Abstract
Purpose
To retrospectively determine whether increased/asymmetric FDG uptake on PET without a correlating morphological lesion on fully diagnostic CT indicates the development of a head and neck malignancy.
Methods
In 590 patients (mean age 55.4 ± 13.3 years) without a head and neck malignancy/inflammation FDG uptake was measured at (a) Waldeyer’s ring, (b) the oral floor, (c) the larynx, and (d) the thyroid gland, and rated as absent (group A), present (group B), symmetric (group B1) or asymmetric (group B2). Differences between groups A and B and between B1 and B2 were tested for significance with the U-test (p < 0.05). An average follow-up of about 2.5 years (mean 29.5 ± 13.9 months) served as the reference period to determine whether patients developed a head and neck malignancy.
Results
Of the 590 patients, 235 (40%) showed no evidence of enhanced FDG uptake in any investigated site, and 355 (60%) showed qualitatively elevated FDG uptake in at least one site. FDG uptake values (SUVmax, mean±SD) for Waldeyer’s ring were 3.0 ± 0.89 in group A (n = 326), 4.5 ± 2.18 in group B (n = 264; p < 0.01), 5.4 ± 3.35 in group B1 (n = 177), and 4.1 ± 1.7 in group B2 (n = 87; p < 0.01). Values for the oral floor were 2.8 ± 0.74 in group A (n = 362), 4.7 ± 2.55 in group B (n = 228; p < 0.01), 4.4 ± 3.39 in group B1 (n = 130), and 5.1 ± 2.69 in group B2 (n = 98, p = 0.01). Values for the larynx were 2.8 ± 0.76 in group A (n = 353), 4.2 ± 2.05 in group B (n = 237; p < 0.01), 4.0 ± 2.02 in group B1 (n = 165), and 4.6 ± 2.8 in group B2 (n = 72; p = 0.027). Values for the thyroid were 2.4 ± 0.63 in group A (n = 404), 3.0 ± 1.01 in group B (n = 186; p < 0.01), 2.6 ± 0.39 in group B1 (n = 130), and 4.0 ± 1.24 in group B2 (n = 56; p < 0.01). One patient developed a palatine tonsil carcinoma (group B1, SUVmax 3.2), and one patient developed an oral floor carcinoma (group B1, SUVmax 3.7).
Conclusion
Elevated/asymmetric head and neck FDG accumulation without a correlating morphological lesion can frequently be found and does not predict cancer development. In populations in which goitre is endemic, FDG uptake by the thyroid is common and not associated with thyroid cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tumour imaging with 2-deoxy-2-[18F]-fluoro-d-glucose (FDG)-PET(/CT) is based on increased glucose metabolism of FDG-avid malignancies. Increased FDG uptake corresponding to a lesion on morphological imaging (e.g. CT) is suspicious for malignancy if not explained otherwise. FDG accumulation, however, is not specific for tumour. Demonstration of a lesion’s status often needs confirmation by biopsy/surgery and histopathological evaluation of the specimen. Only rarely can a benign aetiology be demonstrated by the clinical impression or patient history alone. In the head and neck region [1], there are various nonmalignant conditions that may be avid for FDG uptake [2–9]. Variable physiological FDG uptake is found in normal structures such as muscles, mucosa, lymphoid tissue or tonsils. Furthermore, inflammatory processes and other benign conditions—namely, thyroid adenomas and thyroiditis—may cause increased FDG uptake [10]. Although FDG uptake in primary head and neck neoplasms is usually greater than that observed in metabolically active normal structures/benign conditions, an overlap between tumour and nonmalignant uptake may occur and hamper image interpretation [11].
Since its clinical implementation in 2001, whole-body FDG-PET/CT has been increasingly used for staging, restaging, monitoring treatment, and predicting prognosis in oncological patients, including those with head and neck cancer [1, 12–20]. “Full-diagnostic” FDG-PET/CT with intravenous administration of contrast agent shows advantages over FDG-PET alone by (1) giving a precise anatomical context for any metabolic spot, and (2) by allowing assessment of the morphology of any such spot [21]. The correspondence of a morphological lesion and a metabolic spot may raise the suspicion of a pathological condition such as tumour or inflammation. However, there is no conclusive strategy for how to deal with a PET-positive finding without a correlating lesion on CT. As FDG-PET can provide diagnostic information earlier than morphological imaging [22], such a finding may still indicate a pathological condition namely the development of malignancy. It has been suggested that the symmetry of uptake should be observed in order to differentiate between physiological and pathological FDG accumulation [23, 24]. However, some authors indicate that symmetry may not be a reliable predictor of physiological or nonmalignant uptake. Several malignancies show symmetric FDG uptake, and, conversely, physiological FDG uptake may often be asymmetric [11]. Diagnostic uncertainty may lead to unnecessary diagnostic work-up and may distress the patient.
