Abstract
Background
N-methyl[11C]2-(4′methylaminophenyl)-6-hydroxy-benzothiazole (PIB) is a positron emission tomography (PET) tracer with amyloid binding properties which allows in vivo measurement of cerebral amyloid load in Alzheimer’s disease (AD). Frontotemporal dementia (FTD) is a syndrome that can be clinically difficult to distinguish from AD, but in FTD amyloid deposition is not a characteristic pathological finding.
Purpose
The aim of this study is to investigate PIB retention in FTD.
Methods
Ten patients with the diagnosis of FTD participated. The diagnosis was based on clinical and neuropsychological examination, computed tomography or magnetic resonance imaging scan, and PET with 18Fluoro-2-deoxy-d-glucose (FDG). The PIB retention, measured in regions of interest, was normalised to a reference region (cerebellum). The results were compared with PIB retention data previously obtained from 17 AD patients with positive PIB retention and eight healthy controls (HC) with negative PIB retention. Statistical analysis was performed with a students t-test with significance level set to 0.00625 after Bonferroni correction.
Results
Eight FTD patients showed significantly lower PIB retention compared to AD in frontal (p < 0.0001), parietal (p < 0.0001), temporal (p = 0.0001), and occipital (p = 0.0003) cortices as well as in putamina (p < 0.0001). The PIB uptake in these FTD patients did not differ significantly from the HC in any region. However, two of the 10 FTD patients showed PIB retention similar to AD patients.
Conclusion
The majority of FTD patients displayed no PIB retention. Thus, PIB could potentially aid in differentiating between FTD and AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
N-methyl[11C]2-(40-methylaminophenyl)-6-hydroxy-benzothiazole (PIB) is a novel amyloid binding positron emission tomography (PET) tracer used to detect amyloid depositions in the human brain. The first PET study comparing PIB retention between Alzheimer’s disease (AD) patients and healthy controls (HC) indicates robust group differences [1]. A two-fold higher PIB retention was seen in frontal, parietal, and temporal cortices, as well as striatum, areas known to contain large amounts of amyloid in AD. Conversely, in areas known to be less affected by amyloid deposition, such as subcortical white matter, pons, and the cerebellum, a similar low retention was seen in AD and HC. However, when single individuals were compared, there was an overlap between AD and HC. A further study has substantially replicated these findings, showing significant group differences, although with individuals overlapping [2].
To evaluate the potential clinical use of PIB with PET in differential diagnosis it is necessary to determine the uptake patterns in dementia disorders other than AD. The purpose of this study was to investigate the presence or absence of PIB retention in frontotemporal dementia (FTD) by comparison with PIB retention positive AD patients and PIB negative HC.
Neuropathologically, AD is characterised by the presence of beta amyloid plaques and neurofibrillary tangles (NFTs) deposition [3]. Amyloid pathology is not present in FTD. The current opinion is that several types of neuropathological changes underlie the clinical syndrome of FTD [4]. These are named frontotemporal lobar degenerations (FTLD) to separate them from the clinical syndrome of FTD [4]. All patients have in common the lobar atrophy, neuronal loss, and gliosis. A further subdivision includes the presence or absence of tau, ubiquitin, and the predominant tau isoform, as detected by immunohistochemistry.
