Abstract
Purpose
The anti-CD20 antibody rituximab labelled with the α-particle-emitting radionuclide 227Th is of interest as a radiotherapeutic agent for treatment of lymphoma. Complete regression of human lymphoma Raji xenografts in 60% of mice treated with 200 kBq/kg 227Th-rituximab has been observed. To evaluate possible late side effects of 227Th-rituximab, the long-term radiotoxicity of this potential radiopharmaceutical was investigated.
Methods
BALB/c mice were injected with saline, cold rituximab or 50, 200 or 1,000 kBq/kg 227Th-rituximab and followed for up to 1 year. In addition, nude mice with Raji xenografts treated with various doses of 227Th-rituximab were also included in the study. Toxicity was evaluated by measurements of mouse body weight, white blood cell (WBC) and platelet counts, serum clinical chemistry parameters and histological examination of tissues.
Results
Only the 1,000 kBq/kg dosage resulted in decreased body weight of the BALB/c mice. There was a significant but temporary decrease in WBC and platelet count in mice treated with 400 and 1,000 kBq/kg 227Th-rituximab. Therefore, the no-observed-adverse-effect level (NOAEL) was 200 kBq/kg. The maximum tolerated activity was between 600 and 1,000 kBq/kg. No significant signs of toxicity were observed in histological sections in any examined tissue. There were significantly (p < 0.05), but transiently, higher concentrations of serum bile acids and aspartate aminotransferase in mice treated with either 227Th-rituximab or non-labelled antibody when compared with control mice. The maximum tolerated dose to bone marrow was between 2.1 and 3.5 Gy.
Conclusion
Therapeutically relevant dose levels of 227Th-rituximab were well tolerated in mice. Bone marrow suppression, as indicated by decrease in WBC count, was the dose-limiting radiotoxicity. These toxicity data together with anti-tumour activity data in a CD20-positive xenograft mouse model indicate that therapeutic effects could be obtained with relatively safe dosage levels of the radioimmunoconjugate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We are exploring the potential of 227Th alpha-particle radioimmunotherapy (alpha-RIT), which is a novel cancer treatment modality approaching clinical use. We have chosen 227Th-rituximab and CD20-positive Raji lymphoma xenografts as our model system. Alpha-particles have high energy and short path lengths in tissue (50–100 μm) which are well matched with the size of micrometastases or single cancer cells, indicating the potential for a tumour-selective irradiation. Alpha-particles interact directly with DNA to produce double-strand breaks or with water to produce hydroxyl radicals that are highly reactive against biological materials [1]. However, one major obstacle for clinical alpha-RIT has previously been the lack of production capacities for suitable radionuclides. 227Th (T ½ = 18.7 days) can be produced routinely in clinically relevant amounts from 227Ac, which in turn can be generated by thermal neutron irradiation of 226Ra [2].
We have previously shown that 227Th can be stably conjugated to antibodies [3] and that treatment with 227Th-rituximab effectively inhibits growth of human lymphoma xenografts at dosages of 200–400 kBq/kg [4]. However, when using alpha emitters for medical purposes there is a particular concern for long-term tissue toxicity [5]. For 227Th-rituximab the long-term toxicity is largely unknown. 227Th decays via its alpha- and beta-emitting daughters 223Ra, 219Rn, 215Po, 211Pb, 211Bi and 207Tl to stable non-radioactive 207Pb (Fig. 1). 223Ra will detach from the antibody-DOTA construct because of the substantial kinetic energy of the recoiling nucleus after the alpha-particle emission. However, the long half-life of 227Th permits tumour targeting and normal tissue clearance of the 227Th-labelled radioimmunoconjugate before larger amounts of 223Ra are generated. Nevertheless, free daughters are produced in vivo from circulating unbound antibodies or from antibodies bound to the surface of the target cells and can relocalize to various target organs where they can accumulate and cause radiotoxicity.
Fortunately, the biodistribution of 223Ra is well known [6, 7]. It clears from the blood very rapidly and is either excreted via the intestines or trapped by hydroxyapatite at bone surfaces [8]. Favourably, 223Ra has a half-life of 11.4 days, which means that there is time for binding to hydroxyapatite or excretion before decay occurs. Minor amounts of 223Ra are also retained in tumour even though 227Th-rituximab is not significantly internalized [9]. The half-lives of the 223Ra daughters are in the millisecond to minute range. They are therefore likely to contribute mainly to the radiation dose in the vicinity of the site of 223Ra decay.