The aim of this retrospective study was firstly to evaluate FDG uptake in Waldeyer’s ring, the oral floor and the larynx as a predictor of tumour development in patients in whom the CT scan was unremarkable. FDG uptake was evaluated qualitatively and quantitatively. Secondly, FDG uptake in the thyroid gland was evaluated as a predictor of tumour development in a population in which goitre was endemic. Only patients without a head and neck malignancy and without a clinically apparent head and neck infection were included.
Materials and methods
Subjects
From June 2002 to December 2004, a total of 2,561 PET/CT scans were performed in our institution including scans with several tracers, low-dose scans, follow-up scans in the same patients, scans for head and neck malignancy, scans for cancer of unknown primary, as well as scans for infections. From this population, 960 patients met our inclusion/exclusion criteria listed below. Of these initial 960 patients, 370 were excluded because of missing follow-up data (n = 79) or a follow-up time of less than 1 year (n = 291; none of these patients showed head and neck malignancy during the short-term follow-up). Finally, 590 patients (212 men and 378 women; mean age 55.4 ± 13.3 years, range 14.5–89.2 years) fulfilled the inclusion criteria listed below and additionally had a follow-up period of at least 1 year, and were included in this retrospective analysis. All patients provided informed consent to the use of intravenously administered FDG and CT contrast material with information concerning its rare potential side effects. The study was performed in accordance with the guidelines established by the local ethics committee.
The inclusion criterion was:
-
Availability of a full-dose, contrast-enhanced (“fully-diagnostic”) whole-body FDG-PET/CT scan.
Exclusion criteria were:
-
Blood glucose level at the time of intravenous FDG injection >150 mg/dl.
-
History of head and neck cancer, thyroid malignancy, head and neck metastases.
-
Prior surgery or radiation therapy of the head and neck region.
-
Cancer of unknown primary.
-
Clinical evidence of inflammation of the head and neck region at the time of FDG-PET/CT investigation.
Imaging protocol
All subjects were instructed to fast for at least 6 h prior to FDG-PET/CT. One hour before starting the FDG-PET/CT scan patients drank 1000 ml of a water-equivalent oral contrast agent [25]. Prior to tracer injection a patient history was taken by the nuclear medicine physician including questions concerning current inflammatory disorders. About 1 h after FDG injection dual-modality FDG-PET/CT imaging was performed on a Biograph PET/CT system (Siemens Medical Solutions, Hoffman Estates, IL) comprising a dual-slice CT scanner (Somatom Emotion, Siemens Medical Solutions, Forchheim, Germany) and a full-ring PET scanner with bismuth germinate crystals (ECAT HR+, Siemens Molecular Imaging, Hoffman Estates, IL). CT was performed first, followed by PET. The CT scan (130 mAs, 130 kV, 5-mm sections, 8-mm table feed, 2.4-mm incremental reconstruction) covered a field of view ranging from the skull base to the upper thighs. All CT scans were enhanced by an iodinated contrast medium (Ultravist 300; Schering, Berlin, Germany) administered intravenously at a flow rate of 3 ml/s for the first 90 ml and 1.5 ml/s for the following 50 ml, with a delay of 50 s. A limited breath-hold technique was used to avoid motion-induced artefacts in the area of the diaphragm [26]. Consecutively, PET images were obtained using the following parameters: 15.5 cm axial field of view per bed position; 4.6 mm in-plane spatial resolution; 3-D mode; emission time adapted to the patient’s body weight (<65 kg, 4 min per bed position; 65–85 kg, 5 min per bed position; >85 kg, 6 min per bed position); iterative image reconstruction (FORE and AWOSEM, nonlinear, two iterations, eight subsets, gaussian filter 5.0 mm, scatter correction). Images were reconstructed with and without CT-based attenuation correction.
Image evaluation/allocation of patients to study groups
The following head and neck sites were evaluated on FDG-PET/CT scans:
-
1.
Waldeyer’s ring, consisting of the pharyngeal (roof), palatine (lateral wall) and lingual tonsils (floor) [2, 5],
-
2.
Oral floor (mucosa and adjacent myohyoid muscles),
-
3.
Larynx at the level of the vocal cords;
-
4.
Thyroid gland.
Waldeyer’s ring was measured in total as it has previously been pointed out that differentiation of each tonsil of Waldeyer’s ring can be difficult on FDG-PET/CT scans [27].