Clinically, FTD is a syndrome characterised by emotional blunting, a breakdown of social conduct, loss of empathy, and impaired illness awareness. Typically, the patient acts in an impulsive manner without being able to consider the consequences. Many patients show features associated with Asperger’s syndrome and autism. Early in the course of the disease, cognitive deterioration is often less prominent than these emotional and behavioural changes. The neurodegenerative process may be focal over several years, affecting either the left or the right side, involving predominantly frontal or temporal areas, respectively. Thus, the clinical presentation may vary. Cognitive disturbances typical for FTD include attention deficits, impaired executive function, and language disturbances, characterized by a reduced speech output and/or difficulties understanding the meaning of common words. In contrast to AD, episodic memory, spatial skills, and praxis are relatively well preserved in early stages of FTD. Neuropsychological testing serves as support for the diagnosis, and typically shows reduction on executive tests and reduced verbal fluency, whereas performance on episodic memory and visuospatial tests are relatively spared [5]. In clinical practice, the diagnosis of FTD relies on observations from the patient’s relatives and on identifying typical signs and symptoms [4, 6]. Structural imaging with brain computed tomography or magnetic resonance imaging may or may not demonstrate frontal and/or temporal atrophies [7]. Molecular imaging with FDG PET reveals glucose hypometabolism primarily in the frontal, temporal cortex, and occasionally in subcortical structures such as basal ganglia and thalami [8].
Patient recruitment and data collection
FTD
Ten patients (five men and five women with a mean age of 66, range 62–75 years, Table 1) with the clinical diagnosis of FTD were recruited from the Memory Clinic at the Department of Geriatrics, Uppsala University Hospital (Table 2). The patients were selected in order to have a clinical picture typical for FTD, i.e. no one had a history suggestive of AD, dementia with Lewy bodies, or cerebrovascular disease. Seven patients performed 27 points or higher on the MMSE [9] (Table 3). Seven patients had a family history of dementia. Two patients had mild extrapyramidal signs; none had signs of motor neuron disease. The diagnostic work-up consisted of a thorough clinical examination including interviews with the patients and his/her next-of-kin, a medical and neurological examination, as well as standard laboratory tests. The patients fulfilled the clinical criteria for FTD according to McKhann which state that patients should have developed “...behavioural or cognitive deficits manifested by either (a) early and progressive change in personality, characterised by difficulty in modulating behaviour, often resulting in inappropriate responses or activities, or (b) early and progressive changes in language, characterised by problems with expression of language or severe naming difficulty and problems with word meaning, causing a significant impairment in social or occupational functioning and representing a significant decline from a previous level of functioning, and that the course is characterised by gradual onset and continuing decline in function” [4]. Patients also fulfilled the Neary criteria for FTD [6], except for that illness awareness was present in some of the patients. All patients had a history of behavioural/social changes and executive impairment and five patients also had marked language impairment, although none filled the critera of semantic dementia or progressive nonfluent afasia [6]. A thorough neuropsychological examination verified cognitive and executive dysfunctions consistent with the diagnosis (Table 3). CT scan was either normal (n = 3), showed frontotemporal atrophy (n = 5), or mild unspecified atrophy (n = 2) (Table 2). Regional cerebral glucose metabolism was measured with FDG PET and the pattern of hypometabolism was consistent with FTD in all patients, i.e. a reduction in frontal and/or temporal cortex, or restricted to the anterior cingulum only in one patient (Table 2). Three patients (2, 4, and 10) had repeated FDG scans. In patient 10, the diagnosis of FTD was confirmed neuropathologically 3 months after the investigation. The other nine patients were clinically followed-up for 19–34 months after the study, showing progression in seven patients and only minor worsening in two patients (4, 8).
All FTD patients and/or their relatives agreed to participate after given information about the study. The Ethical Committee and the Radiation Safety Committee at Uppsala University Hospital approved the study.
Alzheimer’s disease patients
Data from 17 patients (mean age 64, range 51–80, Table 1) with a diagnosis of probable AD according to NINCDS-ADRDA criteria [13] and positive PIB depositions after PET scans were included. All patients were recruited from the Department of Geriatric Medicine, Karolinska University Hospital Huddinge, Stockholm, Sweden, and had previously taken part in PET studies with PIB at the Uppsala Imanet PET Centre [1, 14]. Diagnostic work-up included a thorough clinical examination with close informant interview, neuropsychological examination, CT/MRI scan, single-photon emission computed tomography, measurements of Abeta42 and total tau in cerebrospinal fluid, ApoE genotyping, and electroencephalography. The severity of dementia, as measured with MMSE, ranged from very mild to severe (range 9–29, mean 24 p).
