Abstract
Introduction
Vascular prosthesis infection (VPI) is a life-threatening complication that occurs in 0.5–5% of prostheses. Low-grade infections in non-acute patients are a diagnostic challenge requiring a new method with good diagnostic accuracy. The aim of this work was to define the accuracy of 18F-FDG PET/CT in these settings and to identify essential parameters of the evaluation.
Material and methods
PET/CT was performed prospectively in 76 consecutive patients with a total of 96 vascular prosthetic grafts in which infection was suspected. PET/CT scans were analysed in terms of the presence and intensity of focal and diffuse FDG uptake, the presence of an anastomotic pseudoaneurysm, the presence of an irregular boundary of infiltration, a combination of these, and the uptake ratio between the graft and blood background. The gold standard was based on operative/histopathological finding or a clinical follow up of >6 months.
Results
Among the various assessed parameters only focal FDG uptake and an irregular graft boundary were significant predictors of VPI. Focal intense FDG uptake together with an irregular boundary of the lesion on CT scan predicted VPI with 97% probability, while smooth lesion boundaries and no focal FDG uptake predicted a probability of VPI of less than 5%. Even in lesions with nondiagnostic inhomogeneous focal FDG uptake (18/96) an irregular boundary effectively helped in decision-making with a probability of 28% (smooth) or 77% (irregular) for VPI.
Conclusion
PET/CT gave reliable results with an accuracy >95% in 75% of prostheses. PET/CT can identify those prostheses (25% of prosthesis) for which its diagnostic accuracy is diminished to 70–75%. In our series PET/CT was an excellent diagnostic modality for suspected VPI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vascular prosthesis infection (VPI) occurs in 0.5–5% of implantations [1, 2]. Any delay in diagnosis and adequate treatment can lead to life-threatening complications such as anastomotic bleeding and sepsis which results in limb loss or death in a high number of patients. Early and reliable diagnosis of an infected vascular prosthesis is a precondition of adequate treatment. In this indication, the most frequently used diagnostic imaging method is computed tomography (CT), which has very good diagnostic accuracy in a patients with advanced graft infection [3], but fails in low-grade infection with a sensitivity and specificity about 55% and 100%, respectively [4]. Low-grade infections may pose a diagnostic problem and other imaging methods are used including structural ones such as ultrasonography (US) or magnetic resonance imaging (MRI) [5–7] and functional ones such as scintigraphy using labelled white blood cells (111In [8, 9] or 99mTc-HMPAO [10–13]), 99mTc-labelled antigranulocyte antibodies, 67Ga-citrate [14, 15], labelled antibiotics (99mTc-ciprofloxacin) or avidin in combination with 111In-biotin [16, 17]. The diagnostic accuracy of these imaging methods differs. The numbers of patients studied range between 20 and 30 in most studies, and larger studies are still needed. There is a need for prospective studies evaluating the contribution of the various diagnostic methods indicated for VPI especially low-grade VPI and VPI that does not require urgent surgical treatment. For these patients CT is an adequate diagnostic method [3, 4, 18].
Recently there has been unparalleled development of hybrid imaging technology, including PET/CT and SPECT/CT, that combines two different modalities in one device. These new technologies enable the combination of detailed high spatial resolution morphological CT data with functional PET or SPECT data in one image. This results in exact anatomical localization of functional alterations (e.g. increased glucose metabolism) and therefore more exact diagnosis and increased sensitivity and specificity in diagnosing VPI. Some studies favour PET over SPECT in terms of image quality and better spatial resolution.
PET combined with CT (hybrid PET/CT) using 18F-FDG is a well-accepted diagnostic tool for the assessment of cancer. 18F-FDG is incorporated via the same metabolic pathway as glucose, but the intracellular metabolic pathway is different, so that tissue with a high metabolic activity is characterized by cumulative FDG uptake. Pilot studies have shown a high sensitivity for 18F-FDG PET in the diagnosis of infection and inflammation.