Images were evaluated as follows. The CT images were evaluated for the presence of morphological lesions in the head and neck region, with the investigators being blinded to the PET results. Additionally, the thyroid glands were morphologically investigated. FDG-PET/CT images were evaluated both qualitatively and quantitatively for areas of focally increased tracer uptake. For qualitative evaluation, the attenuation-corrected FDG-PET images as well as the FDG-PET/CT images were analysed for the presence or absence of elevated tracer uptake in the four locations mentioned above. The scheme followed by Nakamoto et al. [4] with modification was used: tracer uptake was rated as “present” if there was uptake greater than the blood pool; tracer uptake was graded as “absent” if either no elevated tracer uptake was visible or uptake was comparable to that of the blood pool. To exclude patients with seemingly elevated tracer uptake caused by artefacts, e.g. from dental implants [28, 29], the PET images without attenuation correction were also examined. Patients were categorized separately for each of the four sites investigated. Those with no increase in tracer uptake were categorized as group A, considered as the group with “no suspicion” of malignancy, and those with elevated uptake were categorized as group B. Group B was further divided into subgroups B1 and B2 according to the symmetry of FDG accumulation: symmetric uptake was considered to indicate a “low suspicion” of malignancy; an asymmetric uptake was considered to indicate a “potential malignancy”. FDG-PET/CT images were evaluated in consensus by a nuclear medicine physician with 5 years experience in reading FDG-PET/CT scans and a radiologist with 4 years experience in reading FDG-PET/CT scans.
For quantitative evaluation, SUVmax values were measured in each of the four sites. For SUVmax measurements, a circular region of interest was drawn on the fused FDG-PET/CT images using the toolbar of the AW Suite Volume Viewer Plus software (General Electrics Healthcare, General Electrics, München, Germany). For each site SUVmax was documented. SUVmax was calculated as
A PACS workstation (General Electrics Healthcare, General Electrics, München, Germany) connected to an AW Suite Volume Viewer Plus workstation was used for image evaluation.
To test whether morphological thyroid changes and/or goitre affect the thyroid SUVmax value, patients were divided into a group without morphological thyroid changes seen on CT and a group with thyroid changes seen on CT.
To test whether systemic oncological treatment affects the SUVmax values, patients were divided into a group without systemic oncological treatment within the last 4 months and a group currently receiving systemic treatment.
Standard of reference
Clinical and radiological follow-up of at least 1 year served as the standard of reference to evaluate whether subjects developed a head and neck malignancy after the FDG-PET/CT scan. In order to obtain clinical follow-up a questionnaire and/or the last medical reports were retrieved from the treating clinicians. The questionnaire addressed the development of head and neck malignancies and/or metastases. From the medical reports all information available was used, including clinical examination results, radiological and nuclear medicine follow-up examinations as well as histopathological results. In a subgroup of patients with FDG uptake of more than 2×SD over the mean all available medical charts of our hospital were reviewed in detail. In addition, medical charts of all patients were checked at the time of the FDG-PET/CT scan to rule out present—clinically known—head and neck tumours and/or infections (see exclusion criteria).
Statistical analysis/data evaluation
Mann-Whitney-Wilcoxon’s test (U-test) was used to test for statistically significant differences in SUVmax between:
-
groups (A versus B)
-
subgroups (B1 versus B2)
-
the patient group with morphologically normal thyroids and the patient group with gland enlargement and/or degenerative changes
-
the patient group without systemic oncological treatment within the last 4 months and the patient group currently receiving systemic treatment
A p value less than 0.05 was considered to be statistically significant. All statistical analyses were done with the SPSS software package 15.0 for Windows (SPSS, Chicago, IL).
Results
The mean follow-up time was 29.5 months (range 12–65.3 months, SD 13.9 months) in 590 patients. The reference standard (clinical and radiological follow-up) was based on follow-up data provided by private practitioners/oncologists (n = 326), and/or medical charts of our university hospital (n = 537), and/or follow-up FDG-PET/CT scans (n = 291), and/or follow-up CT scans (n = 73) and/or ear, nose and throat examinations (n = 11). The mean blood glucose level prior to FDG injection was 97 mg/dl (70–149 mg/dl, SD 16 mg/dl). Patients were injected with a mean of 348 MBq FDG (140–408 MBq, SD 35 MBq). The prevalence and distribution of primary malignancies are given in Table 1. Overall, 235 patients (40%) showed no evidence of enhanced FDG uptake in any of the sites investigated, 355 patients (60%) had qualitatively elevated FDG uptake in at least one site, and 290 patients (49%) had at least one site with asymmetric uptake. Qualitative and quantitative results are summarized in Tables 2, 3 and 4. Regarding systemic oncological treatment within the 4 months prior to FDG-PET/CT scan, 116 patients were receiving treatment and 474 patients had had no treatment.
