Abstract
Purpose
To evaluate FDG-PET for staging, grading, preoperative response assessment and posttherapeutic evaluation in children with Wilms tumour (WT).
Methods
In this study, 23 FDG-PET examinations in 12 paediatric patients (female, n = 5; male, n = 7; age, 1–19 years) with WT (primary, n = 9; relapsed, n = 3) were analysed. All patients were examined with conventional imaging methods (CIM) according to the SIOP2001/GPOH trial protocol. Additionally, FDG-PET/PET-CT was performed for staging (n = 12), preoperative response assessment (n = 6) and posttherapeutic evaluation (n = 5). Imaging results of FDG-PET and CIM were analysed regarding the accuracy in tumour visualisation, impact on therapeutic management and preoperative response assessment, with clinical follow-up and histopathology as the standard of reference.
Results
FDG-PET and CIM showed concordant results for staging of primary WT, whereas FDG-PET was superior in 1/3 cases with recurrent WT. Concerning histological differentiation, one case with anaplastic WT had an standard uptake value (SUV) of 12.3, which was remarkably higher than the average SUV in the eight cases with intermediate risk histology. No parameter analysed for PET or CIM was reliably predictive for histological regression or clinical outcome. After completion of therapy, FDG-PET was superior to CIM in 2/5 cases in detecting residual disease with therapeutic relevance.
Conclusion
FDG-PET does not provide additional information to the traditional imaging work-up for staging WT patients, preoperative response assessment and clinical outcome. FDG-PET was advantageous in ruling out residual disease after completion of first line treatment and in pretherapeutic staging of relapse patients. Furthermore, there seems to be a good correlation of initial SUV and histological differentiation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For more than 30 years, Wilms tumour (WT) patients were treated in international therapy optimisation trials initiated by the International Society of Paediatric Oncology/Hematology (SIOP). Within these trials, the 5-year overall survival rates were gradually increased from approx. 64% (SIOP-1) up to 89.5% (SIOP-9) [1, 2]. Currently (SIOP-9), the 5-year overall survival rate of WT patients is 91.7% in localised and 76.3% in metastasised tumours [2]. Because of the inferior prognosis of patients with metastatic spread, intensified therapy regimes, including surgery or radiotherapy of all accessible metastases and, in selected cases, high-dose polychemotherapy with autologous stem cell rescue, has been recommended [2–4]. Therefore, accurate initial staging is needed to assess tumour spread and to assign patients appropriately to the different risk branches.
Although nine out of ten paediatric WT patients can be cured today, there are still issues concerning the management of WT patients, such as anaplastic histology or treatment of recurrent disease [2, 5, 6]. Another important point is the value of neoadjuvant chemotherapy as applied by the SIOP studies vs. primary resection favored by the NWTS (National Wilms tumour study) study group. Although no difference concerning survival rates is known, there are advantages and disadvantages of either method [7, 8]. Advantages of neoadjuvant chemotherapy, as applied in the present study, are the lower rate of tumour ruptures during tumour nephrectomy as compared to primary resection observed within the NWTS studies and the possibility to assess response to chemotherapy as a prognostic factor [5, 7, 9]. However, one important disadvantage described in the literature is the missing histological proof of WT and its subgroups at the time of diagnosis. This may result in over-treatment in case of benign lesions (approximately 1.8%) and, on the other hand, preoperative treatment not adapted to the histological subtypes of WT or even other renal malignancies (e.g. clear cell sarcoma, malignant rhabdoid tumour) [5, 7, 10]. To overcome the disadvantages of preoperative chemotherapy, a noninvasive method for grading of renal tumours suspicious for WT is desirable to enable risk-adapted treatment strategies.
Another group of patients, which is believed to require more intensive therapy because of the higher risk of relapse, is represented by poor response with persisting viable tumour or even progressive disease after neoadjuvant treatment [5, 11]. Thus, a reliable preoperative response assessment by noninvasive imaging even prior to definite surgery is needed to enable modifications of therapy and to gain prognostic information.
