Abstract
Purpose
To evaluate whether 89Zr can be used as a PET surrogate label for quantification of 90Y-ibritumomab tiuxetan (90Y-Zevalin) biodistribution and dosimetry before myeloablative radioimmunotherapy.
Methods
Zevalin was labelled with 89Zr by introducing N-succinyldesferal (N-sucDf) as a second chelate. For comparison of the in vitro stability of 89Zr-Zevalin and 88Y-Zevalin (as a substitute for 90Y), samples were incubated in human serum at 37°C up to 6 days. Biodistribution of 89Zr-Zevalin and 88Y-Zevalin was assessed at 24, 48, 72 and 144 h p.i. by co-injection in nude mice bearing the non-Hodgkin’s lymphoma (NHL) xenograft line Ramos. The clinical performance of 89Zr-Zevalin-PET was evaluated via a pilot imaging study in a patient with NHL, who had undergone [18F]FDG-PET 2 weeks previously.
Results
Modification of Zevalin with N-sucDf resulted in an N-sucDf-to-antibody molar ratio of 0.83±0.04. After radiolabelling and purification, the radiochemical purity and immunoreactivity of 89Zr-Zevalin always exceeded 95% and 80%, respectively. 89Zr-Zevalin showed the same stability in serum as 88Y-Zevalin, with a radiochemical purity >95% during a period of 6 days. The co-injected 89Zr-Zevalin and 88Y-Zevalin conjugates showed a very similar biodistribution, except for liver and bone accumulation at 72 and 144 h p.i., which was significantly higher for 89Zr than for 88Y. PET images obtained after injection of 89Zr-Zevalin showed clear targeting of all known tumour lesions.
Conclusion
89Zr-Zevalin and 88Y-Zevalin showed a very similar biodistribution in mice, implying that 89Zr-Zevalin-PET might be well suited for prediction of 90Y-Zevalin biodistribution in a myeloablative setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The yttrium-90 (90Y) labelled anti-CD20 murine monoclonal antibody (MAb) ibritumomab tiuxetan (90Y-Zevalin, Biogen IDEC and Schering AG) was approved by the U.S. Food and Drug Administration in 2002 for the treatment of patients with relapsed or refractory low-grade, follicular or transformed B-cell non-Hodgkin’s lymphoma (NHL), including rituximab-refractory follicular NHL [1, 2]. In 2004, the European Medicines Agency (EMEA) approved 90Y-Zevalin for the treatment of adult patients with rituximab-relapsed or refractory CD20+ follicular B-cell NHL [3]. The Zevalin radioimmunotherapy (RIT) procedure is preceded by administration of 250 mg/m2 rituximab (Rituxan, Biogen IDEC and Genentech) to clear peripheral B cells and to improve biodistribution of the radiolabelled Zevalin. Recently, promising results have also been obtained in the treatment of aggressive NHL. In these studies, high-dose 90Y-Zevalin RIT was added to high-dose chemotherapy followed by autologous stem cell transplantation (AuSCT) [4, 5]. The optimal use of 90Y-Zevalin in this kind of myeloablative RIT, however, remains to be determined.
90Y has a physical half-life of 64.1 h and emits high-energy β− particles. The absence of γ-ray emission minimises dose radiation burden for medical personnel and relatives and enables outpatient treatment. Whereas these characteristics make 90Y attractive for therapy, the lack of associated photon emission does not allow external imaging of the in vivo distribution of the 90Y-labelled MAb. Therefore, to confirm tumour targeting and to estimate the absorbed dose (dosimetry) by tumours as well as normal tissues, in the studies on myeloablative RIT a pretherapy single-photon emission computed tomography (SPECT) imaging study with indium-111 (111In, t 1/2=67.3 h) labelled Zevalin was performed [4, 5]. For coupling of 111In and 90Y to Zevalin, the chelator 1-isothiocyanatobenzyl-3-methyldiethylene triamine penta-acetic acid designated MX-DTPA (tiuxetan) was used, because it binds these three-valent radionuclides with high stability.
In myeloablative RIT, high doses of 90Y are used and therefore dosimetry as accurate as possible will be highly desirable for prevention of exposure of normal organs to unacceptable doses and for evaluation of dose-response relationships. Positron emission tomography (PET) is better suited than SPECT for tracer quantification [6], while targeting information can be combined with anatomical information when PET-CT is used.