The toxicity of 223Ra has been investigated in mice and in humans [8, 10, 11]. High dosages, 1,250–3,750 kBq/kg, of 223Ra in mice resulted in a dose-related depletion of myeloid precursors in the bone marrow. Dosages of 50–250 kBq/kg 223Ra in humans were well tolerated, with grade III leucopaenia in 12% of the patients and no more than grade I thrombocytopaenia [11]. The mouse therapy data indicate that these levels would be therapeutically relevant also for 227Th-rituximab in clinical use [4].
Bone marrow toxicity has been shown to be the major normal toxicity problem after treatments with alpha-RIT with 211At- and 213Bi-labelled antibodies [12–15]. However, the more long-lived alpha emitter 225Ac has resulted in long-term kidney toxicity [5].
In this study, we investigated the long-term toxicological effects of 227Th-rituximab by combining data from BALB/c mice with data from short- and long-term nude mice studies. In the latter the animals had got their tumour xenografts inactivated by 227Th-rituximab treatment and were followed for signs of long-term toxicity.
Materials and methods
Preparation of 227Th-p-isothiocyanato-benzyl-DOTA-rituximab
227Th, 227Th-p-isothiocyanato-benzyl-DOTA complexes (Macrocyclics Inc., Dallas, TX, USA) and 227Th-p-isothiocyanato-benzyl-DOTA-rituximab (227Th-rituximab) (Mabthera®, Roche, Basel, Switzerland) were prepared as previously described [2, 3, 16]. The specific activity of the radioimmunoconjugate was in the range of 650–5,300 Bq/μg, which equals from 1 227Th atom per 2,700 rituximab molecules to 1 227Th atom per 330 rituximab molecules. The quality of the radioimmunoconjugate was tested using lymphoma cells and a modified Lindmo method [17]. Cell concentrations of up to 108 Raji cells/ml were used to compensate for the modest specific activity of the radioimmunoconjugate [3, 16]. The immunoreactive fractions of conjugates used in the current study were above 60%.
Quantity and purity of 227Th-rituximab
The 227Th products were measured using a germanium detector (GCW6021, Canberra, Meriden, CT, USA). Stronger sources of purified 227Th samples was measured on a Capintec dose calibrator which had been calibrated by pure 227Th sources quantified by a germanium detector. Radionuclide and radiochemical purity was beyond 98%, i.e. less than 2% 223Ra, for all injectates.
Animals
Two different breeds of mice were used in the current study: (1) 59 BALB/c mice (AnNHsd, Harlan UK, Oxfordshire, UK), age 5 weeks, weighing 13–15 g at the start of the experiment and (2) 74 institutionally bred female BALB/c nu/nu (NCR) mice that were 4–8 weeks old and had body weights in the range of 25–30 g at the start of the experiment. The NCR nu/nu mice were divided into three groups: (1) 36 mice without Raji xenografts were used for early (1–8 weeks) time points; (2) 8 mice without Raji xenografts were used as age-matched controls for the late time points (14, 19, 30 and 36 weeks) and 6 mice without Raji xenografts were used as age-matched controls for the 0 time point; and (3) 24 mice with Raji xenografts that had been treated with 227Th-rituximab and in which the xenografts had been inactivated by the treatment. The animals were maintained under pathogen-free conditions, and food and water were supplied ad libitum. All procedures and experiments involving animals in this study were approved by the National Animal Research Authority and carried out according to the European Convention for the Protection of Vertebrates used for Scientific Purposes.
Treatment and blood sampling
BALB/c mice were injected with either saline, 20 μg rituximab or 50, 200 or 1,000 kBq/kg 227Th-rituximab. Table 1 shows an overview of the treatment groups. Half of the mice were killed after 26 weeks and blood was collected by cardiac puncture for complete blood counts (CBCs) and clinical chemistry. From the rest of the group approximately 200 μl blood was collected at 32 and 40 weeks after injection from the vena saphena lateralis for CBCs and by cardiac puncture at 52 weeks for CBCs and clinical chemistry.
In addition, NCR nu/nu mice without tumours were injected in the tail vein with either saline or 200, 400 or 1,000 kBq/kg 227Th-rituximab in an approximate volume of 0.1 ml. Table 2 shows an overview of the treatment groups. Blood samples were drawn and analysed weekly for the first 8 weeks. The mice were divided into three groups, each containing 12–15 mice. Blood was collected from one alternating group every third week during the first 8-week period. Thus, each mouse was sampled two to three times. In addition, some untreated age-matched NCR nu/nu mice without tumour were included (week 0).
Moreover, blood samples were collected at 3, 7, 14, 19, 30 or 36 weeks after injection from NCR nu/nu mice used in therapy experiments and which had complete tumour response. Table 3 shows an overview of the treatment groups. Approximately 200 μl blood was collected from the vena saphena lateralis in 0.5 ml EDTA-coated tubes (BD Microtainer, Franklin Lakes, NJ, USA). At time points 6, 7, 8, 19 and 36 weeks after injection, blood was collected by cardiac puncture for CBCs and clinical chemistry.