Even though second-line therapy in WT patients is effective and improved the survival of recurrent WT over the last decade, there is still a relevant proportion of patients who cannot be cured [6, 12, 13]. Beside the histological category and initially applied therapy, the site of recurrence and the completeness of surgical resection of the recurrent tumour deposits are significantly predictive for survival [6]. Therefore, after first line therapy, it has to be ensured that a complete remission has been achieved. Any residual tumour deposits require continuing individualised treatment, including chemotherapy, as well as local treatment using surgery or radiotherapy [6]. This, as well as restaging in patients suspicious for late locoregional or metastatic relapse, is a diagnostic challenge as there is no reliable tumour marker for WT. Furthermore, scar tissue may be difficult to be differentiated from viable tumour, and patient compliance to the armamentarium of diagnostic tests might be limited [14].
Imaging modalities currently used for staging, characterisation, preoperative response assessment and follow-up comprise ultrasound (US), chest X-ray, magnetic resonance imaging (MRI), computed tomography (CT) and bone scan. The use of FDG-PET was shown to have potential advantages concerning staging, preoperative response assessment and follow-up in many tumour entities of the paediatric patient population [15–26]. Concerning tumour grading, FDG-PET has been shown to be valuable in several tumour entities, i.e. sarcoma patients [27–29]. Such data are not available for WT so far.
If these experiences were transferable to WT, FDG-PET may represent a promising adjunct to current imaging standards in WT patients. If there is a positive effect of using an alternative imaging method, it should not be withheld to the patients. There is only one dedicated report published so far, which describes successful FDG-PET imaging in three WT patients [30]. In order to further define the value of FDG-PET in staging, grading, preoperative response assessment and posttherapeutic evaluation, the present study was initiated.
Materials and methods
Patients
In this study, 12 patients treated for WT (female, n = 5; male, n = 7; mean age, 5.91 years; range of age, 1–19 years; primary WT, n = 9; recurrent WT, n = 3) from two institutions were enrolled consecutively during a pilot phase (n = 6) and within the bounds of a prospective multicentric study on a variety of paediatric malignant diseases (n = 6; Table 1).
All patients were examined according to the standard algorithms for diagnostic imaging of the therapy optimisation trial SIOP2001/GPOH. Adjusted to this conventional imaging algorithm, FDG-PET was performed 1–7 days prior to therapy for initial staging in all patients, after preoperative chemotherapy (1–14 days prior to local therapy) for assessment of therapy response in six patients and 2–4 weeks upon completion of therapy to exclude residual or early recurrent disease in five patients (Tables 1 and 2).
An oral and written informed consent was obtained from the parents of all enrolled children. The study was carried out in accordance with the Declaration of Helsinki and the principles of good clinical practice. The study protocol was approved by the local ethics committee. Furthermore, approval was granted by the German Federal Office on Radiation Protection (Bundesamt für Strahlenschutz), as well as by the corresponding local authorities.
Conventional imaging modalities—acquisition and analysis
For initial staging of WT patients, conventional imaging modalities (CIM) consisted of contrast-enhanced MRI of the primary tumour site, chest X-ray, ultrasound of the abdomen and other suspicious regions and bone scan when clinically indicated.
For assessment of therapy response, the MRI scan of the primary tumour site was repeated prior to tumour nephrectomy. Depending on the initial tumour spread, reevaluation was completed by ultrasound, chest X-ray, CT and bone scan.
After completion of therapy, all imaging procedures were repeated. During follow-up, diagnostic tests were performed according to the schedules of the SIOP/GPOH2001 trial.
All CIM images were reviewed by two experienced radiologists in consensus and blinded to the results of FDG-PET and clinical follow-up information. All findings judged as deposits of WT were documented. For evaluation of response to neoadjuvant chemotherapy by CIM, the reduction of tumour size was evaluated by MRI as an indicator of tumour regression, and a 40% reduction was used as the cut-off for response [2].