Visualisation and quantification of MAb biodistribution with a PET camera (immuno-PET) requires a suitable positron-emitting radionuclide. As a potential PET surrogate for 90Y, the positron emitter 86Y is receiving attention [7, 8]. An advantage in the use of the same element would be that one type of chelator can be used for coupling of both isotopes. 86Y, however, emits prompt gammas, which can hamper accurate quantification; furthermore, its half-life of 14.7 h is relatively short for optimal imaging and dosimetry with an intact MAb like Zevalin, as typically 2–4 days are required to achieve optimal tumour to non-tumour ratios. The positron emitter zirconium-89 (89Zr) might be a better candidate for prediction and monitoring of the biodistribution of 90Y in RIT studies, especially because of its long half-life of 78.4 h. Procedures for the production and purification of large batches of 89Zr have recently been established at our institute. A versatile method for stable coupling of 89Zr to MAbs, including MAb U36, which is at an early stage of development for detection of head and neck cancer, was developed using the desferal-chelate precursor tetrafluorophenol-N-succinyldesferal-Fe (TPF-N-sucDf-Fe) [9]. Preliminary clinical evaluation of 89Zr-MAb U36 revealed that tumour deposits can be clearly visualised after administration of 74 MBq 89Zr [6]. Moreover, in nude mice bearing head and neck cancer xenografts, 89Zr-N-sucDf-U36 and 88Y-DOTA-U36 (88Y as a substitute for 90Y) conjugates showed a highly similar biodistribution, while accurate 89Zr quantification appeared feasible, these being prerequisites to justify the use of 89Zr-immuno-PET for scouting 90Y-RIT [10].
Therefore, introduction of 89Zr-Zevalin-PET in high-dose 90Y-Zevalin RIT might be an attractive option for prediction of 90Y-Zevalin biodistribution and dosimetry, and of toxicity and tumour response upon high-dose 90Y-Zevalin RIT in a myeloablative setting. To enable the use of 89Zr-Zevalin in such a setting, the present study was conducted to develop a route to an 89Zr-Zevalin conjugate capable of monitoring the biodistribution of the therapeutic 90Y-Zevalin conjugate in the clinic. Since only MX-DTPA premodified ibritumomab is available for clinical use, and MX-DTPA does not bind the four-valent 89Zr, N-sucDf was coupled as a second chelate to Zevalin. The resulting newly developed double-chelator modified 89Zr-Zevalin conjugate was measured for stability in serum and for preservation of immunoreactivity, including during storage. For the analysis of the pharmacokinetic behaviour, the biodistribution of 89Zr-Zevalin was compared with that of 88Y-Zevalin upon co-injection in NHL xenograft-bearing nude mice. The preliminary clinical performance of 89Zr-Zevalin-PET was evaluated in a patient with CD20+ B-cell NHL.
Materials and methods
Monoclonal antibody, radioactivity and cell lines
The monoclonal antibody ibritumomab tiuxetan (Zevalin) was obtained from Schering Nederland BV, The Netherlands. 89Zr (2.7 GBq/ml in 1 M oxalic acid) was produced by the BV Cyclotron by a (p,n) reaction on natural 89Y and isolated with the use of a hydroxamate column [9]. 88Y (37 MBq/ml in 0.1 M HCl) was obtained from Isotope Products Europe, and 90Y (18.5 GBq/ml in 0.05 M HCl) was obtained from PerkinElmer. The CD20+ B-cell lymphoma cell line Ramos was obtained from the American Type Culture Collection (ATCC number CRL-1596).
Radiolabelling
Labelling of Zevalin with 89Zr was achieved starting from the chelate TFP-N-sucDf-Fe, as described previously [9]. In short, the TFP-N-sucDf-Fe ester was coupled to the lysine residues of Zevalin (1.6 mg). After removal of Fe(III) by transchelation to ethylene diamine tetra-acetic acid (EDTA) and purification on a PD10 column (Amersham Biosciences; eluent: 0.9% NaCl/gentisic acid 5 mg/ml, pH 5.0), the premodified Zevalin was labelled with 89Zr in 0.5 M N-2-hydroxyethylpiperazine-N-2-ethanesulphonic acid (HEPES) buffer at pH 7.0 to arrive at a specific activity of approximately 74 MBq/mg. Finally, 89Zr-Zevalin was purified on a PD10 column (eluent: 0.9% NaCl/gentisic acid 5 mg/ml, pH 5.0) to remove unbound 89Zr. A schematic representation of 89Zr-Zevalin (i.e. 89Zr-N-sucDf-ibritumomab tiuxetan) in comparison with the routinely used 90Y-Zevalin conjugate is shown in Fig. 1.
