Abstract
Purpose
The widespread interest in 90Y internal radionuclide treatments has drawn attention to the issue of radiation protection for staff. Our aim in this study was to identify personnel at risk and to validate the protection devices used.
Methods
90Y-MoAb (Zevalin, 15 cases, 1.1 GBq/patient) and 90Y-peptide (90Y-DOTATOC) systemic (i.v., 50 cases, 3.0 GBq/patient) and locoregional (l.r., 50 cases, 0.4 GBq/patient) treatments were considered. Radiolabelling was carried out in a dedicated hot cell. Tele-tongs, shielded (PMMA: polymethylmethacrylate) syringes/vials and an automatic dose fractionating system were used. Operators wore anti-X-ray and anti-contamination gloves, with TLD dosimeters placed over the fingertips. For i.v. administration, activity was administered by a dedicated system; for l.r. administration, during activity infusion in the brain cavity, tongs were used and TLDs were placed over the fingertips. The air kerma-rate was measured around the patients.
Results
The use of devices provided a 75% dose reduction, with mean fingertip doses of 2.9 mGy (i.v. MoAbs), 0.6 mGy (i.v. peptides)/radiolabelling procedure and 0.5 mGy/l.r. administration. The mean effective dose to personnel was 5 μSv/patient. The air kerma-rate around the patients administered i.v. 90Y-peptides were 3.5 (1 h) and 1.0 (48 h) μGy/h at 1 m. Patient hospitalisation of 6 h (l.r.)/48 h (i.v.) guaranteed that the recommended limits of 3 mSv/year to family members and 0.3 mSv/year to the general population (Council Directive 97/43/Euratom) were respected.
Conclusions
When specific procedures are adopted, a substantial improvement in 90Y manipulation is attainable, reducing doses and increasing safety. For the widespread clinical use of 90Y-conjugates, a completely automatic labelling procedure is desirable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
90Y is a pure β-emitter with physical characteristics suitable for therapy (T 1/2=2.67 days; E max=2.27 MeV, E mean=939 keV; R 95=5.95 mm, R 50=5.3 mm in tissue [1]). Moreover, its chemical characteristics allow stable labelling of molecules which are able to specifically target tumour cells. Several anti-tumour radionuclide therapies have recently been developed with 90Y compounds. Among these, peptide receptor radionuclide therapy (PRRT) with 90Y-DOTATOC for the treatment of neuroendocrine tumours, pretargeting antibody-guided radiation therapy (PAGRIT) with 90Y-biotin for the treatment of glioblastoma and 90Y-ibritumomax tiuxetan (Zevalin) for the treatment of non-Hodgkin’s lymphoma have been employed [2–5]. The preparation and administration of these radiopharmaceuticals require the manipulation of high activities of 90Y [6]. During the different steps of therapy, hospital personnel may be exposed to intense radiation fields. In our centre, major efforts have been focussed on the improvement of radiation protection, in order to minimise doses to personnel.
However, a serious accident occurred several years ago [7] as a consequence of erroneous manipulation. A radiodermatitis was observed on the fingertips of an operator (Fig. 1). An in-depth investigation of the whole procedure was therefore conducted with a view to its optimisation. Several measurements were carried out in order to analyse the efficacy of the revised procedures. This paper presents the results obtained.
Radiodermatitis of three fingertips of an operator due to an inappropriate labelling procedure. a The inappropriate procedure responsible for the damage: manipulation of the vial without the use of tongs. b The radiodermatitis of the fingertips of the thumb (1), the index finger (2) and the middle finger (3) as it appeared 2 weeks after the exposure. c The lesions progressively recovered, with a sequela of telangiectasis (diagnosed by capillaroscopy)
Materials and methods
The therapies considered using 90Y radiocompounds are described in Table 1. The measurements were carried out during 50 procedures for the intravenous (i.v.) PRRT, 50 for the brain locoregional (l.r.) PRRT and 15 for the Zevalin treatments. Three main phases during which radiation risk can vary significantly were identified: the radiopharmaceutical preparation, its administration and hospitalisation of the patient. Details regarding the devices and instruments used for radiation protection in these different steps are listed in Table 2.
Radiopharmaceutical preparation
The labelling procedures were carried out in a dedicated hot lab, using a hot cell made of special materials to shield both 90Y β- rays and bremsstrahlung spectrum (characterised by a maximum at ∼80 keV and very few photons >300 keV).
