Abstract
Purpose
Elevated total plasma homocysteine (tHcy) levels and cardiovascular autonomic dysfunction are associated with a high mortality in type 2 diabetic patients. We tested the hypothesis that hyperhomocysteinemia is associated with insulin resistance and cardiovascular autonomic dysfunction in type 2 diabetic patients not receiving insulin treatment.
Methods
The study group consisted of 17 type 2 diabetic patients with high tHcy levels (>15 mmol/l, age 58±5 years, high tHcy group). The control group consisted of 23 age-matched type 2 diabetic patients with normal tHcy levels (≤15 mmol/l, age 58±9 years, normal tHcy group). Cardiovascular autonomic function was assessed by baroreflex sensitivity, heart rate variability, plasma norepinephrine concentrations, and cardiac 123I-metaiodobenzylguanidine (MIBG) scintigraphy.
Results
Early and delayed 123I-MIBG myocardial uptake values were lower (p<0.005 and p<0.01, respectively) and the percent washout rate of 123I-MIBG was higher (p<0.001) in the high tHcy group than in the normal tHcy group. The fasting plasma insulin concentrations (p<0.0001) and the homeostasis model assessment (HOMA) index values (p<0.0001) were higher in the high tHcy group than in the normal tHcy group. Multiple regression analysis revealed that the level of tHcy was independently predicted by the HOMA index values and the myocardial uptake of 123I-MIBG at the delayed phase.
Conclusion
Our results demonstrate that high levels of tHcy are associated with depressed cardiovascular autonomic function and insulin resistance in patients with type 2 diabetes mellitus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been reported that an elevated level of total plasma homocysteine (tHcy) is a risk factor for cardiovascular disease [1, 2]. Impaired autonomic neural activity has been recognized as a crucial risk factor of cardiac dysfunction and is strongly associated with an increased risk for harmful events and overall mortality in diabetic patients [3–5]. We have reported that depressed cardiovascular autonomic function is related to insulin resistance in type 2 diabetic patients [6–8]. Furthermore, elevated tHcy levels are reported to be associated with insulin resistance in non-obese healthy subjects [9] and type 2 diabetic patients [10]. Although these results strongly suggest that tHcy levels, insulin resistance, and autonomic dysfunction are related, the significance of increased tHcy levels for diabetic cardiovascular autonomic function has not been adequately investigated.
Technical advances, including measurements of baroreflex sensitivity (BRS), heart rate variability (HRV), and the concentration of norepinephrine, allow cardiac autonomic function to be assessed. The reliability coefficients of these parameters, however, were shown to be around 50% [11]. A reduction in myocardial uptake of 123I-metaiodobenzylguanidine (MIBG) reflects a reduction in the concentration of norepinephrine at presynaptic sites or a reduction in the neural density, whereas an enhanced washout rate of 123I-MIBG reflects enhanced release of norepinephrine from presynaptic sites [12]. Cardiac 123I-MIBG scintigraphy is a sensitive method for detecting sympathetic dysfunction in many clinical disorders, including diabetes mellitus [13, 14].
We hypothesized that increased levels of tHcy are associated with cardiovascular autonomic dysfunction and insulin resistance in type 2 diabetic patients. To test our hypothesis, we compared BRS, HRV, plasma norepinephrine concentrations, and cardiac 123I-MIBG scintigrams, in addition to the metabolic profiles, of Japanese type 2 diabetic patients with normal tHcy levels and those with high tHcy levels; independent predictors of the level of tHcy in these populations were evaluated.
Materials and methods
We screened 83 consecutive Japanese patients with type 2 diabetes mellitus who were admitted to our department between January 2005 and February 2006. Among these subjects, we enrolled 54 patients who did not have organic heart disease as determined by physical examinations, chest X-rays, 12-lead electrocardiography [ECG), echocardiography, treadmill exercise ECG, and 201Tl cardiac scintigraphy. All patients underwent clinical examinations to exclude the presence of secondary hypertension. Essential hypertension was defined as diastolic blood pressure ≥90 mmHg, systolic blood pressure ≥140 mmHg, or self-reported use of antihypertensive medication [15].