Waldeyer’s ring
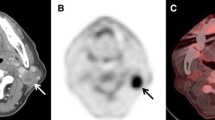
The mean SUVmax was 3.7 (range 1.3–22.7, SD 1.89; Fig. 1). For detailed results see Tables 2, 3 and 4. One 59-year-old woman developed a new carcinoma of the left palatine tonsil during follow-up. In the initial FDG-PET/CT scan she showed minor symmetric FDG uptake in Waldeyer’s ring (group B, subgroup B1, SUVmax 3.2, Fig. 2).
Elevated FDG uptake in the left palatine tonsil without tumour development or clinically evident tonsillitis. Asymmetric FDG uptake in the left palatine tonsil of Waldeyer’s ring (SUVmax: left tonsil 4.3, right tonsil 2.1) of a 50-year-old man with a urothelial carcinoma of the bladder in whom clinically there was no suspicion of malignancy or inflammation at the time of FDG-PET/CT scan. The patient did not develop any malignancy of the head and neck (follow-up time of 13 months)
Oral floor
The mean SUVmax was 3.5 (range 1.1–26.0, SD 2.12). For detailed results see Tables 2, 3 and 4. One 25-year-old woman developed a carcinoma of the oral floor as a second malignancy during follow-up. On the initial FDG-PET/CT scan she showed slightly increased symmetric FDG uptake of the oral floor (group B, subgroup B1, SUVmax 3.7, Fig. 3).
Larynx
The mean SUVmax was 3.4 (range 0.9–18.3, SD 1.65; Fig. 4). For detailed results see Tables 2, 3 and 4. No patient developed laryngeal malignancy during follow-up.
Asymmetric laryngeal FDG uptake without tumour onset or vocal cord paralysis. Asymmetric FDG uptake with accumulation in the left vocal cord (SUVmax: left vocal cord 6.5, right 3.3). The 67-year-old patient had gastric carcinoma and did not develop any laryngeal malignancy. On a follow-up FDG-PET/CT scan 41 months later neither FDG uptake nor a morphological mass was seen on the left vocal cord any more. The patient did not suffer vocal cord paresis
Thyroid gland
The mean SUVmax was 2.6 (range 0.1–6.9, SD 0.83). For detailed results see Tables 2, 3 and 4. Of the 590 patients, 545 (92%) showed morphologically normal thyroids with a mean SUVmax of 2.6 (0.1–6.9, SD 0.84), and 45 (8%) showed enlarged thyroid glands and/or degenerative changes with a mean SUVmax of 2.9 (1.6–5.4, SD 0.96, p = 0.028; Fig. 5). Of these 45 patients, 24 (44%) qualitatively showed no increased FDG uptake in the thyroid (group A), 21 (56%) showed increased uptake (group B), and of these 21 patients, 4 were categorized in group B1 (symmetric uptake) and 17 in group B2 (asymmetric uptake). No patient developed thyroid malignancy during follow-up.
Evaluation of nonmalignant factors possibly increasing FDG uptake
The medical charts of all patients showing elevated FDG uptake of more than 2×SD over the mean SUVmax (80, 13.6%, of patients: SUVmax of Waldeyer’s Ring >7.5, 22 patients; of the oral floor >7.7, 19 patients; of the larynx >6.7, 14 patients; and of the thyroid >4.3, 25 patients) were retrospectively reviewed in order to identify possible nonmalignant factors that may have caused enhanced FDG accumulation. Of these patients, 23 (4% of all patients/29% of patients with SUVmax >2×SD over the mean SUVmax) were receiving systemic treatment. In no patient were any nonmalignant findings explaining a SUVmax of more than 2×SD over the mean SUVmax detectable.
Discussion
Incidentally found, elevated and/or asymmetric FDG accumulation in the head and neck region is a common finding in oncological patients undergoing FDG-PET/CT for reasons other than head and neck cancer [4]. In this study 60% of patients showed elevated FDG uptake in at least one region. In clinical routine, elevated FDG uptake may mandate further diagnostic procedures to exclude malignancy. However, based on the results of this study even strongly enhanced or asymmetric FDG uptake in Waldeyer’s ring, the oral floor, the larynx, and the thyroid gland cannot be considered a reliable predictor of tumour development provided that the corresponding full-diagnostic CT information is unremarkable. Thus, it can be recommended that these incidental findings do not need to be evaluated any further.
Two patients in this study of 590 patients developed head and neck malignancy during follow-up. Both patients were classified as having elevated but symmetric FDG uptake in the respective region. Such an FDG uptake pattern may be considered as one of low suspicion for malignancy. This result also indicates that the pattern and intensity of FDG uptake may not be a useful predictor of cancer development.