Healthy controls
Data from eight subjects previously recruited at the Department of Geriatric Medicine, Karolinska University Hospital Huddinge, Stockholm, Sweden, and the Department of Psychology at Uppsala University, Uppsala, were collected [1]. These volunteers had previously taken part as controls for a PET study with PIB. Mean age was 50, with a range between 21 and 76 years (Table 1). None of these had any history of medical or neurological disease or substance abuse, and all scored normally on neuropsychological testing.
Data from nine healthy volunteers (4 males and 5 females) with mean age 56 (range 48 to 64) used in clinical evaluations at the Uppsala University Hospital were used as reference to assess regional brain glucose metabolic rate (rCMRglu) in the FTD patients.
Methods
Radiotracers
FDG and PIB were produced according to the standard GMP at Uppsala Imanet.
PET procedure
PET was performed in a Siemens ECAT HR+ camera with an axial field view of 155 mm, providing contiguous 2.46 mm slices with a 5.6 mm transaxial and a 5.4 mm axial resolution. Attenuation correction was based on a 10 min transmission scan with rotating 68 Ge rods before PIB administration. The emission data was normalised, corrected for random coincidences, dead time, and scatter. Image reconstruction was performed with standard software (ECAT7.1; CTI PET systems, Knoxville, TN) using Fourier rebinding followed by two-dimensional filtered back-projection applying a 4 mm Hanning filter. Subject’s heads were centred using the orbito-meatal line. The scanner protocol for transmissions, emissions, and reconstructions were the same as used in previous studies [1, 14] at Uppsala Imanet. The subjects were given 242 ±49 (mean and standard deviation) MBq of FDG and 227 ±91 MBq of PIB (mean and standard deviation).
Regions of interest (ROI)
To compare our results with the data from AD patients and HC obtained from a previous study, the same set of ROI as defined in that study was applied. Cortical ROI (1 × 3 cm) were placed in the frontal (three slices) and parietal (four slices) cortices. ROI for the striatum were placed at the level with highest uptake. Other cortical ROI were placed in the occipital and cerebellar cortices at the level of highest radioactivity uptake and in the temporal cortices (five coronal slices). Two ROI (1.5 cm in diameter) were located in the pons and linked, and the subcortical white matter was defined with a traced ROI at the location of centrum semiovale. The parietal, temporal, frontal, and occipital ROI were linked to form volumes of interest [1, 14]. Also, a similar computerised reorientation procedure was used to align the PIB images to the FDG images for accurate intra-individual comparisons [15]. MR images were not used to delineate ROI, nor were such data used for any partial volume correction.
Image quantification
For the FDG examinations, parametric maps of rCMRglc were generated by means of the Patlak method using the time course of the tracer in arterialized venous plasma as input fraction [16]. PIB retention data were given as standard uptake values (SUVs). The mean uptake values of the ROI obtained in a late time interval (40–60 min) were normalized to the corresponding uptake in a reference region (ROI/ref) [17]. The cerebellar cortex was chosen as reference because of its previously reported lack of Congo red- and thioflavin-S positive plaques [18].
Statistical analysis
Statistical analysis was performed using a two-sample, two sided, unequal variance, student’s t-test. Due to multiple comparisons for eight ROI, a Bonferroni correction was applied, setting the statistical significance level to 0.00625 (0.05/8).
Results
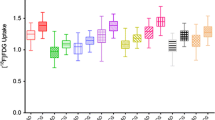
Eight FTD patients had PIB retention similar to healthy controls, whereas two had retention similar to that found in the AD patients (Table 2, Fig. 1). On a group basis, FTD patients showed significantly lower PIB uptake compared to AD in frontal (p < 0.0001, 46% less), parietal (p < 0.0001, 32% less), temporal (p = 0.0001, 28% less), and occipital (p = 0.0003, 29% less) cortices as well as putamina (p < 0.0001, 32% less) (Fig. 2). PIB retention in FTD did not differ significantly from HC in any region. FTD, AD, and HC all had equally low retention in thalami, pons, and white matter. For the two patients (4 and 9, Table 2) whose PIB retention levels overlapped those of AD patients, we also obtained values using a modification of the Patlak method [19]. The results were similar to those obtained with the ROI/reference method.