Laboratory methods
Blood was taken at 7:00 a.m. from the antecubital vein with the patient in the recumbent position after an overnight fast. All patients underwent routine laboratory tests including assays for serum electrolytes, serum total cholesterol, serum triglycerides, serum high-density lipoprotein, fasting plasma glucose, and fasting immunoreactive insulin. Insulin resistance was evaluated by the homeostasis model assessment (HOMA) index: [fasting plasma insulin (μU/ml) × fasting plasma glucose (mmol/l)]/22.5 [16]. Serum total homocysteine levels were determined using the homocysteine microplate enzyme immunoassay assay (Bio-Rad Laboratories, Oslo, Norway) [17]. Using this assay, 17 patients were determined to have high tHcy levels (>15 mmol/l; high tHcy group). We also included 23 age-matched patients from the original 54 enrolled patients who had normal levels of tHcy (≤15 mmol/l; normal tHcy group), the classification of which has previously been validated [18]. The clinical characteristics of patients in the normal and high tHcy groups are summarized in Table 1. Ten of the 17 patients in the high tHcy group and 13 of the 23 patients in the normal tHcy group met the criteria for essential hypertension and all of these patients were being treated with calcium channel antagonists, angiotensin-converting enzyme (ACE) inhibitors, and/or angiotensin II receptor blockers with diuretics. None of the patients was being treated with beta-blockers or insulin. Dyslipidemia was defined as fasting triglycerides levels ≥200 mg/dl or a HDL cholesterol concentration <45 mg/dl for women and <35 mg/dl for men [15]. Seven of the 17 patients in the high tHcy group and eight of the 23 patients in the normal tHcy group met the criteria for dyslipidemia. Urinary excretion was measured in urine collected during a 24-h period. Patients with abnormal plasma creatinine concentrations (≥1.5 mg/dl) were excluded from the study.
All subjects gave their written informed consent to participate in the study, and the study protocol was approved by the ethics committee of the Oita University Hospital.
Echocardiography
M-mode and two-dimensional echocardiography and cardiac Doppler recordings were obtained using a phase-array echo-Doppler system. Echocardiograms were obtained using standard parasternal, short axis, and apical views. The left ventricular mass was calculated as 1.04×[(LVIDd + IVSTd + PWTd)3−LVIDd3]−14 g, where LVIDd is the left ventricular internal diameter at end-diastole, IVSTd is the intraventricular septal thickness at end-diastole, and PWTd is the posterior wall thickness at end-diastole. The left ventricular mass was divided by the body surface area to calculate the left ventricular mass index. Pulsed Doppler recordings were made from a standard apical four-chamber view. Mitral inflow velocity was recorded with the sample volume at the mitral annulus level taking the average from at least three cardiac cycles. The peak velocity of early (E) and late ventricular filling (A) was determined, and the ratio (E/A) and deceleration time were recorded.
Cardiovascular autonomic function tests
Autonomic function was assessed according to methods described in previous studies [6–8]. During the tests, which were performed between 9:00 and 11:00 a.m., all subjects were in a supine position in a quiet room with dimmed lights. Autonomic function tests were performed in the morning after an overnight (≥12 h) fast. A blood sample was obtained from a venous catheter for measurement of the plasma norepinephrine concentration. The catheter was inserted in the right cubital vein, and arterial blood pressure was recorded noninvasively through a tonometric sensor attached over the left radial artery (Jentow-7700; Nihon Colin, Komaki, Japan). The accuracy of continuous blood pressure monitoring has been demonstrated previously [19]. Arterial blood pressure and a standard 12-lead ECG were monitored simultaneously; data were stored in a PCM data recorder (RD-200T; TEAC, Tokyo). Three-lead precordial Holter ECG recordings (model-459; Del Mar Avionics, Irvine, CA, USA) were also obtained throughout the procedure for analysis of HRV.