In order to measure the dose to the fingertips, the operator wore thin plastic thimbles containing TLD detectors (Fig. 2a), under anti-X and latex gloves. 90Y was supplied as chloride diluted in HCl 0.05 M in a glass vial. Long tongs were always used to handle the vial and to transfer it to the dose calibrator and finally to a PMMA container (Fig. 2b).
Radiopharmaceutical preparation. a Fingertip monitoring: plastic thimbles containing TLD detectors (100 LiF chips) were used to measure the dose to the fingertips during labelling and l.r. injection. b Manipulation in the hot cell: the vial with 90Y was shielded by a PMMA container (2 cm thick) with a hole in the lid for the insertion of the needle of the syringe; the syringe was also shielded by a PMMA sleeve (2 cm thick). c For the i.v. infusion: automatic dose fractionating system inside the hot cell. The activity of the whole volume of the solution was fractionated by weight and activity into the activities prescribed for each patient
Inhalation of the radiopharmaceuticals was negligible, 90Y being non-volatile.
Generally, the preparation included the following steps:
-
1.
Measurement of the 90Y-supplied activity by a dose calibrator, set for the geometry in use [8–10]
-
2.
Labelling of the targeting molecules with 90Y, according to two different procedures that involve a different degree of manipulation:
-
Labelling carried out inside the 90Y original vial, using all the available activity (peptides)
-
Labelling carried out in a different reaction vial, using only a fraction of the available activity (Zevalin)
-
-
3.
Determination of the radiochemical purity by chromatographic techniques
-
4.
Fractionation with an automatic system of the whole radiocompound into the prescribed activities for each patient (Fig. 2c)
Finally, the vials were inserted in a cylindrical PMMA shield, surrounded by lead (i.v.) (Fig. 3a) or in PMMA-shielded syringes inside a PMMA case (l.r.) (Fig. 4).
Systemic administration (i.v.). a The radiopharmaceutical was provided in a vial shielded by a PMMA sleeve (1 cm thick), further surrounded by lead (1 cm thick). b For the i.v. injection, the radiopharmaceutical was slowly administered through a patented (RM 2002 A000071) infusion system. It consists of a PMMA container, to shield efficiently the 90Y beta particles, with a central cavity, in which the sterile glass vial containing the radiopharmaceutical is placed. The container is fitted with a screw cap with a hole through which two needles [a 18 G×1 1/2″ (1.2×40 mm) and a spinal 20 G×3 1/2″ (0.9×90 mm)] are connected with a set of sterile tubing. The shorter needle is connected to a saline bag on a pole; the spinal needle is connected to the patient. The infusion is started by allowing the saline solution to drip into the vial, thus forcing the radiopharmaceutical out to the patient i.v. port
Patient administration
Radiopharmaceuticals were administered systemically or locoregionally (into the brain).
For the i.v. injection, the radiopharmaceutical was administered through a patented infusion system (Fig. 3b, Table 2). The infusion started by allowing the saline solution to drip into the vial, thus forcing the radiopharmaceutical out to the patient i.v. port. This method, used for both PRRT and Zevalin therapies, was developed to avoid the handling of syringes, and hence reduce the radiation burden to the physician; in addition, this technique reduces the risk of vein rupture and extravascular injection.
The duration of the infusion ranged between 10 and 30 min, depending on clinical requirements. The termination of the infusion was checked by measuring the dose rate outside the vial. During the i.v. administration, film badge and TLD ring dosimeters were provided and protective aprons were available.
In the l.r. administrations, small volumes of radiopharmaceutical were injected in a short time (<1 min). Shielded syringes and tongs (to hold the filter between syringe and injection site) were used. Plastic thimbles containing TLD dosimeters were placed over the fingertips under anti-X gloves (Fig. 2a).
Patient hospitalisation
After injection, patients were admitted to a dedicated department until they attained the discharge conditions laid down by the Italian radiation protection regulations [11, 12].
In-patient rooms did not require fixed radiation monitors or particular shielding, but only toilets connected to a system of tanks. Adult relatives, excluding pregnant women, were allowed to assist the patients.
The air kerma rate at various distances from patients was measured by an ionisation chamber. The effective dose to personnel (the equivalent dose at a body depth of 10 mm, defined as Hp10) was evaluated by film badge dosimeters. Personnel also wore TLD ring dosimeters during both injection and patient assistance.
Results
Radiopharmaceutical compounding and l.r. administration were identified as the procedures at higher risk.