90Y-Zevalin was prepared according to the instructions of the supplier entitled: “Preparation and dispensing information on 90Y and 111In-ibritumomab tiuxetan”. With this procedure, 90Y was coupled to Zevalin at a specific activity of approximately 740 MBq/mg MAb, and formulated in a phosphate-buffered saline (PBS) containing 7.5% human serum albumin (HSA) and 1 mM diethylene triamine penta-acetic acid (DTPA), pH 7.2 (formulation buffer). Zevalin was labelled with 88Y according to the same procedure, but with reaction volume modifications.
Analyses
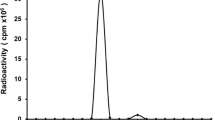
Conjugates were analysed by instant thin-layer chromatography (ITLC) and high-performance liquid chromatography (HPLC) for radiochemical purity, by sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by phosphor imager analyses for integrity, and by a cell-binding assay for immunoreactivity. ITLC analysis of radiolabelled Zevalin was performed on silica gel impregnated glass fibre sheets (Gelman Sciences). As the mobile phase, 20 mM citrate buffer pH 5.0 was used for 89Zr-Zevalin and 0.9% NaCl for 88Y- and 90Y-Zevalin. Antibody-bound 89Zr, 88Y and 90Y remained at the origin (Rf 0.0), whereas unreacted 89Zr, 88Y and 90Y (as 89Zr-citrate or 88Y/90Y-DTPA) moved with Rf 0.8–1.0. HPLC monitoring of the synthesis of 89Zr-Zevalin and of the labelling to arrive at 88Y/90Y-Zevalin was performed on a Jasco HPLC system using a Superdex 200 10/300 GL size exclusion column (Amersham Biosciences). As eluent, a mixture of 0.05 M sodium phosphate/0.15 M NaCl (pH 6.8) was used at a flow rate of 0.5 ml/min.
The N-sucDf-to-MAb molar ratio was quantified using HPLC analysis with TFP-N-sucDf-Fe ester spiked with 59Fe in the modification step, and measuring the amount of MAb bound N-sucDf-59Fe and unreacted N-sucDf-59Fe.
Single isotope counting was performed with a γ-well counter (Compugamma, LKB Wallac) for 89Zr, 88Y and 59Fe, and with a β-counter (Rackbeta, LKB Wallac) for 90Y using Čerenkov radiation. The 511-keV γ-energy of 89Zr and the 1,837-keV γ-energy of 88Y were used for dual-isotope counting in the biodistribution study. Crossover corrections from one radionuclide into the alternate window were performed using a standard of each radionuclide. When 89Zr was measured with the γ-well counter in the presence of 90Y, a similar procedure was applied to correct for background caused by 90Y bremsstrahlung. When 90Y was measured with the β-counter in the presence of 89Zr, corrections were applied to adjust for background caused by 89Zr.
Gel electrophoresis was performed on a Pharmacia Phastgel System (Amersham Biosciences) using preformed 7.5% SDS-PAGE gels (Amersham Biosciences) under non-reducing conditions. In vitro binding characteristics of radiolabelled Zevalin were determined in an immunoreactivity assay essentially as described by Lindmo et al. [11], using Ramos cells fixed with 2.0% paraformaldehyde. Data were graphically analysed in a modified Lineweaver-Burk (double-reciprocal) plot and the immunoreactivity was determined by extrapolating to conditions representing infinite antigen excess.
In vitro stability of radiolabelled Zevalin
Three sets of stability tests were performed, each with a particular design, of relevance for the clinical studies planned: For validation of 89Zr labelling in compliance with Good Manufacturing Practice (GMP), three clinical batches of 89Zr-Zevalin were produced and quality tests were performed. To this end, 89Zr-Zevalin (1.8 mg, 74 MBq) was diluted in 20 ml 0.9% NaCl/gentisic acid 5 mg/ml, pH 5.0 (clinical formulation) and incubated at 4°C or room temperature for 48 h. At 0, 24 and 48 h, aliquots were taken and analysed by ITLC as well as HPLC. In addition, the immunoreactivity was assessed at these time points.
