Abstract
Introduction
Primary hyperparathyroidism (PHPT) is an increasingly diagnosed disease worldwide. In most cases, PHPT is related to the presence of a solitary parathyroid adenoma (PA). Fifty percent or more of newly diagnosed PHPT patients are asymptomatic, and there is debate among endocrinologists and endocrine surgeons about whether or not such patients should be treated.
Localization
Usually, in a PHPT patient with a solitary PA that is well localised pre-operatively, a parathyroidectomy with limited or minimally invasive neck exploration is offered. The diffusion of minimally invasive neck exploration procedures is a consequence of the significant improvement in the accuracy of pre-operative imaging (mainly scintigraphic) techniques; these techniques have changed the surgical strategy to PHPT, from the wide traditional bilateral neck exploration to limited neck exploration.
Review
The present review considers developments during the past 10–15 years with regard to both the accuracy of pre-operative localising imaging techniques and intra-operative minimally invasive procedures in order to provide endocrinologists and endocrine surgeons with further information about the newly available diagnostic and therapeutic tools for use in PHPT patients with a solitary PA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The classic presentation of primary hyperparathyroidism (PHPT), that of “stones, moans and groans”, is rarely seen in contemporary clinical practice. With the introduction of routine biochemical screening in the 1970s, the early detection of modest elevations in serum calcium and early diagnosis of PHPT in patients with very subtle or no demonstrable symptoms of parathyroid disease became possible. This led to an inevitable increase in the prevalence of PHPT worldwide, such that within a very few years after the introduction of multiphasic biochemical screening, the estimated prevalence in the USA had increased by sixfold, with more than 50% of patients reported as asymptomatic [1].
The earlier recognition of PHPT, which in the majority of cases is the result of a parathyroid adenoma (PA), has resulted in an increase in the number of patients undergoing parathyroidectomy. The “traditional” approach to the surgical treatment of hyperparathyroidism with bilateral neck exploration was done to identify all parathyroid glands, followed by removal of an adenoma with or without biopsy of the remaining glands. Although cumbersome and time consuming, this “traditional” approach has continued to find favour amongst endocrine surgeons. However, the development of minimally invasive surgery and its application to parathyroidectomy (MIP) has dramatically reduced morbidity, hospital stay and cost, with better cosmetic results. Successful MIP demands accurate pre-operative localisation of the abnormal gland(s), with reliance on a variety of imaging techniques—most recently on intra-operative guided MIP with a gamma probe, using the in vivo uptake of a radiopharmaceutical that shows a preferential retention in parathyroid adenomas.
In this article we review the value of various imaging modalities in the diagnosis of PHPT and the evolving role of scintigraphy in the pre-operative localisation and intra-operative extirpation of parathyroid adenomas.
The parathyroid gland
Anatomy and physiology of the parathyroid
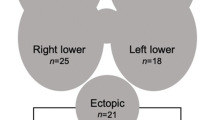
The parathyroid glands originate from the endoderm of the third and fourth pharyngeal pouches and migrate during foetal life toward their final juxtathyroidal location. This migration explains why surgical localisation can sometimes be problematic. In most cases, the upper glands migrate to a position posterior to the middle and upper thirds of the thyroid lobe and posterior to the recurrent laryngeal nerves. The location of the inferior glands is more variable, and in 50% of cases they are found posterior or lateral to the lower pole of the thyroid lobe. They can also be intrathyroidal, intrathymic or within the thyrothymic ligament. It is estimated that up to 20% of parathyroid glands are ectopic and can be found anywhere in the mediastinum and occasionally within the carotid sheath. The position may vary even further when normal glands become adenomatous or hyperplastic.
The secretory balance of the parathyroid and its cellular proliferation is regulated by the calcium ion through a specific receptor expressed on the cell membrane. When activated, these cells secrete parathyroid hormone (PTH), an 84-amino acid single-chain polypeptide with a molecular weight of 9,500 that controls the level of ionised calcium in blood and extracellular fluid. The major determinant of PTH secretion is ionised calcium, where small reductions in extracellular concentrations result in increased PTH secretion with reciprocal effects on the kidney to restore normocalcaemia. This is achieved by increasing tubular reabsorption of calcium, increasing excretion of phosphate and increasing the transformation of precursors into the active form of vitamin D, which, in turn, stimulates increased absorption of calcium in the gastrointestinal tract.
Primary hyperparathyroidism
Primary hyperparathyroidism is a clinical condition characterised by excessive secretion of PTH that is inappropriate for the extracellular calcium concentration. The main abnormality in hyperparathyroidism may relate to an unidentified genetic mutation (or mutations) causing failure of parathyroid cells to reduce PTH secretion when serum calcium is elevated, and this could be a consequence of abnormalities in the expression of calcium receptors located on the cell surface. There is evidence for activation of oncogenes and inactivation of tumour suppressor genes in the non-familial disease. Exposure of the neck and chest during radiation therapy for benign diseases, including 131I treatment for Graves’ disease, is another risk factor for the development of hyperparathyroidism. Interestingly, such risk association has not been demonstrated in 131I treatment of thyroid cancer.