The first published case reports since 2003 [19–26] have shown the possible direction in VPI diagnosis. Pioneering studies by Fukuchi et al. [27] were concerned with infected aortic grafts and showed a high sensitivity of the method, but a lack of specificity, compared to CT. Later published studies were concerned with general analysis of febrile and inflammation status of unknown origin in patients with implanted biomedical materials [28, 29]. Finally, recently studies by Lauwers et al. [19] and a large study evaluating FDG PET/CT in 39 patients with a total of 69 implanted grafts [30] gave excellent results in the diagnosis of VPI, with sensitivity, specificity, NPV and PPV of 93%, 91%, 88% and 96%, respectively. Our study used a different methodology to Keidar’s team who used team image interpretation with the knowledge of the clinical status of the patient and previous diagnostic results.
In general, there are few studies on non-acute VPI. Each of the tested methods gives better results in acute and advanced VPI. Also in mixed cohorts of patients the ability of the tested methods to diagnose non-acute, often low-grade infections cannot be accurately determined. The small numbers of patients studied by most centres leads to a relatively low rate of diagnosis of VPI, and concentration of patients in tertiary centres may lead to better diagnosis and treatment.
The aim of our study was to have sufficient patient numbers, investigate the best methodology for image data evaluation, and to compare subjective and objective criteria for image data evaluation with the gold standard for VPI, and to assess the accuracy of FDG PET/CT in the diagnosis of VPI.
Materials and methods
Patient population
Between May 2004 and May 2007 76 consecutive patients with a suspected VPI were prospectively evaluated using 18F-FDG PET/CT. The patient population was recruited from the two largest tertiary Czech vascular surgery referral centres and consisted of 52 men and 24 women with a mean age 63 years (range 41–83 years). A total of 96 vascular prosthetic grafts had been implanted.
Inclusion and exclusion criteria for 18F-FDG PET/CT examination
All patients with implanted vascular prosthetic grafts and VPI suspected on the basis of clinical signs of infection (e.g. local pain, cellulitis, secreting surgical wound, sonographic finding of periprosthetic fluid, fever of unknown origin, and positive blood bacteriological culture) who did not require urgent surgical treatment were enrolled in this study. Patients with fulminant sepsis, anastomotic bleeding and prosthesoenteric fistula were excluded. Each patient provided signed informed consent form before examination.
Patients with a follow-up period shorter than 6 months were excluded from the study, except those who underwent reoperation.
Gold standard
VPI was considered present in those with operative, necropsy and/or histopathological findings meeting the criteria for VPI of Yeager and Porter [31], i.e. positive microbiological findings of aetiological agents in the prosthesis, presence of pus around the prosthesis, prosthesis seen in a disrupted wound, prosthesoenteric fistula or thrombosed prosthesis with surrounding fluid and cellular detritus including white blood cells. VPI was considered absent in those with negative operative findings, i.e. well-incorporated vascular prosthesis without periprosthetic fluid and negative microbiology, or in those who were not operated upon with a negative clinical and imaging follow-up for at least 6 months after PET/CT.
PET/CT acquisition and processing
Data acquisition
The PET/CT protocol required a 6-h fast prior to intravenous administration of 18FDG (median activity 383 MBq, range 256–565 MBq according to the patient’s weight). Water-soluble iodine contrast material diluted to 1,000 ml was given orally and 70–100 ml iodine contrast agent (400 mg I/ml) was administered intravenously just prior to CT scanning. Data were acquired on a dedicated PET/CT scanner Biograph Duo LSO (Siemens). The PET/CT examination started 45–151 min (median 85 min) after the intravenous injection of 18FDG. Patients were positioned on the table in the prone head-first position, and rarely in the foot-first position. The CT settings were as follows: 80 mAs, 130 kV, slice width 5.0 mm, collimation 4.0 mm, table feed 12.0 mm/rotation, reconstruction increment 3.4 mm. The field of view covered the entire region of the vascular prosthesis with sufficient extension at either end. The CT examination was immediately followed by corresponding PET acquisition in 3-D mode (3 min per bed position). An iterative approach including CT-based attenuation correction was used for PET data reconstruction.