FDG-PET—acquisition and analysis
A total of 23 FDG-PET examinations were performed at two dedicated stand-alone full ring scanners (n = 18 examinations; Ecat Exact 47 and Ecat Exact HR Plus, Siemens, Erlangen, Germany) and one PET-CT device (n = 5 examinations; Biograph 16, Siemens, Erlangen, Germany). Details of the examination protocol can be read elsewhere [17].
FDG-PET images were transferred to a workstation with dedicated PET software (e.soft 4.0 Leonardo, Siemens, Erlangen, Germany) and reviewed by two experienced nuclear medicine specialists in consensus and blinded to the results of CIM and clinical follow-up information.
For initial staging, all regions with pathologically increased FDG uptake identified as tumour deposits by visual analysis were recorded. For assessment of response to therapy, the maximal standard uptake value (SUV; corrected to body weight and injected FDG dose; determined with a 3D volume of interest covering the entire hypermetabolic tumour), the SUV reduction in the primary tumour after neoadjuvant therapy as compared with baseline, SUV after therapy and visual interpretation were tested.
Standard of reference
All imaging results were reviewed by an interdisciplinary tumour board consisting of paediatric oncologists, paediatric surgeons, radiologists, nuclear medicine physicians, as well as pathologists. To determine the benign or malignant status of every lesion revealed by diagnostic imaging, clinical data, including all staging examinations, histopathology of resected primary tumours and locoregional lymph nodes, as well as clinical and imaging follow-up, were used. Lesions, which were positive in both PET and CIM, were judged as tumour deposits if clinical data were concordant. In case of discordant findings, images of the different modalities were directly correlated, and lesion status was defined in consensus, taking histopathology and clinical data into account.
Based on these results, the risk branch assignment according to the SIOP/GPOH2001 protocol for the preoperative chemotherapy was performed for every patient. The histological regression of resected tumour sites as a parameter of response to therapy was determined using the proportion of residual viable tumour cells (responder, <10%; non-responder, >10%). Furthermore, the clinical outcome of patients concerning progression-free survival and overall survival was compared with the results of preoperative response assessment by PET and CIM, as well as the final control examinations.
Results
Initial staging of primary WT
FDG-PET, as well as the standard diagnostic algorithm, correctly visualised primary tumours in all nine patients (Table 1; #1–9). The mean SUV of the primary tumours was 6.8 [±2.4 standard deviation (SD); range, 4.2–12.3].
Among the nine patients with primary WT, there was one case (#6) with diffuse anaplastic WT with an SUV of 12.3. The patient developed an early relapse 2 months after completion of first line treatment. In contrast, the remaining eight patients showed a lower SUV ranging from 4.2 to 7.5 [mean, 6.1 (±1.4 SD)] and had intermediate risk histology. During a follow-up period of up to 92 months, relapse occurred in two out of these eight patients 3 (#2, SUV 6.4) and 34 months (#8, SUV 7.1) after completion of therapy, respectively.
Of the nine patients with primary WT, three (#2, 4, 8) had retroperitoneal lymph node involvement. Both FDG-PET and CIM identified all lymph node metastases concordantly.
None of the nine patients had distant metastases at the time of initial staging. By ruling out systemic tumour spread, all patients were concordantly assigned to the risk branches by both FDG-PET and CIM.
Staging recurrent disease
There were three patients (Table 1; #10–12) who were staged for relapsed WT (first relapse, n = 2; second relapse, n = 1) after a complete remission had been achieved by the initial course of therapy. The recurrent disease was detected by CIM at a routine follow-up visit (#10, 11) or triggered by clinical symptoms (#12) 1, 3 and 64 months after completion of first line treatment, respectively.
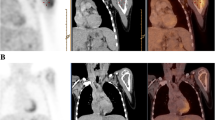
In one case (#10), CIM correctly identified recurrent tumour within the former kidney region concordantly to FDG-PET. In the second case (#12), FDG-PET, as well as MRI, precisely visualised the recurrent tumour infiltrating the brachial plexus (Fig. 1). In the remaining patient (#11), PET and CIM revealed disseminated disease with multiple lung metastases, as well as mediastinal and retroperitoneal lymph node involvement; in this patient, FDG-PET depicted additional tumour deposits in the retroperitoneum, pelvic lymph nodes, as well as peritoneal spread leading to extension of surgery, as well as the radiation field (Fig. 2).