In the majority of cases (80–85%), PHPT is caused by one or more parathyroid adenomas, but it can be the result of parathyroid hyperplasia in 15–20% of cases. Hyperparathyroidism due to hyperplasia may also be a component of familial syndromes such as multiple endocrine neoplasia type 1 (MEN-1) (87–97%), MEN-2 (5–20%) and familial hypocalciuric hypercalcaemia. Parathyroid carcinoma is a rare cause of PHPT, accounting for less than 1% of all cases.
Other forms of hyperparathyroidism
Secondary hyperparathyroidism is commonly associated with chronic renal failure. Renal impairment results in phosphate retention leading to hypocalcaemia, which in turn stimulates PTH secretion. In addition, there is an impaired renal response to increased PTH stimulation coupled with skeletal resistance to the action of PTH, due in part to a direct inhibiting effect of phosphorus retention. All these mechanisms progressively augment and perpetuate the hyperparathyroid state.
Tertiary hyperparathyroidism follows some cases of secondary hyperparathyroidism when biochemical abnormalities persist despite successful renal transplantation. In this instance, hyperparathyroidism is attributed to the development of functional autonomy in one or more parathyroid glands. This term is also used for patients in whom the PTH response caused by secondary hyperparathyroidism exceeds the hypocalcaemic demand, resulting in persistent hypercalcaemia.
Persistent hyperparathyroidism occurs in 5–10% of all patients who undergo surgery for PHPT, with continuation of the pre-operative abnormalities in calcium metabolism in the immediate postoperative period. It may result from failure of localisation of adenomas, inadequate resection of unrecognised multigland disease and/or the presence of metastatic parathyroid carcinoma. It is particularly frequent in patients with familial hyperparathyroidism, especially the MEN-1 syndrome. Hyperparathyroidism presenting after a period of more than 6 months of normocalcaemia following surgery is termed “recurrent hyperparathyroidism”, and is commonly due to continued growth of the remaining parathyroid tissues.
Surgery in PHPT
Bilateral neck exploration
Bilateral neck exploration is considered standard treatment of PHPT [2]. It consists in the excision of any grossly enlarged gland with or without biopsy of the remaining glands and, in experienced hands, has a 95% success rate with minimal morbidity. When multiple glands are enlarged, the operative techniques include a 3 1/2-gland resection or, less commonly, four-gland resection with subsequent autotransplantation. Success depends primarily on the experience of the surgeon in recognising the difference between enlarged and normal-sized glands, taking into consideration that the size of the gland does not always correlate with the secretion of PTH. In the absence of pre-operative or intra-operative localisation, the only certain method of finding the offending glands is a thorough neck and mediastinal exploration.
Minimally invasive parathyroidectomy
The development of fast and efficient scintigraphic techniques for pre-operative localisation of parathyroid adenomas, particularly with the use of 99mTc-MIBI, encouraged the introduction of “focussed” or minimally invasive parathyroidectomy (MIP) [3–5]. MIP is associated with less morbidity and comparable cure rates [5, 6], and is gradually replacing the traditional four-gland exploration as the procedure of choice in many institutions. Other ancillary procedures, including gamma probe-guided exploration and endoscopic techniques, have improved the accuracy and feasibility of MIP. Once successful localisation has been achieved, MIP can be employed with obvious advantages: the incision is small with minimal dissection, postoperative complications are minimal and hospital stay is shorter. MIP is less costly and may be performed as an outpatient procedure under local anaesthesia.
When available, quick PTH (QPTH) assay is extremely helpful in confirming the successful removal of the adenoma. A fall in the QPTH level by greater than 50% compared with the pre-operative level, assessed at 5–10 min after excision of a suspected adenoma, is considered indicative of successful surgery. The sensitivity, specificity and overall accuracy of QPTH are 98%, 94% and 97%, respectively [7, 8].
Role of imaging in pre-operative localisation
Anatomical imaging
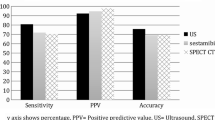
Imaging plays a key role in pre-operative localisation of parathyroid adenomas (Table 1). Ultrasound (US) is commonly the first diagnostic imaging method used in the localisation of a parathyroid adenoma. US is widely available, non-invasive and cost effective, but remains highly operator dependent [9] with a sensitivity that varies as a function of the size and location of the adenoma. The typical US appearance is of a round or oval, homogeneous, hypoechoic, hypervascular nodule with an echogenic capsule, but other atypical appearances are common and may lead to false negative results. Further limitations are encountered in the presence of a multinodular goitre, which can mimic or mask a parathyroid adenoma, and in substernal, retrotracheal and retro-oesophageal adenomas due to acoustic shadowing from overlying bone or air. Therefore, an ectopic adenoma cannot be excluded on the basis of a US examination alone. The reported sensitivity of US in the detection of parathyroid adenoma is 65–85% [10]. However, the sensitivity falls to 40% in patients who have had prior failed surgical exploration [11].