Our results are in contrast to those of previous studies recommending further diagnostic work-up to exclude malignancy for any FDG uptake asymmetry in the head and neck region [23, 24, 30, 31]. However, those studies were not performed using FDG-PET/CT, but stand-alone FDG-PET. Therefore, morphological lesions could not be ruled out in those studies. For this reason recommendations as to the diagnostic work-up of incidental FDG uptake need to be adapted to FDG-PET/CT. Full-diagnostic CT data offer relevant additional information over FDG-PET alone or over FDG-PET/CT with a low-dose CT scan [32]. As the pattern and magnitude of FDG accumulation in nonmalignant conditions and in malignant head and neck tumours [33] overlap substantially, the CT information is highly relevant when deciding on the presence or absence of a tumour. A “full-diagnostic” CT scan performed as part of a combined FDG-PET/CT examination improves the assessment of the head and neck region as also shown for other body regions [32, 34–36]. Our study indicates that an unremarkable full-diagnostic CT scan in the head and neck region has the potential to virtually rule out head and neck cancer in cases with incidental positive FDG accumulations.
Blodgett et al. have pointed out that physiological FDG uptake can be asymmetric and that FDG uptake in the presence of malignancy can be symmetric [11]. Our results are in accordance with these findings. In a substantial proportion of subjects physiological and asymmetric FDG accumulation was detectable and did not mark early tumour onset, whereas both head and neck malignancies developing during follow-up occurred together with symmetrically elevated uptake. However, one of these tumours (oral floor) developed more than 4 years after the FDG-PET/CT scan and was probably not linked to the previous FDG accumulation.
Our results suggest that elevated FDG uptake without a morphological correlate may be attributable to nonmalignant factors such as muscle activity [37] or immune processes that may run at a subclinical level [38]. It has been shown that FDG uptake may be seen in the laryngeal muscles. Excessive oral floor and laryngeal uptake may be a result of muscular activity when patients ignore the instruction to keep silent or when coughing after FDG administration [5, 27, 39]. Increased FDG accumulation caused by immune reactions is explicable by the recruitment of activated granulocytes, macrophages and lymphocytes which have enhanced levels of glucose transport proteins (e.g. GLUT 1 and GLUT 3 [40]) and increased glucose metabolism [38, 41–43]. An interesting aspect is that SUVmax values measured at symmetrically enhancing Waldeyer’s rings are significantly higher than values of asymmetrically enhancing Waldeyer’s rings. This finding may be attributed to the fact that a subclinical tonsillitis/pharyngitis may start at only one pharyngeal wall (right or left wall, with only a limited level of inflammatory process) and, in the course of the inflammatory process, subsequently affect the contralateral pharyngeal wall with an overall higher level of inflammation. The magnitude of pharyngeal and laryngeal SUVmax found in this study corresponds well to the “standardized uptake value atlas” of Wang et al. [44] and Zincirkeser et al. [45]. However, the range of SUVmax observed in this study exceeded the minimum and maximum values given in these atlases. This fact is most probably related to the substantially higher number of investigated patients compared to those studies [44, 45].
Our results reveal significantly higher SUVmax values in patients receiving systemic oncological treatment when evaluating Waldeyer’s ring, the oral floor and the larynx, in contrast to the thyroid gland where no significant difference was detectable. These findings are probably based on mucositis as a well-known complication of systemic oncological treatment [46], as well as on lymphatic inflammatory reactions. Of note, quantitative assessment in Waldeyer’s ring showed only slightly higher SUVmax values in group B than in group A. This most probably goes back to the difficulty—at times—of qualitatively distinguishing between “uptake” and “no uptake”.
In contrast to other studies [47], FDG uptake in the thyroid gland was detected in a substantial proportion of patients (31%)—more often symmetric than not. This rather high frequency of thyroid gland uptake may represent the high prevalence of benign thyroid gland disease (in particular goitre and nodular disease) in the patient population under investigation. A variety of benign thyroid gland diseases, such as thyroid adenoma and Hashimoto’s thyroiditis, have the potential to accumulate FDG [48, 49]. In combination with the very low prevalence of thyroid carcinoma in Germany (about 1 per 1,000 persons) it is reasonable that in our study no thyroid carcinoma developed during follow-up. In areas in which is endemic symmetric or asymmetric FDG uptake thus appears to be no indicator of thyroid cancer. This is in sharp contrast to studies in nonendemic areas such as the US, Japan or South Korea. In those countries a low prevalence of incidental FDG uptake in the thyroid gland has been found (1–2% [50–52]) with a rather high rate of thyroid cancer (10–50% [50–52]).
This study was designed to analyse the relevance of incidental FDG foci in the head and neck region. Patients with known or suspected head and neck cancer/inflammation or those with a history of head and neck cancer were excluded from the study. In addition, patients with cancer of unknown primary were ruled out. For this reason, our results cannot be transferred to patients with a history of, or actually suffering from, head and neck cancer due to the much higher probability of malignant head and neck lesions in such “high-risk” patients [53]. Further studies must elucidate the significance of FDG foci in the head and neck region without a morphological correlate on fully diagnostic CT in patients at a high a priori risk for head and neck cancer.