Recurrent WT (patient #12) encasing the brachial plexus and the subclavian vessels well demonstrated by both MRI (a, coronal image) and FDG-PET-CT (b, c, d). Whole-body FDG-PET rules out further tumour deposits (e). Despite good metabolic response after chemotherapy (f), the partially resected tumour showed poor histological regression
Recurrent WT (patient #11) with recurrent tumour in the lung (a), mediastinum (a) and retroperitoneum (b) well demonstrated by both CT (upper row) and FDG-PET (middle row and d). FDG-PET additionally depicted a peritoneal deposit (c). Despite good metabolic response (e), disease progression occurred 6 months later
Local regression and preoperative response assessment
FDG-PET was performed just after preoperative therapy in six patients (Table 2); four with primary WT (#1–4) and two treated for recurrent disease (#11, 12). In five patients (#1–4 and #12), the tumour was resected 1–4 weeks after PET. Regarding local regression, patients with primary WT showed 10% or less residual viable tumour cells within the resected specimen. The patient with recurrent WT (#12) had a poor response with 20% viable tumour cells (Fig. 1). So far, one (#2) out of these five patients experienced a relapse after definite tumour resection (range of follow-up period, 3–30 months).
SUV reduction of tumours undergoing subsequent definite resection within 1–4 weeks after FDG-PET ranged from 36% to 78%. Volume reduction measured on MRI ranged from 42% to 95%. The patient (#12) with the poorest histological response had moderate SUV reduction (53%) and shrinkage (61%). The only patient (#2; intermediate risk histology) in this group who suffered a relapse during follow-up had the highest SUV and volume reduction.
In one patient (#11; multifocally relapsed WT) systemic therapy was continued and followed by delayed surgical tumour reduction 6 months after response assessment with CT, MRI and FDG-PET. Despite the good response with 97% volume reduction (CT, MRI) and metabolic inactivation of all tumour deposits (SUV reduction, 93%), evaluation of the resected specimen revealed large proportions of viable tumour (40%). The patient subsequently developed progressive disease and died (Fig. 2).
Summarising the results of preoperative response assessment, neither morphologic assessment of response nor PET parameters (including SUV reduction, SUV at baseline, SUV after therapy and visual interpretation) revealed a reliable indicator for therapy success regarding local control or progression-free survival.
Posttherapeutic evaluation
Among the five patients (Table 3) who underwent FDG-PET upon completion of standard treatment including local and systemic therapy, there were two (#4 and #10) in whom both FDG-PET and CIM correctly showed complete remission. In one patient (#11) with a newly occurred lung metastasis seen on chest X-ray and subsequent CT, FDG-PET depicted multiple additional tumour deposits in the retroperitoneum and the neck as confirmed by histopathology; these were partly missed by the standard diagnostic procedure. In the remaining two patients, there were suspicious lesions with increased locoregional FDG uptake after tumour nephrectomy. In the first patient (#1) with a haematoma and surrounding regenerative tissue activity, the PET finding was false positive as confirmed by CIM and follow-up (29 months). In the second patient (#2), local MRI and US were false negative at the time of the positive PET scan; an abdominal CT scan performed 2 months later confirmed the recurrent tumour growth, which was then histologically proven (Fig. 3); the patient, therefore, underwent continuing therapy and achieved complete remission (follow-up, 16 months). This resulted in a patient-based accuracy of 3/5 for CIM and 4/5 for FDG-PET.
Early recurrent WT (patient #2) after completion of first line treatment including nephrectomy: FDG-PET (a, b; black arrows) clearly reveals a suspicious lesion in the former kidney region. At the same time, MRI (c) was negative. Two months after PET locally recurrent tumour growth was confirmed by CT (d; white arrows) and histopathology
Discussion
In this study, the value of FDG-PET was evaluated in 12 children suffering from histologically proven WT for staging (n = 12), preoperative response assessment (n = 6) and posttherapeutic evaluation (n = 5).