The cumulative sensitivity is increased when US is combined with other imaging modalities, such as scintigraphy, particularly in differentiating enlarged parathyroid glands from thyroid nodules [12–15]—an important consideration in areas with endemic nodular goitre.
Contrast-enhanced computed tomography (CT), with thin collimation, has a sensitivity that varies from 46% to 87%, with adenomas usually demonstrating avid contrast enhancement [10]. The high anatomical definition of CT is useful in ectopic, mediastinal adenomas, particularly those located in retrotracheal, retro-oesophageal and mediastinal spaces, but the performance is poor in ectopic lesions in the lower neck and lesions close to or within the thyroid gland [16–18]. Prior neck surgery affects the sensitivity of CT imaging, and artefacts caused by metallic clips have been shown to reduce sensitivity to 46–58% [18, 19]. Other disadvantages include movement artefacts (respiration and swallowing), the use of iodinated intravenous contrast and relatively high radiation exposure.
The ability of magnetic resonance imaging (MRI) to characterise nodular lesions based on intensity changes in the T1- and T2-weighted images makes it ideal for the detection of parathyroid adenomas, with improved detection by the addition of contrast enhancement and three-dimensional reconstruction [17]. Reported sensitivity reaches 80%, but due to difficulties in distinguishing parathyroid adenomas from hyperplasia or carcinoma, specificity has been found to be consistently low [20–23]. The main disadvantages of MRI are its limited availability, high cost and long scan times. CT and MRI are therefore useful in patients with recurrent or persistent hyperparathyroidism when the probability of ectopic glands is high.
Selective venous sampling (SVS) is another imaging technique employed in the localisation of parathyroid adenoma. It involves collection of venous blood samples from the cervical and mediastinal veins, via a femoral vein approach. Localisation is successful when a 1.5-fold increase in PTH concentration compared with the femoral vein is achieved, followed by a decreasing gradient in consecutive samples [24]. SVS is an invasive procedure and is often used when non-invasive imaging modalities are inconclusive or in cases of recurrent or persistent hyperparathyroidism following surgery.
Scintigraphic imaging
The lack of an ideal anatomical imaging modality to localise parathyroid adenomas prompted a search for alternative or complementary functional imaging procedures, but this endeavour was hampered by the failure to produce radiopharmaceuticals that could specifically target the parathyroid gland. Scintigraphic assessment of parathyroid tissue is made all the more difficult by its proximity to the thyroid gland, and earlier efforts focussed on finding a scintigraphic method to separate these tissues. Earlier success was based on taking advantage of the different uptake mechanisms of radiotracers by the thyroid and parathyroid glands (75Se-methionine or 201Tl chloride) and those preferentially accumulated by the thyroid (iodine or 99mTc-pertechnetate) to obtain a “subtraction image” that represented uptake in the parathyroid only. However, subtraction scans were technically demanding and involved a high radiation burden to the patient [25], while the results were not optimal owing to variability and low reproducibility in interpretation among different centres [26].
The introduction of 99mTc-MIBI scintigraphy in clinical practice in 1989 by Coakley and co-workers [27] substantially improved the role of pre-operative scintigraphic imaging in hyperparathyroid patients. Localisation of 99mTc-MIBI in the parathyroid tissue is based on a combination of blood flow, gland size and mitochondrial activity [28], which is similar to the mechanism of uptake in the thyroid. However, the washout rate from the two glands is different, with faster release of 99mTc-MIBI from the thyroid compared with the parathyroid, allowing for successful visualisation of the parathyroid when imaging is delayed for 1.5–2 h. This differential retention might be related to some down-regulation of the P-glycoprotein system in the parathyroid adenomas, resulting in delayed efflux of 99mTc-MIBI, [29]. However, in parathyroid hyperplasia these so-called multidrug resistance-associated molecules are overexpressed, may result in a faster rate of radiotracer washout and may be a contributing factor to false negative scintigraphy [30, 31]. Further, preliminary data suggest the possibility of inhomogeneity of the histological phenotype expressed in parathyroids of patients with multiglandular disease that could lead to incomplete identification of affected glands and persistent hyperparathyroidism [32].
The single-tracer dual-phase scintigraphy protocol, based on differential washout, was originally described by Taillefer et al. [33] and gained acceptance owing to its ease of use. However, the observation that 99mTc-MIBI can accumulate in solid thyroid nodules reduced specificity, particularly in geographic areas with a high prevalence of nodular goitre [34, 35], while reduced sensitivity was also noted in association with rapid 99mTc-MIBI washout from some parathyroid adenomas [36].
This led to the adoption of a modified protocol, dual-tracer subtraction scintigraphy, whereby a second radiopharmaceutical accumulating specifically in the thyroid gland was added. The suggested protocols (with inclusion of the entire chest in the imaging field) have included:
-
1.