The absorbed doses to the fingertips during labelling procedures, normalised to the manipulation of 3 and 1.5 GBq for 90Y-PRRT and Zevalin, respectively, are listed in Table 3. Despite the lower activity manipulated during radiolabelling of MoAbs (Zevalin), the dose to fingertips was higher owing to the greater complexity of the procedure, compared with PRRT labelling. The PMMA shield containing 90Y vials and syringes, the hot cell, the automatic fractionation system and the anti-X gloves proved to be useful for radiation protection optimisation. In particular, the dose fractionation system allowed a ∼60% reduction in fingertip doses (0.6 vs 1.6 mGy, Table 3). The effective dose to the operator was negligible (<10 μSv/labelling), as expected.
The bremsstrahlung irradiation of the personnel during systemic administration and assistance of patients was very low (physician’s hands <0.1 mGy/patient; nurse’s and physician’s thorax <5 μGy/patient).
The doses to physician fingertips during l.r. injection showed a significant 75% (0.5 vs 1.9 mGy, Table 4) dose reduction when tongs and shielded syringes were used. Results normalised to a single injection of 0.4 GBq of 90Y conjugates are listed in Table 4.
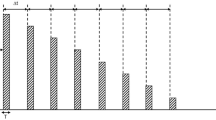
The mean values of the air kerma rate (μGy/h) at different distances (5, 20, 50 and 100 cm) from the patients, at 1, 24 and 48 h p.i. are shown in Fig. 5. Data refer to PRRT (i.v.) treatments, being higher than both the injected activities and the number of measurements. In any case, Zevalin data were comparable to the literature data [6].
The air kerma rate data were used to estimate the effective dose to the patient visitors. Effective dose limits of 3 mSv (family members) and 0.3 mSv/year (population) [11, 12]) were guaranteed the patients being hospitalised for almost 6 h (l.r. PRRT and Zevalin) and for 48 h (i.v. PRRT).
Discussion
In the past decade, 90Y has been widely used for the labelling of tumour targeting molecules. The suitability of its physical characteristics and the encouraging clinical results obtained will lead to increasing use in the future. As a pure β-emitter, the low intensity radiation field, mainly due to bremsstrahlung, represents a consistent advantage (specific bremsstrahlung constant for 90Y in soft tissue: ∼1.4×10−4 C·kg−1·cm2·MBq−1·h−1 for the reference man [13]). In general, restrictive radiation precautions for patient management are not required, as opposed to the case with 131I therapies. In contrast, major attention has to be paid to the preparation and administration of the radiopharmaceutical owing to the possible direct irradiation of the hands by an intense field of high-energy β-particles. This applies especially when very high activities are handled. Some PRRT protocols involve administrations of up to 5 GBq of 90Y peptides per patient, frequently leading to the manipulation of more than 20 GBq for the treatment of a few patients in a single session.
The literature in the field of radiation protection concerning 90Y focusses mainly on the important guidelines for calibration procedures and on the health care of patients and relatives. Data reporting the doses to personnel, on the other hand, are rare [7, 14, 15].
In our centre, 90Y radiocompounds have been used over the past 10 years. The accuracy and planning of radiation protection procedures has improved over time, with strict monitoring of the doses to personnel by means of TLD ring and film badge dosimeters. The results of these controls have always been well below the prescribed ICRP limits for the exposed workers. However, a serious accident occurred 8 years ago [7]. A few days after performing a labelling procedure, a radiodermatitis was observed on a limited and well-defined area of the tips of the thumb, the index finger and the middle finger of the left hand of an operator (Fig. 1b).
Despite the use of anti-X and disposable gloves, skin lesions were induced during a manual radiopharmaceutical fractionation, as the operator directly held a vial containing 16.7 GBq of 90Y for about 10 s without using the available tongs (Fig. 1a). The estimated dose to the damaged tissues (<1 cm2/finger) was 12 Gy [16], while the readout of the TLD ring dosimeter, worn for 1 month, indicated 70 mGy, including routine work. A complete recovery occurred within about 6 months (Fig. 1c). However, a sequela of telangiectasis is still detectable by capillaroscopy.
This unexpected accident, which occurred at the early stages of our activity, led us to conduct an in-depth analysis of the different phases of therapy. For safety purposes, it was decided to make use of a second operator to supervise all radiochemical procedures, in order to avoid oversights. To develop more appropriate radiation protection rules, systematic measurements were planned.