Due to the retrospective design of this study, in the majority of patients the reference standard was neither a histological one nor an ear, nose and throat examination, but was rather based on clinical and radiological follow-up data. A prospective study would allow a dedicated verification of elevated FDG uptake. However, such studies would be hampered by a administrative, logistic and financial burden and may not be justified for ethical reasons in large patient numbers. In contrast, the clinical course over on average 2.5 years, as used as the standard in this study considering a mean doubling time in solid tumours of 100–150 days [54], the mean follow-up time in this study, would allow for an increase in tumour volume by a factor 64 to 500.
In conclusion, elevated and/or asymmetric FDG accumulation in Waldeyer’s ring, the oral floor or the larynx is a common incidental finding in oncological patients undergoing FDG-PET/CT for reasons other than head and neck cancer. However, when accompanied by an unremarkable full-diagnostic CT scan as part of the FDG-PET/CT scan, such a finding virtually never predicts the development of head and neck cancer. Further diagnostic work-up thus seems not to be justified. Incidental FDG uptake in the thyroid gland is frequently observed in the studied population in which goitre is endemic (as opposed to populations in which goitre is not endemic) is no indicator of thyroid cancer.
References
Pentenero M, Cistaro A, Brusa M, Ferraris MM, Pezzuto C, Carnino R, et al. Accuracy of 18F-FDG-PET/CT for staging of oral squamous cell carcinoma. Head Neck. 2008;30:1488–96.
Chen YK, Su CT, Chi KH, Cheng RH, Wang SC, Hsu CH. Utility of 18F-FDG PET/CT uptake patterns in Waldeyer’s ring for differentiating benign from malignant lesions in lateral pharyngeal recess of nasopharynx. J Nucl Med. 2007;48:8–14.
Cook GJ, Maisey MN, Fogelman I. Normal variants, artefacts and interpretative pitfalls in PET imaging with 18-fluoro-2-deoxyglucose and carbon-11 methionine. Eur J Nucl Med. 1999;26:1363–78. doi: 10.1007/s002590050597.
Nakamoto Y, Tatsumi M, Hammoud D, Cohade C, Osman MM, Wahl RL. Normal FDG distribution patterns in the head and neck: PET/CT evaluation. Radiology 2005;234:879–85. doi: 10.1148/radiol.2343030301.
Kostakoglu L, Hardoff R, Mirtcheva R, Goldsmith SJ. PET-CT fusion imaging in differentiating physiologic from pathologic FDG uptake. Radiographics 2004;24:1411–31. doi: 10.1148/rg.245035725.
Rosenbaum SJ, Lind T, Antoch G, Bockisch A. False-positive FDG PET uptake – the role of PET/CT. Eur Radiol 2006;16:1054–65. doi: 10.1007/s00330-005-0088-y.
Strauss LG. Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med 1996;23:1409–15. doi: 10.1007/BF01367602.
Stahl A, Dzewas B, Schwaiger M, Weber WA. Excretion of FDG into saliva and its significance for PET imaging. Nuklearmedizin 2002;41:214–6.
Wong RJ. Current status of FDG-PET for head and neck cancer. J Surg Oncol 2008;97:649–52. doi: 10.1002/jso.21018.
Del Rocio Estrada-Sanchez G, Altamirano-Ley J, Ochoa-Carrillo FJ. Normal variants and frequent pitfalls with (18)FDG PET/CT study. Cir Cir. 2007;75:491–7.
Blodgett TM, Fukui MB, Snyderman CH, Branstetter BF 4th, McCook BM, Townsend DW, et al. Combined PET-CT in the head and neck: part 1. Physiologic, altered physiologic, and artifactual FDG uptake. Radiographics 2005;25:897–912. doi: 10.1148/rg.254035156.
Blodgett TM, Meltzer CC, Townsend DW. PET/CT: form and function. Radiology 2007;242:360–85. doi: 10.1148/radiol.2422051113.
Antoch G, Saoudi N, Kuehl H, Dahmen G, Mueller SP, Beyer T, et al. Accuracy of whole-body dual-modality fluorine-18-2-fluoro-2-deoxy-D-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol 2004;22:4357–68. doi: 10.1200/JCO.2004.08.120.
Antoch G, Vogt FM, Bockisch A, Ruehm SG. Whole-body tumor staging: MRI or FDG-PET/CT? Radiologe 2004;44:882–8. doi: 10.1007/s00117-004-1093-x.
Antoch G, Vogt FM, Freudenberg LS, Nazaradeh F, Goehde SC, Barkhausen J, et al. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA 2003;290:3199–206. doi: 10.1001/jama.290.24.3199.