After an interval of 30 min to permit stabilization of the cardiovascular baroreflex mechanism, the patient was asked to breathe at a rate of 15 breaths/min using a metronome to stabilize the relationship between respiration and cardiovascular function. BRS was assessed by the phenylephrine method. Briefly, phenylephrine (2–3 μg/kg) was injected for 15 s to obtain a 15- to 40-mmHg rise in systolic blood pressure. BRS was calculated as the slope of the linear regression function relating systolic blood pressure changes to changes in the RR interval. Regression lines with more than 20 data points and a correlation coefficient (r) greater than 0.8 were accepted for analysis. The average of the two slopes was taken as the BRS value.
HRV was analyzed using Holter ECG recordings (Marquette Electronics Inc., Milwaukee, WI, USA). The power spectrum of the RR interval was computed by a fast Fourier transformation and expressed as the area under the power spectrum. We calculated the power of two spectral bands: the normal frequency (LF) component at 0.04–0.15 Hz and the high frequency (HF) component at 0.15–0.40 Hz. Based on their skewed distribution, the measured values of HRV were transformed to natural logarithmic values. The ratio of LF to HF (LF/HF) was also computed.
Planar and single-photon emission computed tomography studies were performed 15 min (early) and 4 h (delayed) after the injection of 111 MBq of 123I-MIBG using a rotating gamma camera (ZLC 7500; Siemens, Munich, Germany). Data were analyzed with analysis software (SCINTIPAC; Shimadzu, Kyoto, Japan). The anterior planar images from early and delayed 123I-MIBG studies were analyzed visually. For semiquantitative analysis, regions of interest were identified within the whole heart, and a 10×10 mm area over the upper mediastinum on the early and delayed planar images was used to calculate the mean heart-to-mediastinum (H/M) ratio. After correcting for the physical decay of 123I, the percent washout rate (WR) of the tracer from the myocardium was determined over a 4-h period.
Anthropometric and body composition measurement
The anthropometric and body composition characteristics of the patients were evaluated using the following parameters: height, body weight, body mass index (BMI), waist circumference, hip circumference, and waist-to-hip ratio. BMI was calculated as weight/(height2) (kg/m2). The waist circumference was measured midway between the lower rib margin and the iliac crest and the hip circumference was measured at the widest circumference over the trochanter in standing subjects after normal expiration.
Statistical analysis
Data are presented as means±SD. Differences between two groups were analyzed with the unpaired Student’s t test, chi-square test, or Fisher’s exact probability test. A p value <0.05 was considered statistically significant. Simple (Spearman’s rank) correlation coefficients between tHcy levels and various parameters were calculated. Stepwise multiple regression analysis was then used to evaluate the association between the levels of homocysteine and other factors, such as the BMI, waist circumference, waist-to-hip ratio, E/A ratio, triglyceride levels, HDL-c levels, uric acid levels, fasting plasma insulin concentrations, HOMA index values, creatinine level, creatinine clearance rates, urinary albumin excretion, the percent WR of 123I-MIBG, and the H/M ratio at the early and delayed phase after 123I-MIBG administration. In our multivariate analysis, F values ≥4 were considered significant.
Results
As shown in Table 1, the mean ages of the high and normal tHcy groups were similar, and there were no significant differences between the groups with respect to gender, duration of diabetes, or administered medications. The BMI values, waist circumferences, and waist-to-hip ratios were larger in the high tHcy group than in the normal tHcy group (p=0.0448, p=0.0282, and p=0.0071, respectively). Regarding glucose metabolism, fasting plasma glucose and insulin concentrations and HOMA index values were higher in the high tHcy group than in the normal tHcy group (p=0.0428, p<0.0001, and p<0.0001, respectively). There was, however, no significant difference in the levels of hemoglobin A1c. With regard to lipid metabolism, the concentration of serum triglyceride was higher and the concentration of serum HDL-cholesterol was lower in the high tHcy group than in the normal tHcy group (p=0.0437 and p=0.0194, respectively), whereas serum total cholesterol levels were not significantly different between the groups. The concentration of uric acid was higher in the high tHcy group than in the normal tHcy group (p=0.0191). Parameters measuring renal function, including the serum creatinine concentration, the creatinine clearance rate, and urinary albumin excretion, were greater in the high tHcy group than in the normal tHcy group (p=0.0010, p<0.0001, and p<0.0001, respectively). The hemodynamic data listed in Table 1 were obtained immediately before BRS assessment. The resting heart rate and the systolic and diastolic blood pressures were not significantly different between the two groups.