The duration of the procedures was measured and compared among different operators. Steps at highest risk were identified as those associated with prolonged duration and/or to reduced distance. These were typically related to greater experience and manual training of the personnel, as well as the radiation dose received. The dosimeter readouts of the operators also usually showed a progressive decrease after a few procedures. These outcomes, quite expected, prompted us to establish an accurate training programme for personnel selected to perform the radiolabelling procedure. The programme consists of a theoretical section on the shielding requirements for beta-emitting radionuclides and two practical dummy preparations in which all the radiolabelling steps are performed without any radioactivity. The practical training is conducted by experienced radiochemists since these professionals are more accustomed to working with tools such as tongues etc. than physicians and/or other nuclear medicine personnel.
This study also highlights the fact that not only the labelling procedure but also l.r. injection into the brain involves a high exposure risk owing to the relatively short distance between the physician’s hands and the injection site, and to the care required. The absorbed dose to the hands (to the fingertips) requires accurate monitoring, possibly with specific dosimeters. Manipulation time must be minimised by training. The use of automatic fractionation systems is very efficient in reducing fingertip doses during labelling, and this is strongly recommended. Tongs and PMMA shields for both vials and syringes are particularly recommended during both radiolabelling and administration. The use of these devices resulted in a consistent dose reduction of up to 75% (0.5 vs 1.9 mGy, Table 4).
The measurements evidenced a wide range of doses, related not only to the specific labelling procedure but also to personal skill and experience and to possible mishaps (radiopharmaceutical quality, catheter viability, etc.). Therefore, the maximum values of the doses listed in Tables 3 and 4 do not represent accidents or serious problems encountered, but the occurrence of minor influencing events and/or incomplete adherence to the radiation protection rules (especially due to prolonged duration of procedures, reduced distance or inadequate shielding). In particular, upon interviewing the staff, the quality of the reagents (isotopes) turned out to be a typical source of prolonged reaction time, along with additional quality control.
The fingertip doses reported in Tables 3 and 4 are higher than those of other high-risk procedures, such as the treatment of hepatic tumours with 90Y-microspheres (mean value ∼0.3 mSv, range 0.01–1.3 mSv, per treatment [15]), or the radiographically guided endovascular treatment of abdominal aortic aneurysms (mean 0.35 mSv per treatment [17]). Just for comparison, the mean finger dose to nuclear medicine staff exposed to diagnostic procedures is of the order of 20 mSv/year [17].
In order to adhere to the dose limit for the hands (500 mSv/year), it is recommended that the dose received be monitored for each procedure, with restriction of the number of allowed procedures/year/operator if need be.
The doses measured by the “conventional” ring dosimeter provide only a rough estimation of the dose to the whole hand, and cannot reveal local “hot spots”. Therefore, the regular monitoring of fingertip doses is strongly recommended.
As for the effective dose due to bremsstrahlung, the results confirmed a negligible degree of radiation exposure to the personnel. The air kerma rate around patients is lower than the air kerma rates related to the most common nuclear medicine examinations [18]. The mean values shown in Fig. 5 (28 and 4 μGy/h at 5 cm and at 1 m from the patient, 1 h p.i.) are also lower than the values reported for other therapeutic procedures (90Y-microspheres injected in the liver for non-resectable hepatic tumours: 135 and 6 μSv/h on contact and at 1 m from patients [15]).
All these considerations guarantee safety to the personnel and relatives during patient assistance. Care must be taken to avoid possible contamination from biological fluids. The prescribed duration of almost 2 days of hospitalisation, adopted in our centre, is related not to the safety requirements of radiation protection but to the need for compliance with Italian regulations [12], which are very restrictive concerning the disposal of radioactivity directly into the environment. The publication ICRP 94 [19] contains detailed indications for the release of patients after radionuclide therapy. Naturally, the hospitalisation of the patients must in many cases be prolonged owing to clinical needs.
Conclusion
Awareness of the real risks involved represents the basis of radiation protection optimisation. Our results confirm that radiolabelling, administration and hospitalisation can be carried out in conditions of acceptable safety. The use of a fractionation system and of PMMA shields is strongly recommended, as is the regular use of dedicated fingertip TLD dosimeters. This study provides useful indications to any centre which is administering therapy with 90Y compounds. In the near future, a marked increase in the number of therapeutic procedures with 90Y conjugates is expected. For this reason, a centralised radiopharmacy and/or a fully automated labelling system should be developed.