In forthcoming clinical myeloablative RIT studies, we anticipate the possibility of administration of 89Zr-Zevalin alone for pretherapy imaging as well as administration in combination with high-dose 90Y-Zevalin (for RIT). In the latter case, the PET and RIT conjugate might be administered together as a mixture. For testing the stability of both conjugates in such a mixture at challenging radioactivity concentrations, 37 MBq 89Zr-Zevalin (0.5 mg) and 720 MBq 90Y-Zevalin (1 mg) in formulation buffer (PBS buffer containing 7.5% HSA and 1 mM DTPA, pH 7.2) were combined in a final volume of 6.5 ml and stored at 4°C. At various time intervals (2, 4, 7 and 24 h), samples were taken for quality testing of both conjugates, including ITLC, HPLC and binding assay.
For testing the in vitro stability in human serum of 89Zr-Zevalin and 88Y-Zevalin, samples of 0.1 ml containing the radiolabelled MAbs were added to 0.9 ml of freshly prepared human serum and incubated at 37°C in a humidified incubator maintained at 5% CO2 and 95% air. In this case, 88Y was used instead of 90Y to facilitate quantification in serum. At various time intervals (1, 2, 4, 6 days), aliquots were taken and analysed by ITLC and HPLC.
Biodistribution
Nude mice bearing the subcutaneously implanted B-cell lymphoma cell line Ramos were used. Female mice (athymic nu/nu, 21–31 g, Harlan CPB) were 10–14 weeks old at the time of the experiment. All animal experiments were performed according to National Institutes of Health principles of laboratory animal care and Dutch national law (“Wet op de dierproeven”, Stb 1985, 336).
The mice (n=16) were injected intravenously with a mixture of 0.37 MBq 89Zr-Zevalin and 0.13 MBq 88Y-Zevalin. Unlabelled Zevalin was added to this mixture so that all animals received 100 μg MAb in total. At 24, 48, 72 and 144 h post injection, groups of four mice were anaesthetised, bled, killed and dissected. After blood, tumour, normal tissues and gastrointestinal contents had been weighed, the amount of radioactivity in each was measured in a γ-well counter. Radioactivity uptake was calculated as the percentage of the injected dose per gram of tissue (%ID/g). Differences in tissue uptake between co-injected conjugates were statistically analysed for each time point with SPSS 11.0 software using Student’s t test for paired data. Two-sided significance levels were calculated and p<0.05 was considered statistically significant.
PET imaging
The preliminary clinical performance of 89Zr-Zevalin PET was evaluated in a male patient (aged 49 years) who had an indolent CD20+ NHL, with transformation to diffuse large B-cell NHL, with relapsed disease after AuSCT. This feasibility study, ahead of the planned study “90Y-Zevalin myeloablative RIT and 89Zr-Zevalin-PET in conditioning for stem cell transplantation in patients with aggressive B-cell NHL”, was reviewed and approved by the Medical Ethics Committee of the VU University Medical Centre. The patient gave written informed consent after receiving a thorough explanation of the study.
PET scanning was performed using a dedicated full ring PET scanner (ECAT EXACT HR+, CTI/Siemens). A PET session with 89Zr-Zevalin was performed to assess biodistribution and to confirm targeting of tumour lesions that had been delineated 2 weeks previously by [18F]fluorodeoxyglucose (FDG) PET scanning.
In the case of 89Zr-Zevalin immuno-PET, whole-body scans were performed, consisting of seven bed positions covering the patient from the base of the skull to the upper femur. At each bed position a 3-min transmission scan, acquired using three germanium-68 rod sources, and a 7-min emission scan in 3D mode were acquired. The patient first received rituximab, 250 mg/m2 over 3.5 h, followed within 4 h by 74 MBq 89Zr-Zevalin. The patient received a total of 1.8 mg Zevalin by adding unlabelled Zevalin. Whole-body scans were performed at 2 and 96 h after intravenous injection of 89Zr-Zevalin. All scans were normalised and corrected for randoms, scatter, attenuation and decay. Reconstructions were performed using an attenuation- and normalisation-weighted ordered subset expectation maximisation (OSEM) algorithm (ECAT software, version 7.2, CTI/Siemens) with two iterations and 16 subsets followed by post-smoothing of the reconstructed image using a 5-mm FWHM Gaussian filter. OSEM reconstructions without attenuation correction were performed as well. Interpretation of the scans was performed using these non-attenuation-corrected images and was based on asymmetry and retention of activity on the late image.