Data evaluation
The PET/CT study was assessed on the basis of subjective as well as semiquantitative analysis of FDG uptake and subjective and quantitative analysis of the morphological and anatomical pattern. Subjective evaluation of diffuse and/or focal FDG uptake around the graft was assessed using a three-point scale: none, inhomogeneous, intense. Any accidental foci of FDG uptake outside the vascular prosthesis (e.g. in scars) was ignored for the purposes of this study. Homogeneous FDG uptake in the course of the vascular prosthesis was classified as “no focal uptake”, clearly identifiable focal uptake was classified as “intense focal uptake”, and inhomogeneous FDG uptake without clearly identifiable focal uptake within the course of the vascular prosthesis was classified as “inhomogeneous uptake”.
For the purposes of semiquantitative analysis of FDG uptake, a region of interest (ROI) was drawn around the infiltrate or a graft and maximum FDG uptake (in becqerels per millilitre) within the region (Gmax) expressed was recorded. Blood background served as reference. It was measured as the average FDG uptake in a ROI demarcated by the wall of the abdominal aorta above the origin of the renal arteries. The uptake ratio between graft and aorta was calculated from the formula: uptake ratio = Gmax/(blood background uptake).
The following morphological parameters were evaluated on CT slices: area of infiltrate in a plane perpendicular to the long axis excluding the area of lumen of the graft expressed in square centimetres, presence of an irregular graft boundary, and the presence of pseudoaneurysm. CT signs of irregularity of the graft boundary included fine stripes radial to graft boundary and/or a blurred outer margin of the prosthesis.
Statistical analysis
All PET and CT image data were evaluated by the same radiologist trained in FDG PET/CT with no knowledge of the patient’s clinical or other diagnostic status. The gold standard was reported by the operating surgeon. In patients not operated upon, clinical follow-up was reported by the patient’s surgeon. Sensitivity, specificity, overall accuracy, positive predictive value (PPV) and negative predictive value (NPV) were determined for each test or their combination. Continuous quantitative variables were assessed using receiver operating characteristic (ROC) curve analysis, and the Mann-Whitney and Kruskal-Wallis tests. Multiple parameters were evaluated by stepwise logistic regression.
Results
The study included 76 patients with a total of 96 implanted vascular prostheses (Table 1) investigated for a clinically suspected infection. Patients underwent PET/CT examination within a median of 6.9 months after prosthesis implantation (range 0.4–246 months). The distributions of time intervals between grafting and PET/CT are shown in Fig. 1, separately for those patients with an infected and those with a noninfected prosthesis according to the gold standard. No significant difference in timing of PET/CT after grafting was found between these two subgroups (p = 0.99). VPI was present in 55 prostheses, and absent in 41 prostheses. Of these, 13 were assessed by reoperation, and in 28 there was a stable clinical follow-up with median of 11.9 months (range 6.2–35.4 months). For all examined vascular prostheses the total prevalence of VPI or fatal follow-up was 57.3% (95% CI 46.8–67.2%, 55/96 prostheses).
Of 55 infected prostheses the microbiology was positive in 35, negative in 17 and not available in 3. The overall sensitivity of positive microbiological cultures in this group is 67.3% (95% CI 52.8–79.3%). Among the 35 microbiologically positive prostheses, there was no focal FDG uptake in 1, and at least inhomogeneous (in 6) or intense (in 28) focal FDG accumulation as shown by subjective evaluation.
FDG image evaluation showed intense focal FDG uptake as intense in 46 prostheses, inhomogeneous FDG uptake in 18, and no uptake in 32 (Table 2). When inhomogeneous FDG uptake was considered as a negative result of PET, sensitivity, specificity, overall accuracy, PPV and NPV for predicting VPI were 78.2%, 92.7%, 84.4%, 93.5 and 76.0%, respectively. However, when inhomogeneous FDG uptake was considered as a positive result of PET, sensitivity, specificity, overall accuracy, PPV and NPV were improved at 98.2%, 75.6%, 88.5%, 84.4% and 96.9%, respectively.
Inhomogeneous FDG uptake was present in 18 prostheses (18.8%), and of these 11 were infected (61%) and 7 (39%) were not infected. Thus inhomogeneous focal FDG uptake hampered the accuracy of PET and can be considered as a nondiagnostic result. When this identifiable subgroup of findings was excluded from analysis, the sensitivity, specificity, overall accuracy, PPV and NPV for predicting VPI were 97.7%, 91.2%, 94.9%, 93.5% and 96.9%, respectively.
