw JR, Voll RJ, Williams L, Camp VM, Owens MJ, et al. Carbon-11 HOMADAM: a novel PET radiotracer for imaging serotonin transporters. Nucl Med Biol 2005;32:211–24.
Nye JA, Votaw JR, Jarkas N, Purselle D, Camp V, Bremner JD, et al. Compartmental modeling of 11C-HOMADAM binding to the serotonin transporter in the healthy human brain. J Nucl Med 2008;49:2018–25.
Ginovart N, Wilson AA, Meyer JH, Hussey D, Houle S. Positron emission tomography quantification of [11C]-DASB binding to the human serotonin transporter: modeling strategies. J Cereb Blood Flow Metab 2001;21:1342–53.
Nabulsi N, Williams W, Planeta-Wilson B, Labaree D, Ropchan J, Neumeister A, et al. The serotonin transporter tracer [11C]AFM provides high specific binding signals in humans. J Nucl Med 2008;49:80P.
Lundberg J, Odano I, Olsson H, Halldin C, Farde L. Quantification of 11C-MADAM binding to the serotonin transporter in the human brain. J Nucl Med 2005;46:1505–15.
Oya S, Choi SR, Coenen H, Kung HF. New PET imaging agent for the serotonin transporter: [18F]ACF (2-[(2-amino-4-chloro-5-fluorophenyl)thio]-N,N-dimethyl-benzenmethanamine). J Med Chem 2002;45:4716–23.
Shiue GG, Fang P, Shiue CY. Synthesis of N,N-dimethyl-2-(2-amino-4-[18F]fluorophenylthio)benzylamine as a serotonin transporter imaging agent. Appl Radiat Isot 2003;58:183–91.
Peng CJ, Huang YY, Huang WS, Shiue CY. An automated synthesis of N,N-dimethyl-2-(2-amino-4-[18F]fluorophenylthio)benzylamine (4-[18F]-ADAM) for imaging serotonin transporters. Appl Radiat Isot 2008;66:625–31.
Huang Y, Bae SA, Zhu Z, Guo N, Roth BL, Laruelle M. Fluorinated diaryl sulfides as serotonin transporter ligands: synthesis, structure-activity relationship study, and in vivo evaluation of fluorine-18-labeled compounds as PET imaging agents. J Med Chem 2005;48:2559–70.
Wang JL, Parhi AK, Oya S, Lieberman B, Kung MP, Kung HF. 2-(2′-((Dimethylamino)methyl)-4′-(3-[18F]fluoropropoxy)-phenylthio)benzenamine for positron emission tomography imaging of serotonin transporters. Nucl Med Biol 2008;35:447–58.
Shiue GG, Choi SR, Fang P, Hou C, Acton PD, Cardi C, et al. N,N-dimethyl-2-(2-amino-4-[18F]-fluorophenylthio)-benzylamine (4-[18F]-ADAM): an improved PET radioligand for serotonin transporters. J Nucl Med 2003;44:1890–7.
Ma KH, Huang WS, Kuo YY, Peng CJ, Liou NH, Liu RS, et al. Validation of 4-[18F]-ADAM as a SERT imaging agent using micro-PET and autoradiography. Neuroimage 2009;45:687–93.
Belanger MJ, Simpson NR, Wang T, Van Heertum RL, Mann JJ, Parsey RV. Biodistribution and radiation dosimetry of [11C]DASB in baboons. Nucl Med Biol 2004;31:1097–102.
Herzog H, Elmenhorst D, Winz O, Bauer A. Biodistribution and radiation dosimetry of the A1 adenosine receptor ligand 18F-CPFPX determined from human whole-body PET. Eur J Nucl Med Mol Imaging 2008;35:1499–506.
Tipre DN, Lu JQ, Fujita M, Ichise M, Vines D, Innis RB. Radiation dosimetry estimates for the PET serotonin transporter probe 11C-DASB determined from whole-body imaging in non-human primates. Nucl Med Commun 2004;25:81–6.
Cupp CJ, Uemura E. Body and organ weights in relation to age and sex in Macaca mulatta. J Med Primatol 1981;10:110–23.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res 1993;10:1093–5.
Stabin MG, Tagesson M, Thomas SR, Ljungberg M, Strand SE. Radiation dosimetry in nuclear medicine. Appl Radiat Isot 1999;50:73–87.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 2005;46:1023–7.
International Commission on Radiological Protection. 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication 60. New York: Pergamon Press; 1991.
Kauppinen TA, Bergström KA, Heikman P, Hiltunen J, Ahonen AK. Biodistribution and radiation dosimetry of [123I] ADAM in healthy human subjects: preliminary results. Eur J Nucl Med Mol Imaging 2003;30:132–6.
Lin KJ, Liu CY, Wey SP, Hsiao IT, Wu J, Fu YK, et al. Brain SPECT imaging and whole-body biodistribution with [123I]ADAM – a serotonin transporter radiotracer in healthy human subjects. J Nucl Med 2006;33:193–202.
Lu JQ, Ichise M, Liow JS, Ghose S, Vines D, Innis RB. Biodistribution and radiation dosimetry of the serotonin transporter ligand 11C-DASB determined from human whole-body PET. J Nucl Med 2004;45:1555–9.