Table 2 presents a summary of the echocardiographic findings. The left ventricular dimensions at end-diastole and end-systole, the intraventricular septal and posterior wall thicknesses at end-diastole, the ejection fraction, and the left ventricular mass index values were similar in the two groups. With regard to left ventricular diastolic function, the peak velocity of late ventricular filling (A) was higher and the E/A ratio was lower in the high tHcy group compared with the normal tHcy group (p=0.0117 and p=0.0032, respectively). The deceleration time was longer in the high tHcy group than in the normal tHcy group (p=0.0169).
Figure 1 summarizes the results of the cardiovascular autonomic function tests. BRS was lower in the high tHcy group than in the normal tHcy group (9.0±4.1 vs 11.0±3.3 ms/mmHg, p=ns; Fig. 1a). The plasma norepinephrine concentrations were similar in both groups (high tHcy group: 232±71 pg/ml; normal tHcy group: 213±84 pg/ml; p=ns; Fig. 1b). Furthermore, analysis of HRV in the high and low tHcy groups revealed that the HF power (3.3±1.0 and 3.7±1.1 ln-ms2, respectively; p=ns) and the LF/HF ratios (1.6±1.0 and 1.4±0.7, respectively; p=ns, Fig. 1c) were not significantly different between the two groups. Cardiac 123I-MIBG scintigraphy disclosed that the H/M ratios at early and delayed phases in the high tHcy group were significantly smaller than those in the normal tHcy group (early phase: 2.02±0.13 vs 2.24±0.23, respectively, p<0.005; delayed phase: 1.87±0.20 vs 2.07±0.24, respectively, p<0.01; Fig. 1d). The percent WR of 123I-MIBG was higher in the high tHcy group than in the normal tHcy group (45.2±7.3% vs 35.4±9.2%, p=0.0008; Fig. 1d).
Comparison of autonomic function tests of type 2 diabetes mellitus patients with normal plasma homocysteine levels (normal tHcy) or high homocysteine levels (high tHcy). A Baroreflex sensitivity (BRS). B Plasma norepinephrine (NE) concentration. C Heart rate variability (HRV). Power of the high-frequency component (HF; 0.15–0.40 Hz, a) and the ratio of the low-frequency power (LF; 0.04–0.15 Hz) to HF power (LF/HF, b). The distribution of HRV values was skewed and the values were thus transformed to natural logarithmic values. D Cardiac 123I-MIBG scintigraphic findings. Myocardial uptake of 123I-MIBG at the early a and delayed b phases. Myocardial uptake of 123I-MIBG is expressed as the mean heart-to-mediastinum (H/M) ratio. c Percent washout rate (WR) of 123I-MIBG. Data are mean±SD. ns not significant
Table 3 depicts the correlation between the tHcy level and age, the body mass index, and other variables in both the high and the normal tHcy group. tHcy levels were positively correlated with body mass index values, waist circumference, waist-to-hip ratio, triglyceride levels, uric acid levels, fasting plasma insulin concentration, HOMA index values, creatinine levels, urinary albumin excretion, and percent WR of 123I-MIBG, and were negatively correlated with HDL-c levels, the creatinine clearance rate, the E/A ratio, and the H/M ratio at the early and the delayed phase.
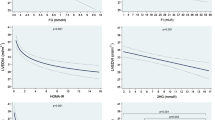
Figure 2 shows the positive correlation between the tHcy level and the HOMA index values (r=0.578, p<0.0001; Fig. 2a), and the negative correlation between the tHcy level and the H/M ratios measured using cardiac 123I-MIBG scintigraphy at the delayed phase (r=−0.535, p=0.0004; Fig. 2b) in type 2 diabetes mellitus.
Multiple regression analysis was performed using the stepwise procedure. The level of tHcy was predicted from the HOMA index values and the H/M ratio at the delayed phase (Table 4).