Cardiac puncture was only performed on anaesthetized mice before termination. Mice were anaesthetized using Sevoflurane® (Abbott, North Chicago, IL, USA) and terminated after sampling by cervical dislocation. All available blood was collected in 2.0 ml syringe and divided between 1.8 ml Eppendorf tubes without anticoagulant and 0.5 ml EDTA-coated tubes. After coagulation the blood was centrifuged to get serum. Complete blood cell counts and several clinical chemistry parameters were analysed by the Central Laboratory at the Norwegian School of Veterinary Science (clinical chemistry: Advia 1620, haematology: Advia 2120, Bayer, Leverkusen, Germany).
In weeks 1 and 5 the white blood cells (WBC) were counted by a haemolysis method [18]: 100 ml blood was mixed with 1 ml lysing solution (VersaLyse, Beckman Coulter, California, USA) for at least 15 min to destroy red blood cells. Subsequently, the cells were counted in an automatic viability analyser (Vi-Cell-XR, Beckman Coulter, Fullerton, CA, USA). Initially WBC counts were counted in paired samples by both methods and the results showed strong correlation (data not shown). With the haemolysis method it was not possible to measure the number of RBC and platelets for weeks 1 and 5.
Measurements of 227Th-rituximab activity in bone marrow
Uptake of 227Th-rituximab in bone marrow was measured by scratching out bone marrow from femur with a needle. The bone marrow sample was smeared on a pre-weighed piece of Parafilm (American National Can, Menasha, WI, USA) and subsequently put in a pre-weighed airtight tube to avoid drying. Activities from 227Th and 223Ra were measured by their most characteristic γ-rays employing a solid-state photon detector with a well (GCW6021, Canberra, Meriden, CT, USA) coupled to a digital gamma ray spectrometer and analysed using the computer software Apex (Canberra, Meriden, CT, USA). Activities from the same samples were also measured with a calibrated gamma detector (Cobra II auto-gamma detector, Packard Instrument Company, Meriden, CT, USA), and data from these measurements were used if the activity was too low to be measured by the solid-state photon detector. It was assumed that 223Ra was in secular equilibrium with its daughters.
In one experiment femur was frozen, cut into sections and put on object slides, which were dipped in photographic emulsion (LM-1 Hypercoat emulsion, GE Healthcare, Buckinghamshire, UK). The emulsion was exposed for 13 days before developing according to the manufacturer’s description.
Histology
All mice were autopsied after cervical dislocation. The kidneys and the spleen were weighed. The heart, lungs, small and large intestines, stomach, kidney, spleen, liver and bone marrow (femur) were fixed in 4% formaldehyde in phosphate buffer (pH ~ 7), embedded in paraffin, cut to a nominal thickness of approximately 5 μm and stained with haematoxylin and eosin. A pathologist examined all sections. The nude mice with Raji tumours did not survive to reach the latest time points because of tumour growth. Therefore, age-matched nude mice without tumours served as controls in the histology experiments for the latest time points.
Statistical analysis
Differences in weight of kidneys and spleen were tested for significance using a t-test. Differences in histological findings were tested for significance using two-way analysis of variance (ANOVA). Clinical chemistry parameters were transformed to normal distribution (mean ~ 0.0, SD ~ 1.0) by fitting the logarithm of the data points to an exponential function. The data were tested for statistically significant differences with respect to time from injection and treatment type using two-way ANOVA. Pairwise comparisons were performed with the Holm-Sidak method or the Mann-Whitney rank sum test.
Results
Weight of mice, kidneys and spleen
There were no significant differences in the body weight development for the different BALB/c treatment groups, except for the group receiving 1,000 kBq/kg 227Th-rituximab (n = 12), which resulted in inhibition of weight gain between weeks 1 and 15 and weight loss and death after week 15 (Fig. 2). There were no significant differences in the mean weight of kidneys or spleen 26 weeks after injection (ANOVA on ranks, p = 0.153 and p = 0.51, respectively). However, for the 1,000 kBq/kg 227Th-rituximab group (n = 5) one mouse had 27% lower kidney weight and 63% lower spleen weight than the mean of the control mice and one mouse had 32% higher kidney weight and 176% higher spleen weight than the mean of the control mice.
The nude mice in the therapy experiments were also weighed but here the body weights were significantly influenced by the weight and development of tumour, and the control mice were euthanized because of large tumour size at earlier time points than the mice treated with 227Th-rituximab.