PIB retention in FTD (n=10), AD (n=17), and HC (n=8) expressed as ROI/reference region (cerebellum) as means ± SD
*Statistically significant differences between FTD and AD (p < 0.00625). There was no statistically significant difference between FTD and HC. Occ occipital cortex, Front frontal cortex, Par patietal cortex, Temp temporal cortex, Put putamina, Thal thalami, WhM cerebral white matter
Discussion
This was a study to investigate PIB retention in patients diagnosed with FTD according to both clinical picture and pattern of regional glucose metabolism.
The mean age for the HC examined with FDG was 56 years, which is 10 years lower than the mean age for the FTD patients. Metabolic changes produced by aging could induce wrong interpretations. To avoid this, the definition of hypometabolism was based on a difference in uptake lower than 2 SD.
To compare PIB retention, we selected eight healthy controls with negative PIB retention: five age matched HC (mean age 67 years) and 3 younger HC (mean age 21 years). Data from 17 AD patients (mean age 64) with positive PIB retention were included. The mean age for the FTD patients was 66 years. The reason to include the three young HC is because they represent true negative PIB controls [1]. Because we did not find differences in PIB retention between young HC and the older matched group, we include them all in the same group. Since comparison with PIB positive HC (or PIB negative AD) does not contribute to answering the question whether or not FTD shows PIB retention, HC with PIB positive scans were excluded from this study.
The results indicate that there is a significant difference in the level of PIB retention between the majority of patients with FTD and AD patients. This is as expected from the assumptions that PIB binds to amyloid and that amyloid deposition is a characteristic neuropathological feature of AD, but not of FTD. The PIB uptake in these FTD patients is close to that seen in negative healthy controls, indicating lack of amyloid depositions. Therefore, PIB could aim to differentiate FTD from AD in cases with atypical symptomatology. Two of the ten patients with clinical diagnosis FTD (4 and 9, Table 2) had a PIB retention similar to the AD patients suggesting that (1) their true diagnosis is AD, (2) they have FTD with coexistent AD or coexistent age-dependent amyloidosis, and (3) PIB might bind to other structures than amyloid.
A 10–20% frequency of false positive diagnosis of FTD is in accordance with studies on clinicopathological correlations in FTD. In a prospective autopsy series [20], 4 out of 18 patients (22%) clinically classified as FTD (with or without semantic dementia or progressive aphasia as a second syndrome) were given a non-FTLD postmortem diagnosis (two of which had AD). Litvan and colleagues [21] found a positive predictive value of about 85% for the diagnosis of Picks disease (elder terminology of FTD) when compared to pathology, although there were no cases of AD among the false positive ones. In our study, both patients 4 and 9 had a typical history of FTD at the time of the PET scan. Clinical and neuroimaging data were further reviewed in order to check for diagnostic accuracy. Patient 9 initially had typical frontal behavioural changes, showing rudeness, fixation to sex and money, in clear contrast to her former personality. These changes had developed slowly over several years. Her FDG-PET scan showed a typical picture with hypometabolism in frontal, temporal, and anterior cingulated cortices (Table 2). However, during follow-up after the PIB PET examination, she shows a more global, AD-like cognitive impairment. Furthermore, her brother had recently been diagnosed with AD. Her neuropsychological profile was not typical of FTD, with relatively poor performance on visuospatial and episodic memory tests (Table 3). It seems therefore plausible that patient 9 suffered from AD with frontal involvement, and not FTD. The symptoms of patient 4 were not suggestive of AD. This patient had facial agnosia early in the course of the disease, with changes in social behaviour and mild motor restlessness. His results on neuropsychological testing were typical for FTD, i.e. relatively good performance on visuospatial tests and episodic memory test (Table 3). The initial FDG-PET scan (hypometabolism in frontal cortex, anterior cingulum, and thalami bilaterally, Table 2) was supportive of FTD and was confirmed later by two repeated FDG PET scans, 17 and 21 month apart. These showed a progression of the temporal hypometabolism and, in addition, the development of parietal hypometabolism, a progression in accordance with FDG follow-up studies on other patients with FTD [22].