Stahl A, Stollfuss J, Ott K, Wieder H, Fink U, Schwaiger M, et al. FDG PET and CT in locally advanced adenocarcinomas of the distal oesophagus. Clinical relevance of a discordant PET finding. Nuklearmedizin 2005;44:249–55.
Kapoor V, Fukui MB, McCook BM. Role of 18FFDG PET/CT in the treatment of head and neck cancers: principles, technique, normal distribution, and initial staging. AJR Am J Roentgenol 2005;184:579–87.
Kapoor V, Fukui MB, McCook BM. Role of 18FFDG PET/CT in the treatment of head and neck cancers: posttherapy evaluation and pitfalls. AJR Am J Roentgenol 2005;184:589–97.
Ng SH, Chan SC, Yen TC, Chang JT, Liao CT, Ko SF, et al. Staging of untreated nasopharyngeal carcinoma with PET/CT: comparison with conventional imaging work-up. Eur J Nucl Med Mol Imaging 2008;36:12–22.
Ha PK, Hdeib A, Goldenberg D, Jacene H, Patel P, Koch W, et al. The role of positron emission tomography and computed tomography fusion in the management of early-stage and advanced-stage primary head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 2006;132:12–6. doi: 10.1001/archotol.132.1.12.
Schoder H, Yeung HW, Gonen M, Kraus D, Larson SM. Head and neck cancer: clinical usefulness and accuracy of PET/CT image fusion. Radiology 2004;231:65–72. doi: 10.1148/radiol.2311030271.
Abouzied MM, Crawford ES, Nabi HA. 18F-FDG imaging: pitfalls and artifacts. J Nucl Med Technol 2005;33:145–55.
Cook GJ, Fogelman I, Maisey MN. Normal physiological and benign pathological variants of 18-fluoro-2-deoxyglucose positron-emission tomography scanning: potential for error in interpretation. Semin Nucl Med 1996;26:308–14. doi: 10.1016/S0001-2998(96)80006-7.
Basu S, Houseni M, Alavi A. Significance of incidental fluorodeoxyglucose uptake in the parotid glands and its impact on patient management. Nucl Med Commun 2008;29:367–73.
Antoch G, Kuehl H, Kanja J, Lauenstein TC, Schneemann H, Hauth E, et al. Dual-modality PET/CT scanning with negative oral contrast agent to avoid artifacts: introduction and evaluation. Radiology 2004;230:879–85. doi: 10.1148/radiol.2303021287.
Beyer T, Antoch G, Blodgett T, Freudenberg LF, Akhurst T, Mueller S. Dual-modality PET/CT imaging: the effect of respiratory motion on combined image quality in clinical oncology. Eur J Nucl Med Mol Imaging 2003;30:588–96.
Wong WL, Gibson D, Sanghera B, Goodchild K, Saunders M. Evaluation of normal FDG uptake in palatine tonsil and its potential value for detecting occult head and neck cancers: a PET CT study. Nucl Med Commun 2007;28:675–80. doi: 10.1097/MNM.0b013e32829152b1.
Goerres GW, Hany TF, Kamel E, von Schulthess GK, Buck A. Head and neck imaging with PET and PET/CT: artefacts from dental metallic implants. Eur J Nucl Med Mol Imaging 2002;29:367–70. doi: 10.1007/s00259-001-0721-1.
Kamel EM, Burger C, Buck A, von Schulthess GK, Goerres GW. Impact of metallic dental implants on CT-based attenuation correction in a combined PET/CT scanner. Eur Radiol 2003;13:724–8.
Schoder H, Yeung HW. Positron emission imaging of head and neck cancer, including thyroid carcinoma. Semin Nucl Med 2004;34:180–97. doi: 10.1053/j.semnuclmed.2004.03.004.
Macapinlac HA. FDG-PET in head and neck, and thyroid cancer. Chang Gung Med J 2005;28:284–95.
Pfannenberg AC, Aschoff P, Brechtel K, Muller M, Bares R, Paulsen F, et al. Low dose non-enhanced CT versus standard dose contrast-enhanced CT in combined PET/CT protocols for staging and therapy planning in non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2007;34:36–44. doi: 10.1007/s00259-006-0186-3.
Syed R, Bomanji JB, Nagabhushan N, Hughes S, Kayani I, Groves A, et al. Impact of combined (18)F-FDG PET/CT in head and neck tumours. Br J Cancer 2005;92:1046–50. doi: 10.1038/sj.bjc.6602464.
Pfannenberg AC, Aschoff P, Brechtel K, Muller M, Klein M, Bares R, et al. Value of contrast-enhanced multiphase CT in combined PET/CT protocols for oncological imaging. Br J Radiol 2007;80:437–45. doi: 10.1259/bjr/34082277.
Antoch G, Forsting M. How much CT does PET/CT need? Nuklearmedizin 2004;43:141–2.