Haematology
Two-way ANOVA were performed for WBC counts, red blood cell (RBC) counts and platelet counts for all time points and for both mouse strains. The WBC count was significantly higher in control mice (6.5 ± 3.3 · 109 WBC/l) than in mice treated with 600 (4.0 ± 1.1 · 109 WBC/L) and 1,000 (3.6 ± 2.3· 109 WBC/l) kBq/kg 227Th-rituximab (Mann-Whitney rank sum test, p < 0.005). The RBC count was higher in control mice (9.8 ± 0.9 · 1012 RBC/l) than in mice treated with 1,000 kBq/kg 227Th-rituximab (8.6 ± 0.9 · 1012 RBC/l) (t test, p < 0.001). The platelet count was higher in control mice (982 ± 378 · 109 PLT/l) than in mice treated with 1,000 kBq/kg 227Th-rituximab (781 ± 193 · 109 PLT/l) (Mann-Whitney rank sum test, p < 0.005).
Subsequently, data for both mouse strains were divided into two subgroups with regard to time after injection (1–19 weeks and 26–52 weeks). In the 1–19 week subgroup there were significantly lower platelet and WBC counts in mice treated with 400 and 1,000 kBq/kg 227Th-rituximab as compared with control mice, while for RBC counts only the treatment with 1,000 kBq/kg 227Th-rituximab had a significant effect (Table 4). In the 26–52 weeks period, there were no significant differences between mice treated with 227Th-rituximab or rituximab and control mice for any parameter (not shown).
The WBC, platelet and RBC counts varied with time in the first period (Fig. 3). The nadir for WBC count for mice treated with 1,000 kBq/kg was at 2–3 weeks, and after 7–8 weeks there were no significant differences between treated and untreated mice (Fig. 3a). The nadir for platelets was at 2–3 weeks for mice treated with 400 and 1,000 kBq/kg, and after 6 weeks there were no significant differences between treated and untreated mice. For RBC counts the significant difference between 1,000 kBq/kg and control treatments was constant in the whole period (almost parallel regression lines).
Blood counts. a Number of WBC as a function of time after injection of NaCl or 200, 400, 600 and 1,000 kBq/kg 227Th-rituximab. b Number of platelets as a function of time after injection of NaCl or 200, 400, 600 and 1,000 kBq/kg 227Th-rituximab. c Number of RBC as a function of time after injection of NaCl or 200, 400, 600 and 1,000 kBq/kg 227Th-rituximab. Points plotted are individual data. Data for weeks 1 and 5 are missing for platelets and RBC because a haemolysis method was used for WBC counting
Uptake of 227Th-rituximab in red bone marrow
The uptake of 227Th-rituximab in bone marrow scraped out of the femur of NCR nu/nu mice without tumour was measured for several time points after injection of 600 kBq/kg 227Th-rituximab (Fig. 4). From the activity versus time curve the cumulated activity was calculated and the absorbed radiation dose was estimated by multiplying with the energy per alpha-particle (5.9 MeV). The absorbed radiation dose after injection of 600 kBq/kg 227Th-rituximab was estimated to be 2.4 Gy, assuming that all alpha-particle energy was absorbed by the red marrow and that the activity in bone marrow decreased with the same half-life after the 14-day time point as between the 7-day and 14-day time points.
Retention of 227Th-rituximab in blood and bone marrow as a function of time after injection. Uptake in bone marrow was measured by scratching bone marrow out of the femur. Error bars = standard error from three mice per time point. The mice were injected with 521 kBq/kg of 227Th-rituximab, which was normalized to 600 kBq/kg for calculation of dose
No 223Ra was detected in red marrow for early time points (up to 7 days). However, previous studies have shown that around 80% of the absorbed radiation dose to femur comes from 223Ra and daughters [4]. For the 14-day time point there was between 20 and 70% 223Ra in the bone marrow. However, it was assumed that this was contamination due to the scraping since previous studies had shown that 223Ra has a very high affinity for bone [7]. Microautoradiography of femur with red marrow 4 days after injection showed a high number of alpha-particle tracks close to the inner bone surface, while there were less alpha-particle tracks in the red marrow (Fig. 5). Figure 5 shows that the absorbed radiation dose to red marrow might be somewhat higher than that calculated from the data in Fig. 4 since the red marrow would also be struck by alpha-particles emitted from the bone surface. However, the tracks from the bone surface did not reach far into the red marrow.