Occurrence of amyloid plaques in cognitively normal subjects is an established phenomenon, although its relation to the development of AD is controversial. For example, 45% in a population of cognitively intact elderly [23] (mean age of subjects 85.9) were defined as having “possible AD” by CERAD criterion at autopsy. The frequency was 18% in another sample [24] (mean age of subjects 85.4). Recent study evidence shows that cerebral amyloid deposition precedes neurodegeneration and symptoms by an extended period of time [14]. If confirmed, the presence of amyloid in the brain sooner or later will produce AD (if the patient lives long enough). Thus, clinically nonsignificant amyloid plaques could theoretically account for the PIB retention in patients 4 and 9. In addition, concomitant AD and FTD, i.e. symptomatic amyloid plaques and symptomatic FTLD pathology together (mixed disease), is possible. These explanations would be more likely for patient 4 (75 years of age), who was the oldest patient in our group. To our knowledge, no study has explored the frequency of amyloid plaque deposition, or concomitant AD, in FTD.
Preclinical data [25] have shown that at nanomolar concentrations such as those achieved intracerebrally in PET scans, PIB binds to amyloid but not to other protein aggregates (such as NFTs) in brains of AD patients. Brains from patients with Picks disease, motor neuron disease-inclusion dementia, and dementia lacking distinct histopathological features (subtypes of FTLD) were also analysed, showing no PIB binding at studied concentrations [25]. No research has, to our knowledge, been done on PIB binding in the remaining pathological entities included in the FTLDs (corticobasal degeneration, progressive supranuclear paralysis, and FTLD-17). With this in mind, it can be argued that our knowledge of PIB binding in the pathological conditions underlying FTD is not complete.
Conclusion
In an effort to find new ways to differentiate dementia diseases, based on in vivo assessment of pathology, we used an amyloid-detecting tracer for PET and studied its retention in FTD comparing it with PIB-negative HC and PIB-positive AD. Most of the FTD patients did not have amyloid depositions but instead showed images similar to those found in the healthy controls. Two out of ten FTD patients had PIB PET scans similar to the AD patients. This finding opens up possibilities for further research in this area, especially in order to monitor the efficacy of anti-amyloid therapies. Studies with larger and more naturalistic patient samples, with postmortem pathology, will be necessary to determine the potential of PIB PET as a clinical differential diagnostic tool. New multi-tracer approaches detecting other neuropathological changes such as astrocytosis, microgliosis, and the presence of tau protein will contribute to improve the accuracy of differential diagnoses. PIB may become an important tool in this approach.
References
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound B. Ann Neurol 2004;55:306–319.
Price JC, Klunk WE, Lopresti BJ, Lu X, Hodge JA, Ziolko SK, et al. Kinetic modelling of amyloid binding in humans using PET imaging and Pittsburgh compound-B. J Cereb Blood Flow Metab 2005;25:1528–1547.
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD) part II. Standardization of the neuropathological assessment of Alzheimer’s disease. Neurology 1991;41:479–486.
McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia. Arch Neurol 2001;58:1803–1809.
Hodges JR. Frontotemporal dementia (Pick’s disease): clinical features and assessment. Neurology 2001;56(Suppl 4):S6–10.
Neary D, Snowden JS, Gustavsson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554.