Discussion
In this study, type 2 diabetic patients with high tHcy levels had decreased uptake and enhanced clearance of 123I-MIBG in the heart relative to the values measured in type 2 diabetic patients with normal tHcy levels. In addition, multiple regression analysis revealed that the level of tHcy in the patients could be independently predicted by the HOMA index values and the H/M ratio at the delayed phase.
There are several reports indicating that an elevated tHcy concentration is associated with insulin resistance in non-obese healthy subjects [9] and patients with type 2 diabetes mellitus [10]. Giltay et al. [9] investigated the association between plasma tHcy levels and insulin resistance using a hyperinsulinemic–euglycemic clamp in 24 non-obese healthy subjects. They found a significant increase in the plasma tHcy levels of healthy subjects with insulin resistance. Emoto et al. [10] demonstrated that insulin resistance was an independent determinant of elevated plasma tHcy levels in 75 type 2 diabetes patients. In the present study, the level of tHcy correlated with the body mass index, triglyceride levels, HDL-cholesterol levels, the fasting plasma insulin concentration, and the HOMA index values.
Although the specific mechanism that links the tHcy level and insulin resistance remains to be elucidated, several mechanisms could explain our observations. Homocysteine may induce and/or enhance insulin resistance through oxidant stress. Homocysteine causes angiotoxicity and inhibits the nitric oxide system by inducing oxidant stress [20]. Oxidant stress, in turn, reduces insulin responsiveness in vitro by interrupting insulin signaling [21]. Moreover, Najib and Sanchez-Margalet [22] reported that homocysteine induces insulin resistance in vitro at high concentrations (50 μM) by inhibiting insulin signaling, and this effect may be mediated by oxidative stress, because it can be prevented by glutathione administration.
On the other hand, hyperinsulinemia associated with insulin resistance may affect the activities of enzymes involved in homocysteine metabolism and thereby influence plasma homocysteine levels. Jacobs et al. [23] have also reported that tHcy levels decreased owing to the increase in the activity of cystathionine β-synthase (CBS) in streptozotocin-induced diabetic rats, but returned to the initial plasma levels after administration of insulin. Fonseca et al. [24] have recently shown that hyperinsulinemia in the high fat-sucrose rat with insulin resistance induced a decrease in CBS activity and compensatorily increased the activity of 5,10-methylene tetrahydrofolate reductase, which was followed by hyperhomocysteinemia.
The relationship between homocysteine levels and cardiovascular autonomic function has been examined in previous studies with conflicting results [25–27]. Elevated homocysteine concentrations have been shown to correlate with the presence of diabetic peripheral or autonomic neuropathy by measuring HRV [25, 26]. Spoelstra-De Man et al. [27], however, reported that the concentration of homocysteine was not related to cardiac autonomic function in Caucasian subjects with or without type 2 diabetes mellitus. The authors used ten different methods to measure autonomic function: the mean of all sinus rhythm RR intervals, the standard deviation of all sinus rhythm RR intervals, LF power, HF power, LF/(LF+HF), expiration–inspiration difference RR intervals, BRS, RR max, RR max/min, and systolic blood pressure difference. HRV is mainly determined by vagal efferent activity [28], whereas BRS is determined using reflex parasympathetic nerve activity [29]. These parameters had reliability coefficients around 50% [11]. In the present study, BRS, HRV, and the plasma norepinephrine concentrations were not different between the two groups. Cardiac 123I-MIBG scintigraphy, however, disclosed that myocardial uptake of 123I-MIBG at the delayed phase was an independent predictor of the level of tHcy. These results were supported by previous studies from our group [6–8] and other groups [13, 30, 31], which demonstrated that 123I-MIBG scintigraphy is a fairly sensitive method for detecting cardiac sympathetic dysfunction in diabetic patients.
Although the precise mechanisms underlying the interactions between high tHcy levels and impaired autonomic function remain unclear, several possibilities could connect these phenomena. Firstly, increased tHcy levels could target neural function though direct cytotoxic effects [32]. Secondly, tHcy may affect autonomic function through endothelial dysfunction and impairment of the nitrate oxide system. As mentioned above, homocysteine angiotoxicity seems to affect the nitric oxide system by inducing oxidant stress [20]. Nitric oxidant damage and endothelial dysfunction are associated with cardiac autonomic dysfunction and increased HRV [33, 34]. In fact, in a recent report demonstrating the association between elevated levels of tHcy, insulin resistance, and microalbuminuria, the authors stressed the central role of endothelial dysfunction [35]. Moreover, we have recently reported that the HOMA index and the myocardial uptake of 123I-MIBG at the delayed phase are independent predictors of urinary albumin excretion [7].