123I/99mTc-MIBI dual-tracer subtraction technique. This protocol is difficult to adopt clinically owing to the high cost and limited availability of 123I.
-
2.
99m\({\text{TcO}}^{{\text{ - }}}_{{\text{4}}} \)/99mTc-MIBI dual-tracer subtraction technique. Patients are injected with 40 MBq of 99mTc-pertechnetate and imaging is performed 20 min post injection. Immediately after completion, patients are injected with 400–500 MBq 99mTc-MIBI without changing the patient’s position, and a 20-min dynamic acquisition is performed. Using this protocol, Geatti and co-workers achieved 95% sensitivity, without any false-positive result [37]. The addition of potassium perchlorate \({\left( {{\text{KClO}}^{{\text{ - }}}_{{\text{4}}} } \right)}\), to achieve a rapid washout of 99mTc-pertechnetate from the thyroid tissue, and neck US enhances the sensitivity of this protocol [12, 38].
With the application of an appropriate imaging protocol, hyperfunctioning parathyroid glands as small as 100 mg can be detected [12, 39–42], with an estimated sensitivity of 85–95%. The addition of single-photon emission computed tomography (SPECT) considerably improves localisation of ectopic adenomas in the retro-oesophageal space or mediastinum [43–47]. It is recommended that SPECT be acquired early rather than late as maximum parathyroid uptake takes place shortly after 99mTc-MIBI injection [48], and in order to avoid early, rapid washout seen in some adenomas [5, 44].
In addition to SPECT, other scintigraphic modifications such as dynamic imaging, pinhole SPECT [49] and hybrid SPECT/CT imaging [50] have been suggested to improve sensitivity. The use of 99mTc-MIBI scintigraphy has resulted in an appreciable improvement in pre-operative localisation and has improved the success rate of MIP with unilateral neck exploration [51–54].
99mTc-tetrofosmin shares some qualities with 99mTc-MIBI though its mechanism of uptake is different, with retention occurring primarily in the cytosol rather than within mitochondria. When used for parathyroid imaging, it has shown slow washout from the thyroid, making it unsuitable for single-tracer, dual-phase scintigraphy [55]. However, the sensitivity of 99mTc-tetrofosmin is substantially improved when it is used in a dual-tracer subtraction protocol with SPECT [56].
Positron emission tomography (PET) using 18F-fluorodeoxyglucose (18F-FDG) has been investigated to detect increased metabolic activity in adenomas, with variable success. Some reports suggest that 18F-FDG PET is more sensitive but less specific than 99mTc-MIBI SPECT [57], while others have reported very low sensitivity [58].
The use of PET with a labelled amino acid such as 11C-methionine has been investigated in parathyroid adenomas. In a study involving 34 patients with primary or recurrent adenomas, Sundin et al., using semi-quantitative standard uptake values (SUV) and transport rate constants, achieved a true positive rate of 85% compared with 59% and 57% for CT and US respectively [59]. In another study, Beggs and Hain investigated 51 patients with suspected adenomas who had negative or equivocal conventional imaging, using 11C-methionine. Their results showed a sensitivity of 83%, a specificity of 100% and an accuracy of 88% [60]. The major drawback of using 11C is its extremely short half-life of 20 min, which limits use to centres in close proximity to a cyclotron.
Gamma probe-guided minimally invasive parathyroidectomy (GP-MIP)
99mTc-MIBI is currently the only radiopharmaceutical employed in the pre- or intra-operative localisation of parathyroid adenoma(s). Intra-operative guidance with a hand-held gamma probe is commonly used at 2–3 h after 99mTc-MIBI injection. A number of protocols have been introduced with the aim of improving parathyroid-to-thyroid count ratios and optimising the performance of the gamma probe.
A single-day protocol has been advocated whereby a full imaging dose of 99mTc-MIBI (740 MBq) is administered, followed by a standard dual-phase imaging protocol at 20 min and 2 h. Gamma probe-guided surgery follows approximately 2.5–3 h later. This protocol offers the advantage of performing both parathyroid scintigraphy and surgery on the same day [61]. However, a prerequisite is that the adenoma has been identified by prior parathyroid scintigraphy and/or other imaging modalities.
A separate-day protocol involves performing 99mTc-MIBI dual-tracer subtraction scintigraphy a few days before surgery to localise the adenoma. On the day of surgery, a small dose of 99mTc-MIBI (37 MBq) is injected in the theatre before the start of the operation, followed by a search in the relevant area using the gamma probe [62, 63]. Intra-operative PTH monitoring is also employed to confirm complete removal of the hyperfunctioning parathyroid tissue. This low-dose protocol offers reduced radiation exposure to the surgical team and is particularly useful in patients with concomitant nodular goitre when separating parathyroid adenomas from adjacent thyroid nodules becomes difficult [12, 37].