Kitagaki H, Mori E, Yamaji S, Ishii K, Hirono N, Kobashi S, et al. Frontotemporal dementia and Alzheimer disease: evaluation of cortical atrophy with automated hemispheric surface display generated with MR images. Radiology 1998;208:431–439.
Jeong Y, Cho SS, Park JM, Kang SJ, Lee JS, Kang E, et al. 18F-FDG PET findings in frontotemporal dementia: an SPM analysis of 29 patients. J Nucl Med 2005;46:233–239.
Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198.
Claeson L-E, Esbjornsson E, Carte’ B-M, Wahlbin M. Manual to Claeson-Dahls learning test for clinical use. Stockholm: Psykologiförlaget AB; 1971.
Schmidt M. Rey auditory verbal learning test: a handbook. Los Angeles, California: Western Psychological Services; 1996.
Rascovsky K, Salmon DP, Ho GJ, Galasko D, Peavy GM, Hansen LA, et al. Cognitive profiles differ in autopsy-confirmed frontotemporal dementia and AD. Neurology 2002;58:1801–1808.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 1984;34:939–944.
Engler H, Forsberg A, Almkvist O, Blomquist G, Larsson E, Savitcheva I, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain 2006;129:2856–2866.
Andersson JL, Thurfjell L. Implementation and validation of a fully automatic system for intra- and interindividual registration of PET brain scans. J Comput Assist Tomogr 1997;21:136–144.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalisations. J Cereb Blood Flow Metab 1983;3:1–7.
Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, et al. Simplified quantification of Pittsburgh compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med 2005;46:1959–1972.
Yamaguchi H, Hiriai S, Morimatsu M, Shoji M, Nakazato Y. Diffuse type of senile plaques in the cerebellum of Alzheimer-type dementia demonstrated by beta protein immunostain. Acta Neuropathol (Berl) 1989;77:314–319.
Blomquist G, Ringheim A, Estrada S, Höglund U, Frändberg P, Nylén G, et al. Influx and net accumulation of PIB compared with CBF in a rhesus monkey. EANM05, Istanbul, Turkey. EJNM 2005;32(Suppl 1):S263.
Kertez A, Mc Monagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain 2005;128:1996–2005.
Litvan I, Agid Y, Sastrj BS, Jankovic J, Wenning GK, Goetz CG, et al. What are the obstacles for an accurate clinical diagnosis of Pick’s disease? A clinicopathological study. Neurology 1997;48:62–69.
Diehl-Schmid J, Grimmer T, Drzezga A, Bornschein S, Riemenschneider M, Förstl H, et al. Decline of cerebral glucose metabolism in frontotemporal dementia: a longitudinal 18F-FDG-PET-study. Neurobiol Aging 2007;28(1):42–50.
Hulette CH, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological changes in “normal” aging: evidence for preclinical Alzheimer’s disease in cognitively normal individuals. J Neuropathol Exp Neurol 1998;57:1168–1174.
Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 2003;62:1087–1095.
Klunk WE, Wang Y, Huang G, Debnath ML, Holt DP, Shao L, et al. The binding of 2-(4′-Methylaminophenyl)Benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J Neurosci 2003;23:2086–2092.
Acknowledgements
The authors wish to thank the Uppsala University Amersham’s Fund (project number UU0058) and the Emma Pettersson Foundation, Sweden, for providing economic support. We thank the staff of Uppsala Imanet for their dedication and professionalism performing this study, in addition to the patients and their relatives for their participation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Henry Engler and Alexander Frizell Santillo have contributed in equal part to the content of this article.
Rights and permissions
About this article
Cite this article
Engler, H., Santillo, A.F., Wang, S.X. et al. In vivo amyloid imaging with PET in frontotemporal dementia. Eur J Nucl Med Mol Imaging 35, 100–106 (2008). https://doi.org/10.1007/s00259-007-0523-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0523-1