Microautoradiography image of epiphyseal region of femur containing red marrow from a mouse autopsied 4 days after injection of 227Th-rituximab. The microautoradiography emulsion was exposed for 14 days. a Phase contrast image (×200) showing bone and red marrow cells. Alpha-particle tracks (black lines) can be seen dimly. b Bright field image of the same area showing the distribution of alpha-particle tracks on bone surface and in the red marrow. Black arrows point to alpha-particle tracks. The section was 5 μm thick
Clinical chemistry
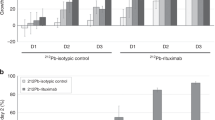
Log transformation of the clinical chemistry data was necessary in order to do statistical analysis because of large variations within treatment groups. Two-way ANOVA analyses of the clinical chemistry parameters for both mouse strains with treatment and time as factors were performed (Table 5, columns 3 and 4). The mean aspartate aminotransferase (AST), urea and alanine aminotransferase (ALT) concentrations in serum varied significantly with the treatment type (Table 5, column 3). The treatments were also compared pairwise using the Holm-Sidak method (Table 5, column 5). The NaCl control group had significantly lower serum AST concentration than the 50 kBq/kg 227Th-rituximab group (three of six mice had fivefold higher values than the control) and the rituximab treatment group had significantly higher serum urea concentrations than the 1,000 kBq/kg 227Th-rituximab group (Table 5, column 5).
No systematic trends in responses were detected with increase in 227Th-rituximab dosage. Therefore, the 227Th-rituximab group was pooled. Figure 6 shows log-transformed data for AST, urea, ALT and bile acids for the 227Th-rituximab group, the rituximab group and the control group. Figure 6 shows that the significant variations of clinical chemistry parameters with time were not systematic. For AST, urea and ALT there were significant differences when the data were pooled over all time points. However, Fig. 6 shows that only for urea there were significant differences between the 227Th-rituximab group and the control group when the data were plotted as a function of time. For bile acids no significant differences between treatments were found for data pooled over time (Table 5). Nevertheless, there was a significantly higher concentration of serum bile acids in the blood of 227Th-rituximab mice than in the blood of NaCl control mice between weeks 12 and 37 (p = 0.003, Mann-Whitney rank sum test). However, also the mean values for rituximab treatment alone were elevated compared with the NaCl values (p = 0.222, Mann-Whitney rank sum test). Therefore, the significant difference between 227Th-rituximab and NaCl for AST and bile acids might to some extent be due to the rituximab part of 227Th-rituximab and not the 227Th part.
Clinical chemistry. Log-transformed concentration of AST, urea and bile acids in serum after injection of NaCl, 20 μg rituximab or different concentrations of 227Th-rituximab pooled in one group. For NaCl and 227Th-rituximab individual measurement points, trend line (solid lines) and 95% confidence intervals (dotted lines) are given. For rituximab, values are given as mean ± SD since we only had values for two time points. Because of large variation in the data the concentrations were log-transformed to a normal distribution with mean 0.0 and SD 1.0
Histology
No significant signs of toxicity were found for any tissue except for some lymphoid infiltration in the liver of one mouse treated with 1,000 kBq/kg 227Th-rituximab.
Discussion
The results of preclinical studies with 227Th-rituximab have generated sanguinity for potential human clinical use [3, 4, 9, 16]. One advantage of using 227Th is its relatively long half-life (18.7 days) compared with other alpha-particle-emitting radionuclides used for radioimmunotherapy (213Bi, T ½ = 45.6 min; 211At, T ½ = 7.2 h). This permits enough time to target less readily accessible tumour cells before decay occurs. Another benefit of 227Th is the availability of large amounts of its raw material, 227Ac [2]. Lack of raw material is a problem for other alpha-particle-emitting radionuclides, including 225Ac (T ½ = 10 days), 213Bi and 211At. Furthermore, 227Th can be stably conjugated to IgG and the pharmacokinetics of free 227Th and daughters are well known [3, 6, 7].
The no-observed-adverse-effect level (NOAEL) is an important parameter in preclinical risk assessment. To be adverse, an effect has to be dose responsive, and statistically and biologically significant [19]. There was a dose-dependent statistically and biologically significant decrease in WBC and platelet counts for 400 and 1,000 kBq/kg 227Th-rituximab and for RBC counts for 1,000 kBq/kg. Only the highest dosage level induced death of mice. Thus, an adverse but tolerable effect on bone marrow is induced by treatment with 400 kBq/kg 227Th-rituximab. The decreases in WBC and platelet counts for 600 kBq/kg 227Th-rituximab were not significant, but the number of animals was small for this dosage group. Liver toxicity was assessed both by histology and clinical chemistry. The level of AST was not dose responsive. However, AST values were significantly higher for mice treated with 227Th-rituximab than for untreated mice and the effect seemed to increase slightly with time. Nevertheless, since there were no dose responses, an increase in AST cannot be used to determine the NOAEL. Thus, the NOAEL for the 227Th-rituximab treatment was 200 kBq/kg. The maximum tolerated activity was probably between 600 and 1,000 kBq/kg
Other studies of alpha-particle radioimmunotherapy have shown increased levels of blood urea nitrogen and creatinine after treatment with 225Ac-HuM195, which is indicative of kidney damage [5, 20]. The present study showed no increase in these parameters.