Within the subgroup of prostheses with inhomogeneous FDG uptake, the morphological appearance of the graft boundaries was analysed (Table 3). In this subgroup the presence of an irregular graft boundary had a sensitivity, specificity, overall accuracy, PPV and NPV for predicting VPI of 72.7%, 85.7%, 77.8%, 88.9% and 66.7%, respectively, .
Pseudoaneurysm was found in 17 anastomoses and not found in 79. The sensitivity, specificity, overall accuracy, PPV and NPV of this finding on the CT images for predicting VPI were 23.6%, 90.2%, 52.1%, 76.5% and 46.8%, respectively. An irregular graft boundary on CT images (present in 52 prostheses and not present in 44) showed a sensitivity, specificity, overall accuracy, PPV and NPV for predicting VPI of 87.3%, 90.2%, 88.5%, 92.3% and 84.1%, respectively. Pseudoaneurysm together with an irregular graft boundary (in 14 prostheses) showed a specificity of 97.6% and a PPV of 92.9% for predicting VPI, although the other statistical parameters were low (sensitivity, overall accuracy, and NPV were 23.6%, 55.2% and 48.8%, respectively).
The ratio of FDG uptake between graft and reference ROIs served as input for ROC analysis (Fig. 2). A trade-off between sensitivity (74.5%) and specificity (82.9%) was achieved when a ratio of >1.7443 was considered as positive.
The three-point subjective assessment of focal and diffuse uptake of FDG, the presence of pseudoaneurysm and an irregular graft boundary, the uptake ratio and area of infiltrate, blood levels of C-reactive protein (CRP) and the white blood cell (WBC) count, age and sex served as inputs in stepwise logistic regression. Both focal FDG uptake and an irregular graft boundary apparent in the CT image were significant independent factors for the prediction of VPI. The values of combinations of these significant factors for predicting VPI for are shown in Table 4. Other parameters added no significant value.
The WBC counts and the levels of CRP were analysed separately between prosthesis with positive and negative gold standard findings. There was no significant difference (p = 0.4932) in WBC counts (Fig. 3) or in CRP levels (p = 0.9614; Fig. 4).
A secondary aim of this work was to assess the dependence of intensity of FDG uptake around noninfected prostheses on the between operation and PET imaging that might influence the results. No significant correlation (p = 0.239) was found in 41 prostheses without VPI (Fig. 5).
Discussion
The incidence of VPI approaches 1% in both our vascular surgery centres. The total prevalence of VPI in our cohort was 57.3% (55/96 prostheses) and is a reflection of disease in those patients in whom there was a clinical suspicion of non-urgent VPI who thus fulfilled our selection criteria. The prevalence of 57.3% indicates a 50% pretest probability of the disease, and therefore the need for an accurate diagnostic method for use in this subgroup of VPI patients is evident in order to prevent all possible complications resulting from chronic inflammation and specific life-threatening situations due to an infected vascular prosthesis. From the diagnostic point of view our cohort represented a completely different problem in comparison to groups of patients with acute graft infection. Our work concerning this subgroup of patients provided specific information. The overall sensitivity of a positive microbiological culture in this group was 67.3% (95% CI 52.8–79.3%) and this result corresponds with previously reported rates in patients with low-grade infection.
Subjective evaluation of intense focal FDG uptake was specific for VPI in 92.7% of prostheses (Fig. 6). This is linked with a very high PPV of 93.5% for predicting VPI. On the other hand a low rate of 1.8% of false-negative PET findings in prostheses with no focal FDG uptake excludes VPI with a very high probability of 96.9% (Fig. 7). In nearly one-fifth of prostheses, FDG uptake was inhomogeneous and should be considered as nondiagnostic (Fig. 8). This suggests that focal FDG uptake represents the almost perfect diagnostic test, after discarding the nondiagnostic results for one-fifth of the prostheses.