Antoch G, Freudenberg LS, Beyer T, Bockisch A, Debatin JF. To enhance or not to enhance? 18F-FDG and CT contrast agents in dual-modality 18F-FDG PET/CT. J Nucl Med 2004;45(Suppl 1):56S–65S.
Jackson RS, Schlarman TC, Hubble WL, Osman MM. Prevalence and patterns of physiologic muscle uptake detected with whole-body 18F-FDG PET. J Nucl Med Technol 2006;34:29–33.
Kawabe J, Okamura T, Shakudo M, Koyama K, Sakamoto H, Ohachi Y, et al. Physiological FDG uptake in the palatine tonsils. Ann Nucl Med 2001;15:297–300. doi: 10.1007/BF02987850.
Barrington SF, Maisey MN. Skeletal muscle uptake of fluorine-18-FDG: effect of oral diazepam. J Nucl Med 1996;37:1127–9.
Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992;33:1972–80.
Scadding GK. Immunology of the tonsil: a review. J R Soc Med 1990;83:104–7.
Paik JY, Lee KH, Choe YS, Choi Y, Kim BT. Augmented 18F-FDG uptake in activated monocytes occurs during the priming process and involves tyrosine kinases and protein kinase C. J Nucl Med 2004;45:124–8.
Babior BM. The respiratory burst of phagocytes. J Clin Invest 1984;73:599–601. doi: 10.1172/JCI111249.
Wang Y, Chiu E, Rosenberg J, Gambhir SS. Standardized uptake value atlas: characterization of physiological 2-deoxy-2-[18F]fluoro-D-glucose uptake in normal tissues. Mol Imaging Biol 2007;9:83–90. doi:10.1007/s11307-006-0075-y.
Zincirkeser S, Sahin E, Halac M, Sager S. Standardized uptake values of normal organs on 18F-fluorodeoxyglucose positron emission tomography and computed tomography imaging. J Int Med Res 2007;35:231–6.
Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am 2008;52:61–77. doi: 10.1016/j.cden.2007.10.002.
Kang KW, Kim SK, Kang HS, Lee ES, Sim JS, Lee IG, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for metastasis evaluation and cancer screening in healthy subjects. J Clin Endocrinol Metab 2003;88:4100–4. doi: 10.1210/jc.2003-030465.
Mikosch P, Wurtz FG, Gallowitsch HJ, Kresnik E, Lind P. F-18-FDG-PET in a patient with Hashimoto’s thyroiditis and MALT lymphoma recurrence of the thyroid. Wien Med Wochenschr 2003;153:89–92. doi: 10.1046/j.1563-258X.2003.02007.x.
Rohren EM. Intense FDG uptake in a benign Hurthle cell adenoma. Clin Nucl Med 2004;29:664–6. doi: 10.1097/00003072-200410000-00021.
King DL, Stack BC Jr, Spring PM, Walker R, Bodenner DL. Incidence of thyroid carcinoma in fluorodeoxyglucose positron emission tomography-positive thyroid incidentalomas. Otolaryngol Head Neck Surg 2007;137:400–4. doi: 10.1016/j.otohns.2007.02.037.
Kurata S, Ishibashi M, Hiromatsu Y, Kaida H, Miyake I, Uchida M, et al. Diffuse and diffuse-plus-focal uptake in the thyroid gland identified by using FDG-PET: prevalence of thyroid cancer and Hashimoto’s thyroiditis. Ann Nucl Med 2007;21:325–30. doi: 10.1007/s12149-007-0030-2.
Kim TY, Kim WB, Ryu JS, Gong G, Hong SJ, Shong YK. 18F-fluorodeoxyglucose uptake in thyroid from positron emission tomogram (PET) for evaluation in cancer patients: high prevalence of malignancy in thyroid PET incidentaloma. Laryngoscope 2005;115:1074–8. doi: 10.1097/01.MLG.0000163098.01398.79.
Donta TS, Smoker WR. Head and neck cancer: carcinoma of unknown primary. Top Magn Reson Imaging 2007;18:281–92. doi:10.1097/RMR.0b0113e3181570c6c.
Revel MP, Merlin A, Peyrard S, Triki R, Couchon S, Chatellier G, et al. Software volumetric evaluation of doubling times for differentiating benign versus malignant pulmonary nodules. AJR Am J Roentgenol 2006;187:135–42. doi: 10.2214/AJR.05.1228.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heusner, T.A., Hahn, S., Hamami, M.E. et al. Incidental head and neck 18F-FDG uptake on PET/CT without corresponding morphological lesion: early predictor of cancer development?. Eur J Nucl Med Mol Imaging 36, 1397–1406 (2009). https://doi.org/10.1007/s00259-009-1113-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1113-1