227Th decays through four alpha-emitting daughters (223Ra, 219Rn, 215Po and 211Bi) and two beta-emitting daughters ( 211Pb and 207Tl). Although the daughter nuclides enhance the dose to tumour cells, they also may result in increased toxicity since they can relocalize to and irradiate normal tissues. The absorbed dose to normal tissues in mice with and without tumour xenografts had earlier been determined after injection of 227Th-rituximab [3, 4]. The resulting absorbed radiation doses after injection of 200 kBq/kg 227Th-rituximab in nude mice were 1.9 Gy in tumour, 1.7 Gy in femur, 1.1 Gy in skull, 0.5 Gy in liver and spleen, and 0.3 Gy in kidney and blood [4]. Both the contribution from 227Th and the contribution from the daughters were included in the calculation, and it was assumed that the 223Ra daughters decayed in the same tissue as 223Ra, although 211Pb has a half-life of 36 min and might relocalize to other sites in the body. However, Henriksen, et al. [7] have shown that 211Bi (and thus 211Pb) is well retained in the bone of mice at 6 h and 3 days after intravenous injection of dissolved 223RaCl2. The radium localized in bone with little uptake elsewhere. It is likely that the bone-seeking radium generated in vivo in this study will have a similar localization as the mother nuclide and the daughter products, i.e. at least for bone, it is a good approximation to assume equilibrium between the mother nuclide and the progenies. Thus, based on absorbed radiation doses, one would expect to get the highest toxicity in bone marrow, liver and spleen. The relative biological effect (RBE) of 227Th-rituximab for tumour treatment is in the range 2.5–7.2 [21]. For normal tissues the RBE of alpha-RIT has been reported to be in the range of 1–5 for myelotoxicity and 3–9 for toxicity to tissues other than blood [12, 22–24]. Assuming that an RBE of 5 applies, the RBE-adjusted absorbed dose to the liver of 2.5 Sv is likely to be tolerable, while a dose to femur of 8.5 Sv seems high.
To investigate bone marrow toxicity further, the uptake and retention of an injection of 600 kBq/kg 227Th-rituximab in the red marrow of femur was measured. The red marrow was scratched out of the femur which may lead to cross-contamination of the red marrow from 223Ra on the inner surface of the femur. However, flushing out the marrow could not be done since it is necessary to know the weight of the red marrow in order to calculate the absorbed radiation dose. Furthermore, at the 1-h to 7-day time points no 223Ra was detected in the red marrow suggesting contamination below the detection limit. However, after 14 days the fraction of 223Ra varied between 20 and 70%, which implies that there might have been contamination of 223Ra in some of the samples at this time point. Thus, injection of 600 kBq/kg 227Th-rituximab resulted in an absorbed radiation dose to red marrow of 1.4 Gy, or 7 Sv when adjusting for RBE. However, the total dose to femur was more than twice as high [4], indicating that a large fraction of the radioactivity in femur decayed in the bone matrix or at the bone surfaces and were due to 223Ra and daughters. However, trabecular bone at the metaphyseal ends probably accumulate more 223Ra since more hydroxyapatite is exposed with possible increased absorbed radiation dose in interspersed marrow. Microautoradiography of sections of femur from a mouse treated with 227Th-rituximab confirmed that there was a high amount of alpha-tracks emitted from the inner surface of femur, but it also showed some activity in the red marrow. However, Fig. 5 shows the distribution of alpha-particles at day 4 after injection. It is likely that the elevated level at bone surfaces mainly relates to 223Ra taken up by the hydroxyapatite, while the activity in the red marrow probably significantly relates to radiolabelled antibody circulating in the bone marrow compartment (as indicated in Fig. 4). Geometrical considerations suggest that about half of the activity on the inner bone surface will be absorbed by the red marrow. Thus, the dose to red marrow can be as much as 50% higher than estimated from activity measured only in bone marrow. An injected dosage of 600 kBq/kg 227Th-rituximab may therefore result in an absorbed radiation dose to red marrow of up to 2.1 Gy or 10.5 Sv delivered over a time period of at least 4–6 weeks.