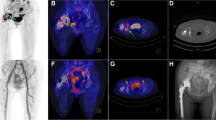
True-positive findings. There is a high focal FDG uptake and irregularity of the boundary of the distal portion of the left femorotibial bypass 6 months after grafting (axial slices in the lower row and maximum intensity projection on the right). There is a normal finding 15 mm above this focus (upper row). Surgery revealed infection by MRSA
A fluid collection around the graft and pseudoaneurysm are typical morphological signs of prosthetic infection, but they did not provide any additional diagnostic information in our study. There are some statements in the literature concerning the same findings in patients after aortic surgery. Fluid collections around aortic grafts were evaluated by Sundaram et al. They detected fluid collections in 20 of 39 patients 1 week to 30 months after surgery. Ten of these patients had no clinical symptoms of graft infection and were not treated for the imaging finding [32]. Yamamoto et al. concluded that a fluid collection around the graft might have be the result of postoperative seroma and/or inflammatory oedema developing as a result of an allergic reaction to the graft material [33]. Oechslin et al. found that a perfused echo-free space (pseudoaneurysm) between an aortic root homograft and the native aortic wall was a common finding [34]. Willems et al. reported similar findings in 15% of patients (12/79) who had undergone implantation of an aortic valve homograft [35].
CT signs of an irregular graft boundary included fine stripes radial to the graft boundary and/or a blurred outer margin of the prosthesis. The presence of this sign brings a new quality to the diagnostic process and separate evaluation of this criterion showed high diagnostic accuracy that reached approximately 90%. If an irregular graft boundary is not present, the probability of VPI is very low. Whilst an irregular graft boundary (periaortic stranding) correlates well with the presence of an infected aortic aneurysm and/or pseudoaneurysm [36], we have not yet found any information in the literature concerning the importance of this finding in peripheral vascular prostheses. Pseudoaneurysm combined with an irregular infiltration of the graft boundary compared with the gold standard predicts almost certain prosthesis infection (with a specificity 97.6%), but the sensitivity drops to unacceptable levels.
ROC analysis of the ratio between FDG uptake in the graft and reference blood background in the abdominal aorta gives a final sensitivity of 74.5% and specificity 82.9% which means that semiquantitative evaluation is no better than subjective evaluation of focal FDG uptake. Furthermore, the area of infiltration is not a better test than subjective evaluation of focal FDG uptake.
Stepwise logistic regression brought no new findings in comparison with the above-mentioned findings, when it identified only focal FDG uptake and irregularity of the boundary of the prosthesis as independent and significant predictors of VPI. The other markers including haematological and biochemical markers of systemic inflammation are nondiagnostic in those with nonurgent VPI, a group that of course differs from the group of patients with acute VPI, who require urgent surgical treatment. The most practical outcome of stepwise logistic regression is shown in Table 4. When a vascular prosthesis has a smooth border and exhibits no focal FDG uptake, the probability of VPI is less than 5%. On the other hand, when the boundary of the prosthesis is irregular and there is intense focal FDG uptake, the probability of VPI is >96%. In total, 76% of prostheses (73/96) were assessed with <5% probability of error. In prostheses with nondiagnostic inhomogeneous focal FDG uptake, the absence of irregularity of the boundary lowers the probability of VPI to 28%, while its presence increases the probability of VPI to 78%. These less-reliable, but still clinically helpful results were found in 19% of prostheses (18/96). In our cohort there were only five prostheses (5%) with other (discordant) signs, resulting in less-reliable results. A very important finding of this analysis is that in practice, the reliability of the test result can be recognized directly when reading images of a particular patient.
The subgroup of patients with inhomogeneous FDG uptake represents the big challenge, because of nondiagnostic results. A mild inhomogeneous FDG uptake might be explained either by infection of very low grade in which only a weak immune reaction might be anticipated or in immune-compromised patients. In both situations, reduced number of WBC might be present in the tissue around a vascular prosthesis and thus only a small amount of FDG is accumulated by them. On the other hand even in the case of no infection, sterile inflammation can be present around a foreign body such as prosthetic material. In some cases it might be of higher intensity, thus forming a pattern of mild inhomogeneous FDG uptake.
There was clearly apparent added value of hybrid PET/CT to PET-only imaging. CT to some extent improves diagnostic accuracy in VPI. The morphological appearance of the graft boundary represents an independent predictor of VPI and is of special value in cases of ambiguous inhomogeneous FDG uptake. Moreover, CT was able to localize more precisely FDG uptake and thus in several patients to change the surgical procedure. Localization of pus collection near the prosthesis by CT led to its simple drainage instead of risky exploration of the graft as would be recommended by PET-only imaging.