[18F]FDG-PET was performed essentially as described previously [12]. In short, the patient was required to fast for at least 6 h before the scan. A whole-body scan was performed consisting of eight bed positions from the crown to the midfemur. At each bed position a 7-min emission scan in 2D mode was performed. Scanning started about 60 min after intravenous injection of 400 MBq [18F]FDG (BV Cyclotron, The Netherlands). The scan was corrected in the same way as described above for the 89Zr-Zevalin scans; however, no attenuation correction was performed. The PET image was read visually using standard ECAT software: foci with increased uptake versus background were considered abnormal, taking physiological biodistribution of [18F]FDG into account.
Results
Modification and radiolabelling
Labelling of Zevalin-MX-DTPA with 89Zr resulted in a labelling yield below 0.1%, confirming the lack of affinity of 89Zr for DTPA chelates. Modification of Zevalin with the chelate TFP-N-sucDf-Fe resulted in a reproducible N-sucDf-to-MAb molar ratio of 0.83±0.04 (mean±SD, n=8). Subsequent labelling of N-sucDf-Zevalin with 89Zr resulted in overall labelling yields of >60% and radiochemical purity always exceeded 95% after purification on PD10. Labelling of Zevalin with 88Y or 90Y resulted in overall labelling yields of >96% and the radiochemical purity was more than 96% for both products. Immunoreactivity of 89Zr-Zevalin was more than 80% at infinite antigen excess and similar to the immunoreactivity of 88Y/90Y-labelled Zevalin conjugates. No physical evidence of antibody degradation was observed for any of the three products, as determined by SDS-PAGE followed by phosphor imager analyses.
In vitro stability testing
The in vitro stability was evaluated by three sets of experiments: The results of the first set, the validation productions according to GMP procedures, are shown in Table 1. 89Zr-Zevalin formulated in 20 ml 0.9% NaCl/gentisic acid 5 mg/ml (pH 5.0) can be stored for 48 h at 4°C and also at room temperature without decrease in radiochemical purity to a level below 95%. Immunoreactivity of the conjugate remained above 80% when stored at 4°C for 48 h and at room temperature for 24 h. This procedure resulted in a sterile final product with endotoxin levels <1.28 EU/ml (acceptance level <5 EU/ml).
In the second set, 89Zr-Zevalin and 90Y-Zevalin were joined in a formulation mixture to observe the damaging effect of radiolysis upon storage at high radioactivity concentration (111 MBq 90Y/ml). The radiochemical purity and immunoreactivity of both products were determined at various time points. Upon storage at 4°C for 4 h, the radiochemical purity of 89Zr-Zevalin remained >95%. At 24 h it decreased to 94.3%. The radiochemical purity of 90Y-Zevalin remained >96% during 24 h. For both products, immunoreactivity remained above 80% during this period. These data indicate that both conjugates will meet the clinical release specification as set for 90Y-Zevalin (radiochemical purity >95%), when the mixture of 89Zr-Zevalin with high-dose 90Y-Zevalin is administered to the patient within 4 h after production and formulation.
In the third set, 89Zr-Zevalin and 88Y-Zevalin were individually tested for stability in human serum. Both conjugates were stable in serum (radiochemical purity >95%) for a period of at least 6 days.
Biodistribution
For comparison of the biodistribution of 89Zr-Zevalin and 88Y-Zevalin, the two conjugates were co-injected in nude mice bearing Ramos B-cell tumours. At 24, 48, 72 and 144 h post injection, the average uptake (%ID/g, mean±SD) in tumour, blood, normal tissues and gastrointestinal contents was determined. These results are shown in Fig. 2. In general, 89Zr-Zevalin and 88Y-Zevalin showed similar uptake in tumour and most other organs at all time points. Significant differences (p<0.05) between the two conjugates were found at 72 and 144 h after injection in liver, thigh bone and sternum. Tumour uptake levels of 89Zr-Zevalin were 17.0±7.6%ID/g at 24 h, 20.9±4.4%ID/g at 48 h, 19.6±4.1%ID/g at 72 h and 9.8±2.5%ID/g at 144 h; for 88Y-Zevalin these levels were 16.7±8.3%ID/g at 24 h, 20.6±5.7%ID/g at 48 h, 20.7±4.6%ID/g at 72 h and 10.0±2.5%ID/g at 144 h. Blood levels slowly decreased from 14.6±2.0%ID/g at 24 h to 5.0±0.5%ID/g at 144 h for 89Zr-Zevalin and from 15.2±2.2%ID/g at 24 h to 5.4±0.6%ID/g at 144 h for 88Y-Zevalin.