From a technical point of view, there was no problem in differentiating primary WT from urinary activity in the study population. This is explained by the large size of the tumours, which usually allows for a valid SUV measurement without alteration by high FDG activity inside the renal pelvis. In a larger group, of course, there will be additive value of using PET-CT with direct anatometabolic correlation in case of smaller tumours adjacent to the renal pelvis.
Analysing the SUV at initial examination of primary WT regarding the histological differentiation prior to therapy, the patient with diffuse anaplastic WT had a remarkably higher SUV as compared to the other eight patients with intermediate risk histology. If this were reproducible in larger series of patients, FDG-PET could be used to discriminate high risk from intermediate risk histology. Together with its well-known ability to differentiate benign from malignant lesions, FDG-PET might be a valuable tool for assessment of WT therapy candidates before preoperative chemotherapy, avoiding invasive diagnostics.
Concerning the evaluation of tumour spread, whole-body FDG-PET is known to be very useful in various paediatric tumour entities because of its high sensitivity [15–17, 19–22]. In the present study, FDG-PET accurately detected all locoregional tumour deposits and ruled out distant metastases in all nine patients with primary disease. However, CIM was equally effective in depicting locoregional spread in the present study. Even though not observed in the present study, the most common site of distant metastases is the lung, where the sensitivity of FDG-PET is limited [20, 31]. Considering this, we do not see a major role for PET in staging primary disease.
Concerning staging of recurrent WT, distant metastases are more likely to be present, and the risk of therapy failure is higher as compared with primary disease [6]. Several prognostic factors have been identified, such as stage at first diagnosis, histological subtype, first line treatment and time from first diagnosis to relapse. One important factor associated with survival in cases of recurrent WT is the completeness of surgical resection of all tumour deposits [6]. This emphasises that accurate staging and detection of all tumour deposits throughout the body is substantial in relapsed WT. In the present analysis, FDG-PET depicted occult tumour deposits in one out of three patients with recurrent WT; this indicates a potential therapeutic relevance of FDG-PET applied for staging of recurrent WT.
Concerning the effect of preoperative chemotherapy, there is evidence that response is associated with clinical outcome [5, 9, 11]. In various other tumour entities of the paediatric patient population, there are efforts to predict response to neoadjuvant therapy by diagnostic imaging to gain prognostic information or even to change preoperative or operative therapy. FDG-PET showed promising results using the decrease of SUV between baseline and during or after therapy and other visual and quantitative PET parameters [18, 23–25]. In view of the limited number of patients who underwent the early follow-up PET scan (n = 6) in the present study, it was not possible to find a precise discriminator for response and non-response regarding PET parameters. However, morphologic assessment of local regression was inaccurate as well, even though effective thresholds for volume reduction of primary tumours had been established by other groups [2]. Therefore, there might be bias due to the small number of subjects.
After completion of the first line therapeutic course, posttherapeutic evaluation has been recommended because any residual or early recurrent disease requires further treatment. We observed additional value of FDG-PET in two out of five patients with impact on the treatment strategy. On the other hand, there was one false positive PET result, which was easily clarified by MRI and US. Despite the small number of patients, the relatively high proportion of patients with relevant discordances and additive value of PET indicates a potential role for FDG-PET in the posttherapeutic setting.
As a conclusion, FDG-PET appears to be suitable for imaging of WT. Even though there are discouraging results for preoperative response assessment and limited value of initial staging, there are certain scenarios, i.e. posttherapeutic imaging, noninvasive tumour characterisation and restaging in case of recurrent disease, which deserve further investigation in larger trials.
References
Lemerle J, Voûte PA, Tournade MF, Delemarre JF, Jereb B, Ahstrohm L, et al. Preoperative versus postoperative radiotherapy, single versus multiple courses of Actinomycin D, in the treatment of Wilms tumor. Preliminary results of a controlled clinical trial conducted by the International Society of Pediatric Oncology (SIOP). Cancer 1976;38:647–54.