References
Delacroix D, Guerre JP, Leblanc P, Hickman C. Radionuclide and radiation protection data handbook 2002, 2nd edition. Rad Prot Dos 2002;98:1–168
Bodei L, Cremonesi M, Grana C, Rocca P, Bartolomei M, Chinol M, et al. Receptor radionuclide therapy with 90Y-[DOTA]0-Tyr3-octreotide (90Y-DOTATOC) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2004;31:1038–1046
Schumacher T, Hofer S, Eichhorn K, Wasner M, Zimmerer S, Freitag P, et al. Local injection of the 90Y-labelled peptidic vector DOTATOC to control gliomas of WHO grades II and III: an extended pilot study. Eur J Nucl Med Mol Imaging 2002;29:486–493
Paganelli G, Grana C, Chinol M, Cremonesi M, De Cicco C, De Braud F, et al. Antibody-guided three-step therapy for high grade glioma with yttrium-90 biotin. Eur J Nucl Med Mol Imaging 1999;26:348–357
Witzig TE, Flinn IW, Gordon LI, Emmanouilides C, Czuczman MS, Saleh MN, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol 2002;20:3262–3269
Wagner HN, Wiseman GA, Marcus CS, Nabi HA, Nagle CE, Fink-Bennett DM, et al. Administration guidelines for radioimmunotherapy of non-Hodgkin’s lymphoma with 90Y-labeled anti-CD20 monoclonal antibody. J Nucl Med 2002;43:267–272
Tosi G. Report on one accident occurred in a nuclear medicine department in Italy. In: Proceedings of the 6th European ALARA Network workshop. Occupational Exposure Optimisation in the Medical Field and Radiopharmaceutical Industry. Sponsored by the European Commission Radiation protection. Madrid; 2002;225
Salako QA, DeNardo SJ. Radioassay of yttrium-90 radiation using the radionuclide dose calibrator. J Nucl Med 1997;38:723–726
Siegel JA, Zimmerman BE, Kodimer K, Dell MA, Simon WE. Accurate dose calibrator activity measurement of 90Y-ibritumomab tiuxetan. J Nucl Med 2004;45:450–454
Zimmerman BE, Cessna JT. Experimental determinations of commercial ‘dose calibrator’ settings for nuclides used in nuclear medicine. Appl Radiat Isot 2000;52:615–619
Council Directive 97/43/Euratom 1997 on health protection of individuals against the dangers of ionising radiation in relation to medical exposure. Available on http://europa.eu.int/comm/energy/nuclear/ radioprotection/doc/legislation/9743_en.pdf
Actuation of the Council Directive 97/43/Euratom 1997 in the Italian regulation (Decreto Legislativo 26 maggio 2000, n. 187. Attuazione della direttiva 97/43/EURATOM in materia di protezione sanitaria delle persone contro i pericoli delle radiazioni ionizzanti connesse ad esposizioni mediche). Available on http://www.camera.it/ parlam/leggi/deleghe/testi/00187dl.htm
Zanzonico PB, Binkert BL, Goldsmith SJ. Bremsstrahlung radiation exposure from pure beta-ray emitters. J Nucl Med 1999;40:1024–1028
Aubert B, Guilabert N, Lamon A, Ricard M. Which protection against radiation for new protocols of internal radiotherapy by 90Y. In: Proceedings of the 6th European ALARA Network workshop. Occupational Exposure Optimisation in the Medical Field and Radiopharmaceutical Industry. Sponsored by the European Commission Radiation protection. Madrid 2002;47–49
Sarfaraz M, Kennedy AS, Cao ZJ, Sackett GD, Yu CX, Lodge MA, et al. Physical aspects of yttrium-90 microsphere therapy for nonresectable hepatic tumors. Med Phys 2003;30:199–203
Stabin M, Siegel J, Lipsztein J, Hunt J, Brill A, Sparks R, et al. RADAR (RAdiation Dose Assessment Resource). http://www.doseinfo-radar.com; http://www.ieo.it/radar
Saether HK, Davidson TM, Widmark A, Wohni T. Measurements of finger doses in x-ray guided surgery, nuclear medicine and research. Radiat Prot Dosimetry 2005;113:392–395
Cremonesi M, Ferrari M, Sacco E, Rossi A, Leonardi L, Chinol M, et al. Radiation protection in radioguided surgery of breast cancer. Nucl Med Commun 1999;20:919–925
Release of patients after therapy with unsealed radionuclides. International Commission on Radiological Protection, ICRP Publication 94, 2005. http://www.elsevier.com
Acknowledgement
The authors are especially grateful to William Russell Edu for English revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cremonesi, M., Ferrari, M., Paganelli, G. et al. Radiation protection in radionuclide therapies with 90Y-conjugates: risks and safety. Eur J Nucl Med Mol Imaging 33, 1321–1327 (2006). https://doi.org/10.1007/s00259-006-0151-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0151-1