PET imaging
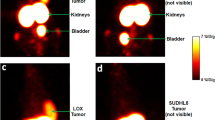
For evaluation of the clinical performance of 89Zr-Zevalin, a pilot PET imaging study (without RIT) was conducted in a patient with CD20+ B-cell NHL. The patient had undergone [18F]FDG-PET scanning previously, the scan indicating cervical, mediastinal, left caput humeri, splenic, para-aortic and inguinal tumour involvement (Fig. 3a, tumour lesions are indicated by arrows). Whole-body images obtained 96 h after administration of 89Zr-Zevalin revealed clear uptake of the conjugate in all tumour lesions (Fig. 3b). No increased uptake in normal organs was observed except for the liver. None of the tumour sites were clearly delineated on the early 89Zr-Zevalin-PET image (2 h p.i.), which showed mainly blood pool activity (diminishing over time) with visualisation of nose, heart, lungs, liver and spleen (Fig. 4).
a [18F]FDG-PET scan of the NHL patient. Coronal images from anterior (left) to posterior (right), with visualisation of cervical, mediastinal, left caput humeri, splenic, para-aortic and inguinal lymphomas. Localisations are indicated by white arrows. b 89Zr-Zevalin immuno-PET scan 96 h p.i. of the same NHL patient. Coronal images from anterior (left) to posterior (right); slices correspond to those of the [18F]FDG-PET scan. Tumour localisations are indicated by white arrows. Note targeting of tumour localisations also visualised by [18F]FDG-PET. Liver uptake (yellow arrow) is probably due to retention of 89Zr after catabolism of the conjugate
Discussion
An important issue in RIT is confirmation of tumour targeting and assessment of dose delivery, especially in high-dose RIT studies. This might be accomplished by performing an immuno-PET imaging procedure prior to RIT. At least three requirements need to be met for optimal use of an imaging radioimmunoconjugate as a predictor of the biodistribution of a therapeutic radioimmunoconjugate. First, imaging and RIT conjugate should have a similar biodistribution. Second, radionuclides used for imaging and RIT should have similar physical half-lives, preferably matching with the biological half-life of the MAb (typically 2–4 days for intact MAbs). Third, procedures for quantification of uptake and subsequent dose calculations should be reasonably accurate.
The choice of the positron emitter is an important factor for a successful pretherapy scouting procedure with PET. Only two long-lived positron emitters seem well suited for imaging intact MAbs, namely 89Zr (t 1/2=78.4 h) and iodine-124 (124I, t 1/2=100.3 h). Of these isotopes, 89Zr can be obtained in high yield and with high radionuclidic purity by a (p,n) reaction on 89Y, which is an attractive target material because of its 100% natural abundance [9]. As a result, production costs are relatively low. Moreover, 89Zr has no prompt gammas that can hamper image quality and accurate quantification [10]. Recent studies at our institute revealed that 89Zr residualises after catabolism, a phenomenon also observed with 90Y, 111In and lutetium-177 (177Lu) [13]. Such residualisation is not observed with, for example, rhenium-186 (186Re), 131I or 124I [14], and therefore 124I-Zevalin cannot be used for monitoring 90Y-Zevalin biodistribution.
89Zr cannot be stably bound by the chelate MX-DTPA. Therefore, we coupled a second chelate, N-sucDf, to Zevalin. The N-sucDf-to-MAb molar ratio was chosen to be kept below 1, since one cannot unlimitedly modify lysine groups without alteration of the biodistribution [15, 16]. Subsequent labelling of this double-chelator modified conjugate with 89Zr resulted in reproducible labelling yields and specific activities of at least 74 MBq/mg Zevalin, while preserving immunoreactivity and integrity of the MAb. Validation productions of 89Zr-Zevalin according to GMP showed reproducibility of labelling procedures, and the possibility of storing 89Zr-Zevalin in a clinical formulation for 48 h at 4°C and for 24 h at room temperature without unacceptable loss of radiochemical purity and immunoreactivity. In addition, we joined 89Zr-Zevalin and 90Y-Zevalin in a formulation mix at clinical dose concentration. The high β-energy of 90Y can result in radiolytic damage to the radiolabelled MAbs. 89Zr-Zevalin remained stable in this formulation solution, without impairment of immunoreactivity. This offers the flexibility to administer both conjugates either together in one single mixture, or separately directly after each other. Preceding the biodistribution study, the in vitro stability of 89Zr-Zevalin and 88Y-Zevalin in human serum was analysed. Stability of both conjugates under these conditions was comparable and high, and radiochemical purity was always above 95% for both radioimmunoconjugates over a period of 6 days. These results indicate that 89Zr had been coupled in a stable way to Zevalin, without affecting the quality of the MAb.