Weirich A, Ludwig R, Graf N, Abel U, Leuschner I, Vujanic GM, et al. Survival in nephroblastoma treated according to the trial and study SIOP-9/GPOH with respect to relapse and morbidity. Ann Oncol 2004;15:808–20.
De Kraker J, Lemerle J, Voute PA, Zucker JM, Tournade MF, Carli M, et al. Wilm’s tumor with pulmonary metastases at diagnosis: the significance of primary chemotherapy. International Society of Pediatric Oncology Nephroblastoma Trial and Study Committee. J Clin Oncol 1990;8:1187–90.
Reinhard H, Semler O, Burger D, Bode U, Flentje M, Göbel U, et al. Results of the SIOP 93-01/GPOH trial and study for the treatment of patients with unilateral nonmetastatic Wilms Tumor. Klin Padiatr 2004;216:132–40.
Weirich A, Leuschner I, Harms D, Vujanic GM, Tröger J, Abel U, et al. Clinical impact of histologic subtypes in localized non-anaplastic nephroblastoma treated according to the trial and study SIOP 9/GPOH. Ann Oncol 2001;12:311–9.
Dome JS, Liu T, Krasin M, Lott L, Shearer P, Daw NC, et al. Improved survival for patients with recurrent Wilms tumor: the experience at St. Jude Children’s Research Hospital. J Pediatr Hematol Oncol 2002;24:192–8.
Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist 2007;10:815–26.
Mitchell C, Pritchard-Jones K, Shannon R, Hutton C, Stevens S, Machin D, et al. For the United Kingdom Cancer Study Group. Immediate nephrectomy versus preoperative chemotherapy in the management of non-metastatic Wilms’ tumour: results of a randomised trial (UKW3) by the UK Children’s Cancer Study Group. Eur J Cancer 2006;42:2554–62.
Boccon-Gibod L, Rey A, Sandstedt B, Delemarre J, Harms D, Vujanic G, et al. Complete necrosis induced by preoperative chemotherapy in Wilms tumor as an indicator of low risk: report of the international society of paediatric oncology (SIOP) nephroblastoma trial and study 9. Med Pediatr Oncol 2000;34:183–90.
De Kraker J, Graf N, van Tinteren H, Pein F, Sandstedt B, Godzinski M, et al. Reduction of postoperative chemotherapy in children with stage I intermediate-risk and anaplastic Wilms’ tumour (SIOP 93-01 trial): a randomised controlled trial. Lancet 2004;364:1229–35.
Ora I, van Tinteren H, Bergeron C, de Kraker J. SIOP Nephroblastoma Study Committee. Progression of localised Wilms’ tumour during preoperative chemotherapy is an independent prognostic factor: a report from the SIOP 93-01 nephroblastoma trial and study. Eur J Cancer 2007;43:131–6.
Grundy P, Breslow N, Green DM, Sharples K, Evans A, D’Angio GJ. Prognostic factors for children with recurrent Wilms’ tumor: results from the Second and Third National Wilms’ Tumor Study. J Clin Oncol 1989;7:638–47.
Miser JS, Tournade MF. The management of relapsed Wilms tumor. Hematol Oncol Clin North Am 1995;9:1287–302.
Carrico CW, Cohen MD, Zerin JM, Weetman R. Wilms tumor imaging: patient costs and protocol compliance. Radiology 1997;204:627–33.
Amthauer H, Furth C, Denecke T, Hundsdoerfer P, Voelker T, Seeger K, et al. FDG-PET in 10 children with non-Hodgkin’s lymphoma: initial experience in staging and follow-up. Klin Padiatr 2005;217:327–33.
Furth C, Amthauer H, Denecke T, Ruf J, Henze G, Gutberlet M. Impact of whole-body MRI and FDG-PET on staging and assessment of therapy response in a patient with Ewing sarcoma. Pediatr Blood Cancer 2006;47:607–11.