The procedure for radio-guided parathyroid surgery starts with external scanning with the gamma probe to locate the highest radioactive spot on the skin surface. After making a small incision, the probe is inserted over the presumed location of the adenoma. The high-pitch signals produced by the gamma probe lead the surgeon towards the location with highest radioactivity. A parathyroid-to-thyroid radioactivity ratio higher than 1.5 suggests the presence of a parathyroid adenoma. After the lesion has been removed, the surgical bed is scanned again to ensure complete removal of the adenoma by establishing a new level of background radioactivity. The errors due to equivocal or false-positive scans are thus decreased by the use of an intra-operative gamma probe. Final assessment of radioactivity in all four quadrants increases confidence in the completeness of the parathyroidectomy. Gamma probe guidance enables the surgeon to perform a small skin incision with an improved cosmetic appearance. The technique can be performed under local anaesthesia with reduced operative time and allows early discharge from hospital [4, 64, 65].
In summary, the GP-MIP is an appropriate approach in the following conditions that are encountered in 60–70% of all cases of PHPT [34, 35, 66]:
-
High probability of a solitary parathyroid adenoma
-
Significant 99mTc-MIBI uptake in the parathyroid adenoma
-
No coexisting 99mTc-MIBI-avid thyroid nodules
-
No history of familial hyperparathyroidism or multiple endocrine neoplasia
-
No history of previous neck irradiation
-
Re-operation for persistent or recurrent hyperparathyroidism and ectopic adenomas
Gamma probe guidance has also been useful when performing a standard bilateral neck exploration, as it increases the accuracy of pre-operative 99mTc-MIBI scintigraphy [42, 67, 68].
Although GP-MIP is helpful in distinguishing a parathyroid adenoma from a thyroid nodule, the presence of a significant nodular goitre is a contraindication to the procedure [62]. Thyroid nodules can give false positive results at both pre-operative and intra-operative localisation [33, 63, 68, 69] owing to 99mTc-MIBI retention [68, 70], and the use of dual-tracer subtraction protocols is generally preferred, particularly in areas where nodular goitre is endemic [68, 71].
Conclusion
The role of scintigraphy in the pre- and intra-operative localisation of parathyroid adenomas, as the main cause of PHPT, has evolved to match the widespread use of minimally invasive parathyroidectomy, which demands accurate pre-operative localisation, particularly in patients undergoing treatment for recurrent or persistent hyperparathyroidism. Anatomical imaging has limited sensitivity and specificity compared with the high efficacy of 99mTc-MIBI using dual-tracer subtraction SPECT protocols. However, the combination of US and scintigraphy offers sufficiently high sensitivity and is successful in accurately identifying parathyroid adenomas. A low dose of 99mTc-MIBI injected immediately before surgery can be easily detected with a hand-held gamma probe and used to guide the surgeon to the location of the parathyroid adenoma during surgery.
References
Heath H 3rd, Hodgson SF, Kennedy MA. Primary hyperparathyroidism. Incidence, morbidity, and potential economic impact in a community. N Engl J Med 1980;302:189–93.
Kaplan EL, Yashiro T, Salti G. Primary hyperparathyroidism in the 1990s. Choice of surgical procedures for this disease. Ann Surg 1992;215:301–17.
Sofferman RA, Nathan MH, Fairbank JT, Foster RS Jr, Krag DN. Preoperative technetium 99m sestamibi imaging. Paving the way to minimal-access parathyroid surgery. Arch Otolaryngol Head Neck Surg 1996;122:369–74.
Norman J, Cheda H. Minimally invasive radioguided parathyroidectomy facilitated by intraoperative nuclear mapping. Surgery 1997;122:998–1004.
Rubello D, Pelizzo MR, Casara D. Nuclear medicine and minimally invasive surgery of parathyroid adenomas: a fair marriage [editorial]. Eur J Nucl Med Mol Imaging 2003;30:189–92.
Sackett WR, Barraclough B, Reeve TS, Delbridge LW. Worldwide trends in the surgical treatment of primary hyperparathyroidism in the era of minimally invasive parathyroidectomy. Arch Surg 2002;137:1055–9.
Irvin GL 3rd. American Association of Endocrine Surgeons. Presidential address: chasin’ hormones. Surgery 1999;126:993–7.
Irvin GL 3rd, Carneiro DM. Rapid parathyroid hormone assay guided exploration. Operative Techniques Gen Surg 1999;1:18–27.
Lloyd MN, Lees WR, Milroy EJ. Pre-operative localisation in primary hyperparathyroidism. Clin Radiol 1990; 41:239–43.
Ahuja AT, Wong KT, Ching AS, Fung MK, Lau JYW, Yuen EHY, et al. Imaging for primary hyperparathyroidism—what beginners should know. Clin Radiol 2004;59:967–76.
Miller DL, Doppman JL, Krudy AG, Shawker TH, Norton JA, Vucich JJ, et al. Localisation of parathyroid adenomas in patients who have undergone surgery. Radiology 1987;162:133–7.