To demonstrate that the newly developed double-chelator modified Zevalin labelled with 89Zr can be used for localisation of the therapeutic Zevalin labelled with 90Y, a biodistribution study was conducted in nude mice bearing Ramos B-cell tumours. This study revealed that 89Zr-Zevalin and 88Y-Zevalin have a very comparable distribution (Fig. 2). It is of note that the apparent loss (in %ID/g) of the residualising 89Zr and 88Y in tumours at 144 h post injection relative to earlier time points is most probably the result of fast tumour growth, since the mean tumour masses at the time of dissection were 192±68 mg at 72 h compared with 406±162 mg at 144 h. Nevertheless, there were some differences (p<0.05) between 89Zr-Zevalin and 88Y-Zevalin in liver and bone at later time points. This difference in uptake in these organs was in the same range as previously observed in biodistribution studies with 89Zr- and 88Y-labelled MAbs [10, 13]. Differences in biodistribution were also reported between 111In- and 90Y-labelled Zevalin [17], with, for example, a difference of 1.7±0.3 versus 3.2±0.3%ID/g in bone at 72 h post injection for 111In-Zevalin versus 90Y-Zevalin, respectively. Deviating bone uptake is probably due to subtle differences in the in vivo stability of the 111In- and 90Y-DTPA complexes [18].
In the case of myeloablative RIT, where bone marrow toxicity is not dose limiting, 89Zr-Zevalin seems well suited to predict 90Y-Zevalin dosimetry. For prediction of dose delivery to bone marrow in non-myeloablative RIT, 89Zr-immuno-PET seems unsuitable owing to differing bone uptake of 89Zr and 90Y. Marrow dosimetry in this setting is challenging anyhow, particularly in the case of tumour involvement of the bone marrow.
For evaluation of the clinical performance of 89Zr-Zevalin PET, a pilot imaging study was conducted in one patient with CD20+ B-cell NHL. Serial PET camera images showed selective uptake in all tumour lesions that had previously been identified by [18F]FDG-PET. Evaluation of these 89Zr-PET images suggests that the favourable biodistribution of 89Zr-Zevalin would make such an NHL patient a suitable candidate for high-dose 90Y-Zevalin RIT.
The general aim of our forthcoming RIT study is to assess whether addition of high-dose 90Y-Zevalin RIT to standard conditioning regimens for AuSCT in the treatment of aggressive NHL is safe, tolerable and effective. Dose escalation will be performed at fixed dose steps (not individualised). The implementation of 89Zr-immuno-PET in these studies will reveal whether it is recommendable to exclude patients with an unfavourable biodistribution (e.g. no tumour targeting or >1,500 cGy to organs) from treatment with standardised high-dose RIT.
Conclusion
To enable clinical PET imaging of Zevalin, procedures were developed for stable and reproducible coupling of the long-lived positron emitter 89Zr. Similar in vitro stability and biodistribution in NHL-bearing nude mice suggest that 89Zr-Zevalin can be safely used for monitoring 90Y-Zevalin biodistribution in a clinical setting. A pilot PET imaging study with 89Zr-Zevalin in a patient with CD20+ B-cell NHL showed clear uptake of 89Zr-Zevalin in all previously known tumour deposits. PET with 89Zr-Zevalin seems attractive for the quantitative prediction of pharmacokinetics, biodistribution and dosimetry of 90Y-Zevalin in high-dose RIT.