Furth C, Denecke T, Steffen I, Ruf J, Voelker T, Misch D, et al. Correlative imaging strategies implementing CT, MRI, and PET for staging of childhood Hodgkin disease. J Pediatr Hematol Oncol 2006;28:501–12.
Franzius C, Sciuk J, Brinkschmidt C, Jürgens H, Schober O. Evaluation of chemotherapy response in primary bone tumors with F-18 FDG positron emission tomography compared with histologically assessed tumor necrosis. Clin Nucl Med 2000;25:874–81.
Franzius C, Sciuk J, Daldrup-Link HE, Jürgens H, Schober O. FDG-PET for detection of osseous metastases from malignant primary bone tumours: comparison with bone scintigraphy. Eur J Nucl Med 2000;27:1305–11.
Györke T, Zajic T, Lange A, Schäfer O, Moser E, Mako E, et al. Impact of FDG PET for staging of Ewing sarcomas and primitive neuroectodermal tumours. Nucl Med Commun 2006;27:17–24.
Korholz D, Kluge R, Wickmann L, Hirsch W, Lüders H, Lotz I, et al. Importance of F18-fluorodeoxy-d-2-glucose positron emission tomography (FDG-PET) for staging and therapy control of Hodgkin’s lymphoma in childhood and adolescence—consequences for the GPOH-HD 2003 protocol. Onkologie 2003;26:489–93.
Völker T, Denecke T, Steffen I, Misch D, Schönberger S, Plotkin M, et al. Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol 2007;25:5435–41.
Hawkins DS, Rajendran JG, Conrad EU, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-d-glucose positron emission tomography. Cancer 2002;94:3277–84.
Hawkins DS, Schuetze SM, Butrynski JE, Rajendran JG, Vernon CB, Conrad EU, et al. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol 2005;23:8828–34.
Hernandez-Pampaloni M, Takalkar A, Yu JQ, Zhuang H, Alavi A. F-18 FDG-PET imaging and correlation with CT in staging and follow-up of pediatric lymphomas. Pediatr Radiol 2006;36:524–31.
Johnson GR, Zhuang H, Khan J, Chiang SB, Alavi A. Roles of positron emission tomography with fluorine-18-deoxyglucose in the detection of local recurrent and distant metastatic sarcoma. Clin Nucl Med 2003;28:815–20.
Folpe AL, Lyles RH, Sprouse JT, Conrad EU, Eary JF. (F-18) fluorodeoxyglucose positron emission tomography as a predictor of pathologic grade and other prognostic variables in bone and soft tissue sarcoma. Clin Cancer Res 2000;6:1279–87.
Lucas JD, O’Doherty MJ, Cronin BF, Marsden PK, Lodge MA, McKee PH, et al. Prospective evaluation of soft tissue masses and sarcomas using fluorodeoxyglucose positron emission tomography. Br J Surg 1999;86:550–6.
Schulte M, Brecht-Krauss D, Heymer B, Guhlmann A, Hartwig E, Sarkar MR, et al. Grading of tumors and tumorlike lesions of bone: evaluation by FDG PET. J Nucl Med 2000;41:1695–701.
Shulkin BL, Chang E, Strouse PJ, Bloom DA, Hutchinson RJ. PET FDG studies of Wilms tumors. J Pediatr Hematol Oncol 1997;19:334–8.
Franzius C, Daldrup-Link HE, Sciuk J, Rummeny EJ, Bielack S, Jürgens H, et al. FDG-PET for detection of pulmonary metastases from malignant primary bone tumors: comparison with spiral CT. Ann Oncol 2001;12:479–86.
Acknowledgement
This study was financially supported by the Deutsche Krebshilfe e.V.
Conflict of interest statement
All authors disclose no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Misch, D., Steffen, I.G., Schönberger, S. et al. Use of positron emission tomography for staging, preoperative response assessment and posttherapeutic evaluation in children with Wilms tumour. Eur J Nucl Med Mol Imaging 35, 1642–1650 (2008). https://doi.org/10.1007/s00259-008-0819-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0819-9