These findings agree with the present observations that the homocysteine levels were associated with higher HOMA index values, the uptake level and percent WR of 123I-MIBG, and microalbuminuria. It is possible that interactions between high tHcy levels, insulin resistance, and autonomic dysfunction reinforce each other through mechanisms associated with endothelial dysfunction.
Compared with the normal tHcy group, patients with high tHcy levels showed cardiac diastolic dysfunction, although no significant difference was found between the groups in the left ventricular mass index. Cardiac diastolic dysfunction is associated with cardiovascular autonomic dysfunction and insulin resistance [15]. Consistent with the present results, Wheeler et al. [34] reported that elevated homocysteine levels are associated with cardiac left ventricular diastolic dysfunction in diabetic patients.
It has also been clearly documented that increased levels of tHcy occur in association with marked degrees of renal dysfunction [36]. Davies et al. [36] demonstrated the relationship between plasma tHcy levels and urinary albumin excretion owing to associated changes in renal function as defined by the creatinine clearance rate in type 2 diabetic patients. Emoto et al. [10], however, demonstrated insulin resistance to be an independent determinant of the plasma tHcy level in type 2 diabetes patients, even when the group of patients with type 2 diabetes was limited to those with normal renal function (creatinine clearance rate >60 ml/min). As in these studies, the present results demonstrate that both insulin resistance and renal dysfunction are associated with high tHcy levels, and insulin resistance is an independent predictor of the concentration of tHcy in type 2 diabetes patients.
There are several limitations to this study. Firstly, subjects in the study population had essential hypertension, which was treated with one or more antihypertensive drugs. These characteristics have been reported to affect insulin resistance [37, 38] and sympathetic nerve function [39–41]. Our small cross-sectional study did not allow us to statistically analyze and exclude the potential effects of these influences on the autonomic function tests. Secondly, our study did not clearly address the prognostic implications of elevated tHcy levels and cardiac autonomic function, although there are prognosis studies of heart failure patients using cardiac 123I-MIBG imaging [42, 43]. Therefore, further studies using cardiac 123I-MIBG scintigraphy are necessary to clarify the significance of the homocysteine concentration for determining the prognosis of cases of type 2 diabetes.
In conclusion, our findings suggest that higher levels of tHcy in patients with type 2 diabetes are associated with depressed cardiovascular autonomic function and insulin resistance.
References
Nygard O, Nordrehaug JE, Refsum H, Ureland PM, farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med 1997;337:230–236
Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. Br Med J 2002;325:1202–1206
Tsuji H, Larson MG, Venditti FJ Jr, Manders ES, Evans JC, Feldman CL, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Study. Circulation 1996;94:2850–2855
La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infraction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infraction) Investigators. Lancet 1998;351:478–484
Reichard P, Pihl M. Mortality and treatment side-effects during long-term intensified conventional insulin treatment in the Stockholm Diabetes Intervention Study. Diabetes 1994;43:313–317
Takahashi N, Nakagawa M, Saikawa T, Ooie T, Yufu K, Shigematsu S, et al. Effect of essential hypertension on cardiac autonomic function in type 2 diabetic patients. J Am Coll Cardiol 2001;38:232–237