Casara D, Rubello D, Pelizzo MR, Shapiro B. Role of preoperative imaging with 99mTc/MIBI scintigraphy combined with neck ultrasound, and of intraoperative 99mTc-sestamibi gamma probe technique in planning unilateral and minimally invasive surgery in primary hyperparathyroidism. Eur J Nucl Med 2001;28:1351–9.
Uden P, Aspelin P, Berglund J. Preoperative localization in unilateral parathyroid surgery. A cost-benefit study on ultrasound, computed tomography and scintigraphy. Acta Chir Scand 1990;156:29–35.
De Feo ML, Colagrande S, Biagini C, Tonarelli A, Bisi G, Vaggelli L, et al. Parathyroid glands: combination of 99mTc-MIBI scintigraphy and US for demonstration of parathyroid glands and nodules. Radiology 2000;214:393–402.
Casara D, Rubello D, Pelizzo MR, Shapiro B. Clinical role of 99mTcO4/MIBI scan, ultrasound and intra-operative gamma probe in the performance of unilateral and minimally invasive surgery in primary hyperparathyroidism. Eur J Nucl Med 2001;28:1351–9.
Eisenberg H, Pallotta J, Sacks B, Brickman AS. Parathyroid localisation, three dimensional modelling and percutaneous ablation techniques. Endocrin Metab Clinics North Am 1989;18:659–700.
Mitchell BK, Merrell RC, Kinder BK. Localization studies in patients with hyperparathyroidism. Surg Clin North Am 1995;75:483–98.
Koong HN, Choong LH, Soo KC. The role of preoperative localisation techniques in surgery for hyperparathyroidism. Ann Acad Med Singapore 1998;27:192–5.
Weber AL, Randolph G, Aksoy FG. The thyroid and parathyroid glands. CT and MR imaging and correlation with pathology and clinical findings. Radiol Clin North Am 2000;38:1105–29.
Lee VS, Spritzer CE. MR imaging of abnormal parathyroid glands. Am J Roentgenol 1998;170:1097–103.
Hishibashi M, Nishida H, Hiromatsu Y, Kojima K, Tabuchi E, Hayabuchi N. Comparison of technetium-99m-MIBI, technetium-99m-tetrofosmin, ultrasound, and MRI for localization of abnormal parathyroid glands. J Nucl Med 1988;39:320–4.
Fayet P, Hoeffel C, Fulla Y, Legmann P, Hazebroucq V, Luton JP, et al. Techetium-99m-sestamibi, magnetic resonance imaging and venous blood sampling in persistent and recurrent hyperparathyroidism. Br J Radiol 1997;70:459–64.
Gotway MB, Reddy GP, Webb WR, Morita ET, Clark OH, Higgins CB. Comparison between MR imaging and 99mTc-MIBI scintigraphy in the evaluation of recurrent or persistent hyperparathyroidism. Radiology 2001;218:783–90.
Seehofer D, Steinmuller T, Rayes N, Podrabsky P, Riethmuller J, Klupp J, et al. Parathyroid hormone venous sampling before reoperative surgery in renal hyperparathyroidism: comparison with noninvasive localization procedures and review of the literature. Arch Surg 2004;139:1331–8.
Waldorf JC, van Heerden JA, Gorman CA, Grant CS, Wahner HW. [75Se]Selenomethionine scanning for parathyroid localization should be abandoned. Mayo Clin Proc 1984;59:534–7.
Samanta A, Wilson B, Iqbal J, Burden AC, Walls J, Cosgriff P. A clinical audit of thallium-technetium subtraction parathyroid scans. Postgrad Med J 1990;66:441–5.
Coakley AJ, Kettle AG, Wells CP, O’Doherty MJ, Collings REC. 99mTc-sestamibi—a new agent for parathyroid imaging. Nucl Med Commun 1989;10:791–4.
Hetrakul N, Civelek AC, Stag CA, Udelsman R. In vitro accumulation of technetium-99m sestamibi in human parathyroid mitochondria. Surgery 2001;130:1011–8.
Bhatnagar A, Vezza PR, Bryan JA, Atkins FB, Ziessman HA. Technetium-99m-sestamibi parathyroid scintigraphy: effect of P-glycoprotein, histology and tumor size on detectability. J Nucl Med 1998;39:1617–20.
Chudzinski W, Niderla J, Lasiecka Z, Wilczynski G, Gornicka B, Wasiutynshi A, et al. P-glycoprotein expression influences the result of 99mTc-MIBI scintigraphy in tertiary hyperparathyroidism. Int J Mol Med 2005;16:215–9.
Grzela T, Chudzinski W, Lazarczyk M, Niderla J, Dziunycz P, Milewski L, et al. Persisted/recurrent hyperparathyroidism associated with development of multi-drug resistance phenotype and proliferation of parathyroid transplants. Int J Mol Med 2004;14:559–99.