Conclusion
So-called low-grade infection of a vascular graft represents a diagnostic challenge, where most additively used diagnostic methods fail. FDG PET/CT is a promising diagnostic method in this clinical situation, combining the advantages of anatomical and functional/metabolic data acquisition in a single session. We identified focal FDG uptake and an irregular graft boundary as independent significant predictors of VPI among other parameters. No other parameters such as the presence of pseudoaneurysm, perigraft fluid collection, diffuse FDG uptake, semiquantitative assessment of focal FDG uptake or WBC count and CRP level added significant clinical information. FDG PET/CT gives reliable results with errors less than 5% in more than 75% of prostheses. Also we can make the right decision in the remaining 25% of patients with a probability of about 70–75%. These are the important findings of this study, in a diagnosis fraught with difficulty.
References
Hallett JW, Marshall DM, Petterson TM, et al. Graft related complications after abdominal aortic aneurysm repair. Population based experience. J Vasc Surg 1977;25:277.
Bandyk DF. Infection of prosthetic vascular grafts. In: Rutherford RB, editor. Vascular surgery. 5th ed. St. Louis, CV Mosby; 1995. p. 566
Orton D, LeVeen R, Saigh J, et al. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics 2000;20:977–93.
Low R, Wall S, Jeffrey R, et al. Aortoenteric fistula and perigraft infection evaluation with CT. Radiology 1990;175:157–62.
Olofsson P, Auffermann W, Higgins C, et al. Diagnosis of prosthetic graft infection by magnetic resonance imaging. J Vasc Surg 1988;8:99–105.
Spartera C, Morettini G, Petrassi C. Healing of aortic prosthetic grafts: a study by magnetic resonance imaging. Ann Vasc Surg 1994;8:536–42.
Spartera C, Morettini G, Petrassi C. Role of magnetic resonance imaging in the evaluation of aortic graft healing, perigraft fluid collection, and graft infection. Eur J Vasc Surg 1990;4:69–73.
Lawrence P, Dries D, Alazraki N, et al. Indium 111-labeled leucocyte scanning for detection of prosthetic vascular graft infection. J Vasc Surg 1985;2:165–73.
Sedwitz M, Davies R, Pretorius H, Vasquez TE. Indium 111-labeled white blood cell scans after vascular prosthetic reconstruction. J Vasc Surg 1987;6:476-81.
Fiorani P, Speziale F, Rizzo L, et al. Detection of aortic graft infection with leucocytes labeled with technetium 99m-hexametazime. J Vasc Surg 1993;17:87–95.
Liberatore M, Iurilli A, Ponzo F, et al. Clinical usefulness of technetium-99m-HMPAO labeled leucocyte scans in prosthetic vascular graft infection. J Nucl Med 1998;39:875–9.
Prats E, Banzo J, Abos M, et al. Diagnosis of prosthetic vascular graft infection by technetium-99m-HMPAO labeled leucocytes. J Nucl Med 1994;35:1303–7.
Krznaric E, Nevelsteen A, van Hoe L, et al. Diagnostic value of 99Tc-HMPAO labelled leucocyte scintigraphy in the detection of vascular graft infections. Nucl Med Commun 1994;15:953–960.
Causey DA, Fajman WA, Perdue GD, et al. 67Ga scintigraphy in postoperative synthetic graft infection. AJR Am J Roentgenol 1980;134:1041–5.
Johnson K, Russ PD, Bair JH, Friefeld GD. Diagnosis of synthetic vascular graft infection: comparison of CT and gallium scans. Am J Roentgenol 1990;154:405–9.
Samuel A, Paganelli G, Chiesa R, et al. Detection of prosthetic vascular graft infection using avidin/indium-111-biotin scintigraphy. J Nucl Med 1996;37:55–61.
Chiesa R, Melissano G, Castellano R, et al. Avidin and 111In-labeled biotin scan: a new radioisotopic method for localising vascular graft infection. Eur J Vasc Endovasc Surg 1995;10:405–14.