Takahashi N, Anan F, Nakagawa M, Yufu K, Ooie T, Nawata T, et al. Microalbuminuria, cardiovascular autonomic dysfunction, and insulin resistance in patients with type 2 diabetes mellitus. Metabolism 2004;53:1359–1364
Anan F, Takahashi N, Ooie T, Nakagawa M, Saikawa T, Yoshimatsu H. High-sensitivity C-reactive protein is associated with insulin resistance and cardiovascular autonomic dysfunction in type 2 diabetic patients. Metabolism 2005;54:552–558
Giltay EJ, Hoogeveen EK, Elbers JM, Gooren LJ, Asscheman H, Stehouwer CD. Insulin resistance is associated with elevated plasma homocysteine levels in healthy, non-obese subjects. Arteriosclerosis 1998;139:197–198
Emoto M, Kanda H, Shoji T, Kawagishi T, Komatsu M, Mori K, et al. Impact of insulin resistance and nephropathy on homocysteine in type 2 diabetes. Diabetes Care 2001;24:533–538
Gerritsen J, TenVoorde BJ, Dekker JM, Kingma R, Kostense PJ, Bouter LM, et al. Measures of cardiovascular autonomic dysfunction nervous function: agreement, reproducibility, and reference values in middle age and elderly subjects. Diabetologia 2003;46:330–338
Sakata K, Shirotani M, Yoshida H, Kurata C. Cardiac sympathetic nervous system in early essential hypertension assessed by 123I-MIBG. J Nucl Med 1999;40:6–11
Spallone V, Menzinger G. Diagnosis of cardiovascular autonomic neuropathy in diabetes. Diabetes 1997;46:S67–S76
Watanabe K, Sekiya M, Tsuruoka T, Funada J, Kameoka H, Miyagawa M, et al. Relationship between insulin resisatnce and cardiac sympathetic nervous function in essential hypertension. J Hypertens 1999;17:1161–1168
Liao D, Sloan RP, Cascio WE, Folsom AR, Liese AD, Evans GW, et al. Multiple metabolic syndrome is associated with lower heart rate variability. The Atherosclerosis Risk in Communities Study. Diabetes Care 1998;21:2116–2122
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419
Donnelly JG, Pronovost C. Evaluation of the Abbott IMx fluorescence polarization immunoassay and the Bio-Rad enzyme immunoassay for homocysteine: comparison with high-performance liquid chromatography. Ann Clin Biochem 2000;37:194–198
Davies L, Wilmshurst EG, McElduff A, Gunton J, Clifton-Bligh P, Fulcher GR. The relationship among homocysteine, creatinine clearance, and albuminuria in patients with type 2 diabetes. Diabetes Care 2001;24:1805–1809
Sato T, Nishinaga M, Kawamoto A, Ozawa T, Takatsuji H. Accuracy of a continuous blood pressure monitor based on arterial tonometry. Hypertension 1993;21:866–874
Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest 1996;98:5–7
Tirosh A, Potashnik R, Bashan N, Rudich A. Oxidant stress disrupts insulin-induced redistribution of insulin receptor substrate-1 and phosphatidylinositol 3-kinase in 3T3-L1 adipocytes. A putative cellular mechanism for impaired protein kinase B activation and glut4 translocation. J Biol Chem 1999;274:10595–10602
Najib S, Sanchez-Margalet V. Homocysteine thiolactone inhibits insulin signaling and glutathione has a protective effects. J Mol Endocrinol 2001;27:85–91
Jacobs RL, House JD, Brosnan ME, Brosnan JT. Effects of treptozotocin-induced diabetes and insulin treatment on homocysteine metabolism rat. Diabetes 1998;47:1967–1970
Fonseca V, Dicker-Brown A, Ranganathan S, Song W, Barnard RJ, Fink L, et al. Effects of a high-fat-sucrose diet on enzymes in homocysteine metabolism in the rat. Metabolism 2000;49:736–741
Ambrosch A, Dierkes J, Lobmann R, Kuhne W, Konig W, Luley C, et al. Relation between homocysteinaemia and diabetic neuropathy in patients with type 2 diabetes mellitus. Diabetic Med 2001;174:185–192
Cohen JA, Jeffers BW, Stabler S, Schrier RW, Estascio R. Increasing homocysteine levels and diabetic autonomic neuropathy. Auton Neurosci 2001;87:268–273