Grzela T, Chudzinski W, Lasiecka Z, Niderla J, Wilczynski G, Gornicka B, et al. The calcium-sensing receptor and vitamin D receptor expression in tertiary hyperparathyroidism. Int J Mol Med 2006;17:779–83.
Taillefer R, Boucher Y, Potvin C, Lambert R. Detection and localization of parathyroid adenomas in patients with hyperparathyroidism using a single radionuclide imaging procedure with technetium-99m-sestamibi (double phase study). J Nucl Med 1992;33:1801–7.
Casara D, Rubello D, Piotto A, Pelizzo MR. 99mTc–MIBI radio–guided minimally invasive parathyroid surgery planned on the basis of a preoperative combined 99mTc-pertechnetate/99mTc–MIBI and ultrasound imaging protocol. Eur J Nucl Med 2000;27:1300–4.
Perrier ND, Ituarte PHG, Morita E, Hamill T, Gielow R, Duh QY, et al. Parathyroid surgery: separating promise from reality. J Clin Endocrinol Metab 2002;87:1024–8, discussion 1028–9.
Bénard F, Lefebvre B, Beuvon F, Langlois MF, Bisson G. Rapid wash-out of technetium-99m-MIBI from a large parathyroid adenoma. J Nucl Med 1995;36:241–3.
Geatti O, Shapiro B, Orsolon P, Proto G, Guerra UP, Antonucci F, et al. Localization of parathyroid enlargement: experience with technetium 99m methoxyisobutylisonitrile and thallium-201 scintigraphy, ultrasound and computed tomography. Eur J Nucl Med 1994;21:17–23.
Rubello D, Saladini G, Casara D, Borsato N, Toniato A, Piotto A, et al. Parathyroid imaging with pertechnetate plus perchlorate/MIBI subtraction scintigraphy. A fast and effective technique. Clin Nucl Med 2000;25:527–31.
Sfakianakis GN, Irvin GL III, Foss J, Mallin W, Georgiou M, Deriso GT, et al. Efficient parathyroidectomy guided by SPECT-MIBI and hormonal measurements. J Nucl Med 1996;37:798–804.
Hindie E, Melliere D, Jeanguillame C, Perlemuter L, Chehade F, Galle P. Parathyroid imaging using simultaneous double window recording of technetium-99m-sestamibi and iodine-123. J Nucl Med 1998;39:1100–5.
Moka D, Eschner W, Voth E, Dietlein M, Larena-Avellaneda A, Schicha H. Iterative reconstruction: an improvement of technetium-99m-MIBI SPECT for the detection of parathyroid adenoma? Eur J Nucl Med 2000;27:485–9.
Rubello D, Casara D, Giannini S, Piotto A, De Carlo E, Muzzio PC, et al. Importance of radio-guided minimally invasive parathyroidectomy using hand-held gamma probe and low 99mTc-MIBI dose: technical considerations and long-term clinical results. Q J Nucl Med 2003;47:224–32.
Billotey C, Sarfati E, Aurengo A, Duet M, Mundler O, Toubert ME, et al. Advantages of SPECT in technetium-99m-sestamibi parathyroid scintigraphy. J Nucl Med 1996;37:1773–8.
Carty SE, Worsey MJ, Virji MA, Brown ML, Watson CG. Concise parathyroidectomy: the impact of preoperative SPECT 99mTc sestamibi scanning and intraoperative quick parathormone assay. Surgery 1997;122:1107–16.
Gallowitsch JH, Mikosch P, Kresnik E, Gomez I, Lind P. Technetium-99m-tetrofosmin parathyroid imaging: results with double-phase study and SPECT in primary and secondary hyperparathyroidism. Invest Radiol 1997;32:459–65.
Neumann DR, Esselstyn CB Jr, Go RT, Wong CO, Rice TW, Obuchowsky NA. Comparison of double-phase 99mTc-sestamibi with 123I-99mTc-sestamibi subtraction SPECT in hyperparathyroidism. Am J Roentgenol 1997;169:1671–4.
Francis IS, Loney EL, Buscombe JR, Thakrar DS, Berger L, Hilson AJW. Technetium-99m-sestamibi dual-phase SPECT imaging: concordance with ultrasound. Nucl Med Commun 1999;20:487–8.
O’Doherty MJ, Kettle AG, Wells P, Collins REC, Coakley AJ. Parathyroid imaging with technetium-99m-sestamibi: preoperative localization and tissue uptake studies. J Nucl Med 1992;33:313–8.
Spanu A, Falchi A, Manca A, Marongiu P, Cossu A, Pisu N, et al. The usefulness of neck pinhole SPECT as a complementary tool to planar scintigraphy in primary and secondary hyperparathyroidism. J Nucl Med 2004;45:40–8.
Gayed IW, Kim EE, Broussard WF, Evans D, Lee J, Broemeling LD, et al. The value of 99mTc-sestamibi SPECT/CT over conventional SPECT in the evaluation of parathyroid adenomas or hyperplasia. J Nucl Med 2005;46:248–52.