Newberg AB, Plossl K, Mozley PD, Stubbs JB, Wintering N, Udeshi M, et al. Biodistribution and imaging with 123I-ADAM: a serotonin transporter imaging agent. J Nucl Med 2004;45:834–41.
Wilson AA, Ginovart N, Hussey D, Meyer J, Houle S. In vitro and in vivo characterisation of [11C]-DASB: a probe for in vivo measurements of the serotonin transporter by positron emission tomography. Nucl Med Biol 2002;29:509–15.
Tipre DN, Fujita M, Chin FT, Seneca N, Vines D, Liow JS, et al. Whole-body biodistribution and radiation dosimetry estimates for the PET dopamine transporter probe 18F-FECNT in non-human primates. Nucl Med Commun 2004;25:737–42.
Seneca N, Andree B, Sjoholm N, Schou M, Pauli S, Mozley PD, et al. Whole-body biodistribution, radiation dosimetry estimates for the PET norepinephrine transporter probe (S,S)-[18F]FMeNER-D2 in non-human primates. Nucl Med Commun 2005;26:695–700.
Takano A, Halldin C, Varrone A, Karlsson P, Sjoholm N, Stubbs JB, et al. Biodistribution and radiation dosimetry of the norepinephrine transporter radioligand (S,S)-[18F]FMeNER-D2: a human whole-body PET study. Eur J Nucl Med Mol Imaging 2008;35:630–6.
Seibyl JP, Wallace E, Smith EO, Stabin M, Baldwin RM, Zoghbi S, et al. Whole-body biodistribution, radiation absorbed dose and brain SPECT imaging with iodine-123-beta-CIT in healthy human subjects. J Nucl Med 1994;35:764–70.
Hart CM, Block ER. Lung serotonin metabolism. Clin Chest Med 1989;10:59–70.
Paczkowski NJ, Vuocolo HE, Bryan-Lluka LJ. Conclusive evidence for distinct transporters for 5-hydroxytryptamine and noradrenaline in pulmonary endothelial cells of the rat. Naunyn Schmiedebergs Arch Pharmacol 1996;353:423–30.
Suhara T, Sudo Y, Yoshida K, Okubo Y, Fukuda H, Obata T, et al. Lung as reservoir for antidepressants in pharmacokinetic drug interactions. Lancet 1998;351:332–5.
Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, et al. Antidepressant-and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A 1993;90:2542–6.
International Commission on Radiological Protection. Radiological Protection and Safety in Medicine. ICRP Publication 73. Ann ICRP 26. Oxford: Pergamon Press; 1996.
European Commission. Radiation Protection 99. Guidance on Medical Exposures in Medical and Biomedical Research. Directorate-General on Environment, Nuclear Safety and Civil Protection. Belgium; 1998.
Fooden J. Comparative review of Fascicularis-group species of macaques (primates: Macaca). Fieldiana Zool 2006;107:1–43.
Mouri T, Agatsuma T, Iwagami M, Kawamoto Y. Species identification by mitochondrial DNA: a case study of macaque remains from Shuri castle, Okinawa (in Japanese with English summary). Primate Res 2000;16:87–94.
Robeson W, Dhawan V, Belakhlef A, Ma Y, Pillai V, Chaly T, et al. Dosimetry of the dopamine transporter radioligand 18F-FPCIT in human subjects. J Nucl Med 2003;44:961–6.
Jones SC, Alavi A, Christman D, Montanez I, Wolf AP, Reivich M. The radiation dosimetry of 2[18F]fluoro-2-deoxy-D-glucose in man. J Nucl Med 1982;23:613–7.
Dhawan V, Belakhlef A, Robeson W, Ishikawa T, Margouleff C, Takikawa S, et al. Bladder wall radiation dose in humans from fluorine-18-FDOPA. J Nucl Med 1996;37:1850–2.
Deterding TA, Votaw JR, Wang CK, Eshima D, Eshima L, Keil R, et al. Biodistribution and radiation dosimetry of the dopamine transporter ligand [18F]FECNT. J Nucl Med 2001;42:376–81.
Dowd MT, Chen CT, Wendel MJ, Faulhaber PJ, Cooper MD. Radiation dose to the bladder wall from 2-[18F]fluoro-2-deoxy-D-glucose in adult humans. J Nucl Med 1991;32:707–12.
Votaw JR, Ansari MS, Mason NS, Schmidt D, De Paulis T, Holburn G, et al. Dosimetry of iodine-23-epidepride: a dopamine D2 receptor ligand. J Nucl Med 1995;36:1316–21.
Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, et al. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage 2008;39:1408–19.
Acknowledgments
We thank the Institute of Nuclear Energy Research of Taiwan for providing the OLINDA 1.0/EXM computer program. The synthesis of 4-F-ADAM was funded by the National Institute of Mental Health (NIMH) under contract no. NO1-MH-32005. Toxicology studies were also funded by NIMH, under contract no. M422-05. This work was supported by the National Science Council of Taiwan (grants NSC 95-2811-B-016-002, 95-2321-B-016-001-MY2 and 96-2811-B-016-004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, YY., Ma, KH., Tseng, TW. et al. Biodistribution, toxicity and radiation dosimetry studies of the serotonin transporter radioligand 4-[18F]-ADAM in rats and monkeys. Eur J Nucl Med Mol Imaging 37, 545–555 (2010). https://doi.org/10.1007/s00259-009-1281-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1281-z