Spoelstra-De Man AM, Smulders YM, Dekker JM, Heine RJ, Bouter LM, Nijpels G, et al. Homocysteine levels are not associated with cardiovascular autonomic function in elderly Caucasian subjects without or with type 2 diabetes mellitus: Hoorn study. J Intern Med 2005;258:536–543
Mantysaari M, Kuikka J, Mustonen J, Tahvanainen K, Vanninen E, Lansimies E, et al. Noninvasive detection of cardiac sympathetic nervous dysfunction in diabetic patients using [123I] metaiodobenzylguanidine. Diabetes 1992;41:1069–1075
Schnell O, Muhr D, Weiss M, Kirsch CM, Haslbeck M, Tatsch K, et al. Three-year follow-up on scintigraphically assessed cardiac sympathetic denervation in patients with long-term insulin-dependent (type I) diabetes mellitus. J Diabetes Complicat 1997;11:307–313
Weir DG, Scott JM. The biochemical basis of neuropathy in cobalamin deficiency. Bailliere’s Clin Heamatol 1995;8:479–497
Chowdhary S, Vaile JC, Fletcher J, Ross HF, Coote JH, Townend JN. Nitric oxide and cardiac autonomic control in humans. Hypertension 2000;36:264–269
Davis JI, Band M, Morris A, Struthers AD. Spironolactone impaires endothelial dysfunction and heart rate variability in patients with type 2 diabetic. Diabetologia 2004;47:1687–1694
Meigs J, Jacques P, Selhub J, Singer D, Nathan D, Rifai N, et al. Fasting plasma homocysteine levels in the insulin resistance syndrome. The Framingham Offspring study. Diabetes Care 2001;24:1403–1410
Wheeler DC, Townend JN, Landray MJ. Cardiovascular risk factors in predialysis patients: baseline data from the Chronic Renal Impairment in Birmingham (CRIB) study. Kidney Int Suppl 2003;84:S201–S203
Bostom AG, Shemin D, Verhoef P, Nadeau MR, Jacques PF, Selhub J, et al. Elevated fasting total plasma homocyateine levels and cardiovascular disease outcomes in maintenance dialysis patients: a prospective study. Arterioscler Thromb Vasc Biol 1997;17:2554–2558
Davies L, Wilmshurst EG, Mcelduff A, Gunton J, Clifton-Bligh P, Fulcher GR. The relationship among homocysteine, creatinine clearance, and albuminuria in patients with type 2 diabetes. Diabetes Care 2001;24:1805–1809
Gavras HP. Issues in hypertension: drug tolerability and special populations. Am J Hypertens 2001;14(Pt 2):231S–236S
Lender D, Arauz-Pacheco C, Breen L, Mora-Mora P, Ramirez LC, Raskin P. A double blind comparison of the effects of amlodipine and enalapril on insulin sensitivity in hypertensive patients. Am J Hypertens 1999;12:298–303
Kontopoulos AG, Athyros VG, Didangelos TP, Papageorgiou AA, Avramidis MJ, Mayroudi MC, et al. Effect of chronic quinapril administration on heart rate variability in patients with diabetic autonomic neuropathy. Diabetes Care 1997;20:355–361
Rodgers JE, Patterson JH. Angiotensin II-receptor blockers: clinical relevance and therapeutic role. Am J Health Syst Pharm 2001;58:671–683
Lefrandt JD, Heitmann J, Sevre K, Castelloano M, Hausberg M, Fallon M, et al. The effects of dihydropyridine and phenylalkylamine calcium antagonist classes on autonomic function in hypertension: the VAMPHYRE study. Am J Hypertens 2001;14:1083–1089
Yamashina S, Yamazaki J. Role of MIBG myocardial scintigraphy in the assessment of heart failure: the need to establish evidence. Eur J Nucl Med Mol Imaging 2004;31:1353–1355
Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, et al. Effects of candesartan on cardiac sympathetic nerve activity in patients with congestive heart faiure and preserved left ventricular ejection fraction. J Am Coll Cardiol 2005;45:661–667
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anan, F., Yonemochi, H., Masaki, T. et al. Homocysteine levels are associated with the results of 123I-metaiodobenzylguanidine myocardial scintigraphy in type 2 diabetic patients. Eur J Nucl Med Mol Imaging 34, 28–35 (2007). https://doi.org/10.1007/s00259-006-0200-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0200-9