Bone marrow toxicity had been evaluated previously after treatments with alpha-RIT with 211At- and 213Bi-labelled antibodies [12–15]. The maximum tolerated dose to bone marrow for 211At-IgG was estimated to be between 0.6 and 0.7 Gy using a microdosimetric model, while it was 4 Gy for 213Bi-CO17-1A calculated using the same method as in the present paper. It is conceivable that the protracted radiation regimen of 227Th-rituximab is more tolerable than both the 211At-IgG and the 213Bi-CO17-1A treatment because the protracted treatment offers more time for repair and repopulation of the red marrow. However, due to the difficulties in estimation of alpha-particle dose to bone marrow in mouse studies and differences in calculation method, it is not possible to draw any conclusions about which treatment has the lowest maximum tolerated dose.
Protracted α-exposure from 224Ra has been linked to increased carcinogenesis in dogs, while it resulted in lower acute haematological toxicity than single injection of 224Ra [25]. Using 227Th-labelled antibody would cause a more protracted exposure compared with cationic 223Ra and the other shorter lived α-emitting nuclides under consideration for tumour therapy. Accordingly, the maximum tolerated doses to blood for therapy with 211At-labelled antibodies are higher than the maximum tolerated dose to blood for 213Bi-labelled antibodies [12–14, 22]. Based on the low dose rate of 227Th-rituximab one would suspect lower toxicity of 227Th-labelled antibodies than for 223RaCl2 or for 213Bi- and 211At-labelled antibodies. However, the toxicity also depends on the microdistribution of the drug. Thus, it is important to evaluate the long-term toxicity of 227Th-rituximab.
In conclusion, treatment with 1,000 kBq/kg 227Th-rituximab resulted in an adverse decrease in mouse body weight, WBC, platelet and RBC counts, which all probably were due to irradiation of the bone marrow. Also 400 kBq/kg of 227Th-rituximab resulted in a significant but tolerable decrease of WBC and platelet counts. Thus, the NOAEL for the 227Th-rituximab treatment was 200 kBq/kg, while the maximum tolerated activity was between 600 and 1,000 kBq/kg, resulting in 2.1–3.5 Gy dose to bone marrow if the contributions from 223Ra and daughters are included. The tolerance of humans to alpha-RIT may be different to that of mice. Therefore, we suggest that 20 kBq/kg is a safe dosage for start of clinical trials.
References
Hall EJ. Radiobiology for the radiologist. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2000.
Henriksen G, Bruland OS, Larsen RH. Thorium and actinium polyphosphonate compounds as bone-seeking alpha particle-emitting agents. Anticancer Res 2004;24:101–5.
Dahle J, Borrebaek J, Melhus KB, Bruland OS, Salberg G, Olsen DR, et al. Initial evaluation of (227)Th-p-benzyl-DOTA-rituximab for low-dose rate alpha-particle radioimmunotherapy. Nucl Med Biol 2006;33:271–9.
Dahle J, Borrebaek J, Jonasdottir TJ, Hjelmerud AK, Melhus KB, Bruland ØS, et al. Targeted cancer therapy with a novel low-dose rate alpha-emitting radioimmunoconjugate. Blood 2007;110:2049–56.
Jaggi JS, Seshan SV, McDevitt MR, LaPerle K, Sgouros G, Scheinberg DA. Renal tubulointerstitial changes after internal Irradiation with alpha-particle-emitting actinium daughters. J Am Soc Nephrol 2005;16:2677–89.
Henriksen G, Breistøl K, Bruland ØS, Fodstad Ø, Larsen RH. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res 2002;62:3120–5.
Henriksen G, Fisher DR, Roeske JC, Bruland ØS, Larsen RH. Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med 2003;44:252–9.
Nilsson S, Larsen RH, Fosså SD, Balteskard L, Borch KW, Westlin JE, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 2005;11:4451–9.
Melhus KB, Larsen RH, Stokke T, Kaalhus O, Selbo PK, Dahle J. Evaluation of the binding of radiolabeled rituximab to CD20-positive lymphoma cells: an in vitro feasibility study concerning low-dose-rate radioimmunotherapy with the alpha-emitter 227Th. Cancer Biother Radiopharm 2007;22:469–79.
Larsen RH, Saxtorph H, Skydsgaard M, Borrebaek J, Jonasdottir TJ, Bruland OS, et al. Radiotoxicity of the alpha-emitting bone-seeker 223Ra injected intravenously into mice: histology, clinical chemistry and hematology. In Vivo 2006;20:325–31.
Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 2007;8:587–94.
Elgqvist J, Bernhardt P, Hultborn R, Jensen H, Karlsson B, Lindegren S, et al. Myelotoxicity and RBE of 211At-conjugated monoclonal antibodies compared with 99mTc-conjugated monoclonal antibodies and 60Co irradiation in nude mice. J Nucl Med 2005;46:464–71.