References
Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol 2002;20:2453–2463
Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol 2002;20:3262–3269
Hagenbeek A, Lewington V. Report of a European consensus workshop to develop recommendations for the optimal use of 90Y-ibritumomab tiuxetan (Zevalin) in lymphoma. Ann Oncol 2005;16:786–792
Winter JN. Combining yttrium 90-labeled ibritumomab tiuxetan with high-dose chemotherapy and stem cell support in patients with relapsed non-Hodgkin’s lymphoma. Clin Lymphoma 2004;5 Suppl 1:22–26
Nademanee A, Forman S, Molina A, Fung H, Smith D, Dagis A, et al. A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood 2005;106:2896–2902
Verel I, Visser GWM, van Dongen GAMS. The promise of immuno-PET in radioimmunotherapy. J Nucl Med 2005;46 Suppl 1:164–171
Pauwels S, Barone R, Walrand S, Borson-Chazot F, Valkema R, Kvols LK, et al. Practical dosimetry of peptide receptor radionuclide therapy with 90Y-labeled somatostatin analogs. J Nucl Med 2005;46 Suppl 1:92–98
Lövqvist A, Humm JL, Sheikh A, Finn RD, Koziorowski J, Ruan S, et al. PET imaging of 86Y-labeled anti-Lewis Y monoclonal antibodies in a nude mouse model: comparison between 86Y and 111In radiolabels. J Nucl Med 2001;42:1281–1287
Verel I, Visser GWM, Boellaard R, Stigter-Van Walsum M, Snow GB, Van Dongen GAMS. Zr-89 immuno-PET: comprehensive procedures for the production of Zr-89-labeled monoclonal antibodies. J Nucl Med 2003;44:1271–1281
Verel I, Visser GWM, Boellaard R, Boerman OC, Van Eerd J, Snow GB, et al. Quantitative Zr-89 immuno-PET for in vivo scouting of Y-90-labeled monoclonal antibodies in xenograft-bearing nude mice. J Nucl Med 2003;44:1663–1670
Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA. Determination of the immunoreactive fraction of radiolabeled monoclonal-antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods 1984;72:77–89
Zijlstra JM, Hoekstra OS, Raijmakers PGHM, Comans EFI, van der Hoeven JJM, Teule GJJ, et al. 18FDG positron emission tomography versus 67Ga scintigraphy as prognostic test during chemotherapy for non-Hodgkin’s lymphoma. Br J Haematol 2003;123:454–462
Perk LR, Visser GWM, Vosjan MJWD, Stigter-van Walsum M, Tijink BM, Leemans CR, et al. 89Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals 90Y and 177Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J Nucl Med 2005;46:1898–1906
Verel I, Visser GWM, Boerman OC, Van Eerd JEM, Finn R, Boellaard R, et al. Long-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PET. Cancer Biother Radiopharm 2003;18:655–661
van Gog FB, Visser GWM, Klok R, van der Schors R, Snow GB, van Dongen GAMS. Monoclonal antibodies labeled with rhenium-186 using the MAG3 chelate: relationship between the number of chelated groups and biodistribution characteristics. J Nucl Med 1996;37:352–362
van Gog FB, Visser GWM, Stroomer JWG, Roos JC, Snow GB, van Dongen GAMS. High dose rhenium-186-labeling of monoclonal antibodies for clinical application: pitfalls and solutions. Cancer 1997;80:2360–2370
Chinn PC, Leonard JE, Rosenberg J, Hanna N, Anderson DR. Preclinical evaluation of 90Y-labeled anti-CD20 monoclonal antibody for treatment of non-Hodgkin’s lymphoma. Int J Oncol 1999;15:1017–1025
Camera L, Kinuya S, Garmestani K, Brechbiel MW, Wu C, Pai LH, et al. Comparative biodistribution of indium- and yttrium-labeled B3 monoclonal antibody conjugated to either 2-(p-SCN-Bz)-6-methyl-DTPA (1B4M-DTPA) or 2-(p-SCN-Bz)-1,4,7,10-tetraazacyclododecane tetraacetic acid (2B-DOTA). Eur J Nucl Med 1994;21:640–646
Acknowledgements
This project was financially supported by the Dutch Technology Foundation (STW, grant VBC.6120). The authors thank Schering Nederland BV (The Netherlands) for supply of Zevalin and for reviewing the manuscript, the technical staff of BV Cyclotron and the Radionuclide Centre for supply and processing of 89Zr, Jan H. Rector (Solid State Physics, VU university) for sputtering 89Y on copper supports, Adriaan A. Lammertsma for providing PET imaging facilities, and Yvonne W.S. Jauw and Ronald Boellaard for performing PET reconstructions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perk, L.R., Visser, O.J., Stigter-van Walsum, M. et al. Preparation and evaluation of 89Zr-Zevalin for monitoring of 90Y-Zevalin biodistribution with positron emission tomography. Eur J Nucl Med Mol Imaging 33, 1337–1345 (2006). https://doi.org/10.1007/s00259-006-0160-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0160-0