Mark A, Moss A, Lusby R, et al. CT evaluation of complications of abdominal aortic surgery. Radiology 1982;145:409–44.
Lauwers P, Van den Broeck S, Carp L, et al. The use of positron emission tomography with (18)F-fluorodeoxyglucose for the diagnosis of vascular graft infection. Angiology 2007;58:717–24.
Balink H, Reijnen MM. Diagnosis of abdominal aortic prosthesis infection with FDG-PET/CT. Vasc Endovascular Surg 2007;41:428–32.
Burroni L, D’Alessandria C, Signore A. Diagnosis of vascular prosthesis infection: PET or SPECT. J Nucl Med 2007;48:1227–9.
Tegler G, Sörensen J, Björck M, et al. Detection of aortic graft infection by 18-fluorodeoxyglucose positron emission tomography combined with computed tomography. J Vasc Surg 2007;45:828–30.
Stadler P, Belohlavek O, Spacek M, Michalek P. Diagnosis of vascular prosthesis infection with FDG-PET/CT. J Vasc Surg 2004;40:1246–7.
Rohde H, Horstkotte MA, Loeper S, et al. Recurrent Listeria monocytogenes aortic graft infection: confirmation of relapse by molecular subtyping. Diagn Microbiol Infect Dis 2004;48:63–7.
Krupnick AS, Lombardi JV, Engels FH, et al. 18-fluorodeoxyglucose positron emission tomography as a novel imaging tool for the diagnosis of aortoenteric fistula and aortic graft infection-a case report. Vasc Endovascular Surg 2003;37:363–6.
Keidar Z, Engel A, Nitecki S, et al. PET/CT using 2-deoxy-2-[18F]fluoro-D-glucose for the evaluation of suspected infected vascular graft. Mol Imaging Biol 2003;5:23–5.
Fukuchi K, Ishida Y, Higashi M, et al. Detection of aortic graft infection by fluorodeoxyglucose positron emission tomography: comparison with computed tomographic findings. J Vasc Surg 2005;42:919–25.
Bleeker-Rovers CP, Vos FJ, Corstens FH, Oyen WJ. Imaging of infectious diseases using [18F] fluorodeoxyglucose PET. Q J Nucl Med Mol Imaging 2008;52:17–29.
Jaruskova M, Belohlavek O. Role of FDG-PET and PET/CT in the diagnosis of prolonged febrile states. Eur J Nucl Med Mol Imaging 2006;33:913–8.
Keidar Z, Engel A, Hoffman A, et al. Prosthetic vascular graft infection: the role of 18F-FDG PET/CT. J Nucl Med 2007;48:1230–6.
Yeager RA, Porter JM. Arterial and prosthetic graft infection. Ann Vasc Surg 1992;6:485–91.
Sundaram B, Quint LE, Patel S, et al. CT appearance of thoracic aortic graft complications. AJR Am J Roentgenol 2007;188:1273–7.
Yamamoto K, Noishiki Y, Mo M, et al. Unusual inflammatory responses around a collagen-impregnated vascular prosthesis. Artif Organs 1993;17:1010–6.
Oechslin E, Carrel T, Ritter M, et al. Pseudoaneurysm following aortic homograft: clinical implications. Br Heart J 1995;74:645–9.
Willems TP, Van Herwerden LA, Taams MA, et al. Aortic allograft implantation techniques: pathomorphology and regurgitant jet patterns by Doppler echocardiographic studies. Ann Thorac Surg 1998;66:412–6.
Macedo TA, Stanson AW, Oderich SG, et al. Infected aortic aneurysms: imaging findings. Radiology 2004;231:250–7.
Acknowledgment
The authors would like to acknowledge Dr. John W. Frank, M.Sc, FRCP, FRCR, FBIR (immediate past president of the British Nuclear Medicine Society) for his contribution in reviewing the article and language correction.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spacek, M., Belohlavek, O., Votrubova, J. et al. Diagnostics of “non-acute” vascular prosthesis infection using 18F-FDG PET/CT: our experience with 96 prostheses. Eur J Nucl Med Mol Imaging 36, 850–858 (2009). https://doi.org/10.1007/s00259-008-1002-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-1002-z