McLendon RE, Archer GE, Larsen RH, Akabani G, Bigner DD, Zalutsky MR. Radiotoxicity of systemically administered 211At-labeled human/mouse chimeric monoclonal antibody: a long-term survival study with histologic analysis. Int J Radiat Oncol Biol Phys 1999;45:491–9.
Zalutsky MR, Stabin MG, Larsen RH, Bigner DD. Tissue distribution and radiation dosimetry of astatine-211-labeled chimeric 81C6, an alpha-particle-emitting immunoconjugate. Nucl Med Biol 1997;24:255–61.
Behr TM, Sgouros G, Stabin MG, Béhé M, Angerstein C, Blumenthal RD, et al. Studies on the red marrow dosimetry in radioimmunotherapy: an experimental investigation of factors influencing the radiation-induced myelotoxicity in therapy with beta-, Auger/conversion electron-, or alpha-emitters. Clin Cancer Res 1999;5:3031s–43s.
Larsen RH, Borrebaek J, Dahle J, Melhus KB, Krogh C, Valan MH, et al. Preparation of TH227-labeled radioimmunoconjugates, assessment of serum stability and antigen binding ability. Cancer Biother Radiopharm 2007;22:431–7.
Lindmo T, Bunn PA Jr. Determination of the true immunoreactive fraction of monoclonal antibodies after radiolabeling. Methods Enzymol 1986;121:678–91.
Ashmore LM, Shopp GM, Edwards BS. Lymphocyte subset analysis by flow cytometry. Comparison of three different staining techniques and effects of blood storage. J Immunol Methods 1989;118:209–15.
Dorato MA, Engelhardt JA. The no-observed-adverse-effect-level in drug safety evaluations: use, issues, and definition(s). Regul Toxicol Pharmacol 2005;42:265–74.
Miederer M, McDevitt MR, Sgouros G, Kramer K, Cheung NK, Scheinberg DA. Pharmacokinetics, dosimetry, and toxicity of the targetable atomic generator, 225Ac-HuM195, in nonhuman primates. J Nucl Med 2004;45:129–37.
Dahle J, Bruland OS, Larsen RH. Relative biologic effects of low-dose-rate alpha-emitting 227Th-rituximab and beta-emitting 90Y-tiuexetan-ibritumomab versus external beam X-radiation. Int J Radiat Oncol Biol Phys 2008;72:186–92.
Behr TM, Béhé M, Sgouros G. Correlation of red marrow radiation dosimetry with myelotoxicity: empirical factors influencing the radiation-induced myelotoxicity of radiolabeled antibodies, fragments and peptides in pre-clinical and clinical settings. Cancer Biother Radiopharm 2002;17:445–64.
Howell RW, Azure MT, Narra VR, Rao DV. Relative biological effectiveness of alpha-particle emitters in vivo at low doses. Radiat Res 1994;137:352–60.
Howell RW, Goddu SM, Narra VR, Fisher DR, Schenter RE, Rao DV. Radiotoxicity of gadolinium-148 and radium-223 in mouse testes: relative biological effectiveness of alpha-particle emitters in vivo. Radiat Res 1997;147:342–8.
Muggenburg BA, Hahn FF, Griffith WC Jr, Lloyd RD, Boecker BB. The biological effects of radium-224 injected into dogs. Radiat Res 1996;146:171–86.
Moore DM. Hematology of the mouse (Mus musculus). In: Feldman BF, Zinkl JG, Jain NC, editors. Chalm’s veterinary hematology. Philadelphia: Lippincott, Williams & Wilkins; 2000. p. 1219–24.
Acknowledgements
This work was supported by the Norwegian Cancer Society, the Norwegian Research Council and Helse Sør-Øst. We are grateful to the Histology Laboratory at Division for Pathology, The Norwegian Radium Hospital for making and staining the histological sections of mouse organs. We also appreciate the services provided by the Animal Department at the Norwegian Radium Hospital. We also thank Dr. Olav Kaalhus, The Norwegian Radium Hospital, for help with the statistical analysis of the clinical chemistry data. Algeta ASA has a patent pending for using 227Th for radioimmunotherapy and has provided research support for this study. Jørgen Borrebæk is an employee of Algeta ASA. Roy H. Larsen, Thora J. Jonasdottir and Jostein Dahle own stocks in Algeta ASA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dahle, J., Jonasdottir, T.J., Heyerdahl, H. et al. Assessment of long-term radiotoxicity after treatment with the low-dose-rate alpha-particle-emitting radioimmunoconjugate 227Th-rituximab. Eur J Nucl Med Mol Imaging 37, 93–102 (2010). https://doi.org/10.1007/s00259-009-1197-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1197-7