Denham DW, Norman J. Cost-effectiveness of preoperative sestamibi scan for primary hyperparathyroidism is dependent solely upon the surgeons choice of operative procedure. J Am Coll Surg 1998;186:293–304.
George EF, Komisar A, Scharf SC, Ferracci A, Blaugrund S. Diagnostic value of the preoperative sestamibi scan in intraoperative localisation of parathyroid adenomas: a case study. Laryngoscope 1998;108:627–9.
Chen H, Sokoll LJ, Udelsman R. Outpatient minimally invasive parathyroidectomy: a combination of sestamibi-SPECT localisation, cervical block anaesthesia, and intraoperative parathyroid hormone assay. Surgery 1999;126:1016–21.
Kumar A, Cozens NJA, Nash JR. Sestamibi scan-directed unilateral neck exploration for primary hyperparathyroidism due to a solitary adenoma. Eur J Surg Oncol 2000;26:785–8.
Froberg AC, Valkema R, Bonjer HJ, Krenning EP. 99mTc-tetrofosmin or 99mTc-sestamibi for double-phase parathyroid scintigraphy? Eur J Nucl Med Mol Imaging 2003;30:193–6.
Gallowitsch HJ, Mikosch P, Kresnik E, Unterweger O, Lind P. Comparison between 99mTc-tetrofosmin/pertechnetate subtraction scintigraphy and 99mTc-tetrofosmin SPECT for preoperative localization of parathyroid adenoma in an endemic goiter area. Invest Radiol 2000;35:453–9.
Neumann DR, Esselstyn CB, MacIntyre WJ, Go RT, Obuchowski NA, Chen EQ, et al. Comparison of FDG-PET and sestamibi SPECT in primary hyperparathyroidism. J Nucl Med 1996;37:1809–15.
Melon P, Luxen A, Hamoir E, Meurisse M. Fluorine-18-fluorodeoxyglucose positron emission tomography for preoperative parathyroid imaging in primary hyperparathyroidism. Eur J Nucl Med 1995;22:556–8.
Sundin A, Johansson C, Hellman P, Bergstrom M, Ahlstrom H, Jacobson GB, et al. PET and parathyroid L-[carbon-11]methionine accumulation in hyperparathyroidism. J Nucl Med 1996;37:1766–70.
Beggs A, Hain SF. Localization of parathyroid adenomas using 11C-methionine positron emission tomography. Nucl Med Commun 2005:26:133–6.
Norman JG. Minimally invasive radioguided parathyroidectomy: an endocrine surgeon’s perspective. J Nucl Med 1998;39:15N–24N.
Casara D, Rubello D, Cauzzo C, Pelizzo MR. 99mTc-MIBI radio-guided minimally invasive parathyroidectomy: experience with patients with normal thyroids and nodular goiters. Thyroid 2002;12:53–61.
Rubello D, Casara D, Pelizzo MR. Symposium on parathyroid localization. Optimization of peroperative procedures. Nucl Med Commun 2003;24:133–40.
Flynn MB, Bumpous JM, Schill K, McMasters KM. Minimally invasive radioguided parathyroidectomy. J Am Coll Surg 2000;191:24–31.
Goldstein RE, Blevins L, Delbeke D, Martin WH. Effect of minimally invasive radioguided parathyroidectomy on efficacy, length of stay, and costs in the management of primary hyperparathyroidism. Ann Surg 2000;231:732–42.
Koong HN, Choong LH, Soo KC. The role of preoperative localisation techniques in surgery for hyperparathyroidism. Ann Acad Med Singapore 1998;27:192–5.
Norman J, Denham D. Minimally invasive radioguided parathyroidectomy in the reoperative neck. Surgery 1998;124:1088–93.
Bonjer HJ, Bruining HA, Pols HAP, de Herder WW, van Eijck CH, Breeman WA, et al. Intraoperative nuclear guidance in benign hyperparathyroidism and parathyroid cancer. Eur J Nucl Med 1997;24:246–51.
Chapius Y, Fulla Y, Bonnichon P, Tarla E, Abbound B, Pitre J, et al. Values of ultrasonography, sestamibi scintigraphy and intraoperative measurement of 1–84 PTH for unilateral neck exploration of primary hyperparathyroidism. World J Surg 1996;20:835–40.
Giordano A, Rubello D, Casara D. New trends in parathyroid scintigraphy. Eur J Nucl Med 2001;28:1409–20.
Rubello D, Casara D, Shapiro B. Recent advances in preoperative and intraoperative nuclear medicine procedures in patients with primary hyperparathyroidism. Panminerva Med 2002;44:99–105.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rubello, D., Gross, M.D., Mariani, G. et al. Scintigraphic techniques in primary hyperparathyroidism: from pre-operative localisation to intra-operative imaging. Eur J Nucl Med Mol Imaging 34, 926–933 (2007). https://doi.org/10.1007/s00259-007-0388-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0388-3