Abstract
Purpose
To assess 18F-fluorodeoxyglucose (FDG) uptake in bone metastases in patients with and without previous treatment, and compare positive positron emission tomography (PET) with osteolytic or osteoblastic changes on computed tomography (CT).
Methods
One hundred and thirty-one FDG-PET/CT studies were reviewed for bone metastases. A total of 294 lesions were found in 76 patients, 81 in untreated patients and 213 in previously treated patients. PET was assessed for abnormal FDG uptake localised by PET/CT to the skeleton. CT was evaluated for bone metastases and for blastic or lytic pattern. The relationship between the presence and pattern of bone metastases on PET and CT, and prior treatment was statistically analysed using the chi-square test.
Results
PET identified 174 (59%) metastases, while CT detected 280 (95%). FDG-avid metastases included 74/81 (91%) untreated and 100/213 (47%) treated lesions (p<0.001). On CT there were 76/81 (94%) untreated and 204/213 (96%) treated metastases (p NS). In untreated patients, 85% of lesions were seen on both PET and CT (26 blastic, 43 lytic). In treated patients, 53% of lesions were seen only on CT (95 blastic, 18 lytic). Of the osteoblastic metastases, 65/174 (37%) were PET positive and 98/120 (82%), PET negative (p<0.001).
Conclusion
The results of the present study indicate that when imaging bone metastases, prior treatment can alter the relationship between PET and CT findings. Most untreated bone metastases are PET positive and lytic on CT, while in previously treated patients most lesions are PET negative and blastic on CT. PET and CT therefore appear to be complementary in the assessment of bone metastases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many malignancies develop bone metastases which may lead to complications including pain, hypercalcaemia, fracture and spinal cord compression. Early identification of skeletal metastases may lead to changes in patient management. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) is playing an increasing role in the assessment of cancer patients. There are, however, conflicting reports regarding the sensitivity of FDG-PET for the diagnosis of bone metastases [1–5]. PET appears to be superior to conventional bone scintigraphy using 99mTc-methylene diphosphonate owing to its higher spatial resolution and routinely available tomographic images. The pathophysiological mechanism of tracer uptake by which these two functional techniques detect skeletal tumours is different, with bone scintigraphy identifying an osteoblastic response [6, 7] while FDG uptake is related to increased intratumoural glycolysis [8].
FDG-PET has been previously shown to have an increased sensitivity for the detection of predominantly osteolytic metastases in breast cancer as compared with blastic lesions, these findings suggesting that sclerotic bone metastases may have a lower affinity for FDG [9]. There appear to be significant differences in the performance of FDG-PET for the detection of bone metastases among various tumour types, with a lower sensitivity in prostate cancer than in lung neoplasms [10].
Several potential variables may affect FDG uptake in bone metastases. Previously reported discrepancies in anatomical and functional imaging of bone metastases [9, 11] could be explained by the fact that following treatment, previously lytic skeletal lesions may show sclerotic changes on computed tomography (CT) and become metabolically inactive and therefore sometimes FDG non-avid. The present study addresses the issue of a potential difference in the FDG avidity of bone metastases on PET depending on whether a patient has previously received treatment, as well as the question of whether there is a relationship between abnormal FDG uptake in skeletal metastases, prior systemic anti-cancer therapy, and sclerotic or lytic bone lesions on CT.
Materials and methods
Patients
FDG-PET/CT studies performed in 131 cancer patients as part of their routine work-up during a period of 12 months were retrospectively evaluated for the presence of bone metastases on the PET and CT components of the hybrid imaging study. The patients gave their informed consent for performance of the study and for retrospective evaluation of their files.
Bone metastases were found on PET, CT or both imaging modalities in 76 patients, who represent the final study population. Patients showing either only skeletal lesions characterised as benign on CT or only abnormal FDG foci with no further confirmation for bone metastases were not included in the further analysis.
The patient population included 41 female and 35 male patients with a mean age of 55.8 years (range 21–79 years). The primary malignancies included 57 solid tumours (19 patients with lung cancer, 15 with breast cancer, eight with colon cancer, three each with gastric cancer and sarcoma, two each with prostate and renal cell cancer, and one each with thyroid, bladder, cervical and nasopharyngeal cancer and melanoma), as well as 19 patients with lymphoma. Fifteen patients were evaluated at diagnosis and 61 during follow-up or for suspected recurrence. A total of 294 bone metastases were evaluated, these being located in the spine (n=119), pelvis (n=95), thoracic cage (sternum, ribs, scapulae) (n=57) and appendicular skeleton (n=23). In 36 patients a single bone metastasis was detected while 40 patients showed multiple bone lesions. Thirty-one patients showing 81 bone metastases had not received any previous systemic treatment for their primary tumour. Forty-five patients with 213 bone metastases were evaluated following the administration of treatment including chemotherapy (28 patients with 136 lesions), radiotherapy (three patients with seven lesions) or a combination of both (14 patients with 70 lesions). The time interval between the PET/CT study and previous treatment ranged between 1 and 24 months (mean 2.7 months), with 35 patients being evaluated at less than 3 months, five at 3–12 months and five at more than 12 months after therapy. None of the 17 patients who had previously received radiation treatment had received radiotherapy targeting the sites of bone metastases that were evaluated in the present study.
Patients were instructed to fast, except for glucose-free oral hydration, for 4–6 h before the injection of 370–555 MBq (10–15 mCi) 18F-FDG. PET and non-contrast-enhanced CT were acquired consecutively 60–90 min after the injection of FDG, using a PET/CT system (Discovery LS, GE Healthcare Technologies, Milwaukee, USA), combining a third-generation multislice spiral CT with a dedicated full-ring PET scanner with BGO crystals. The PET and CT devices are mechanically aligned back to back, and share a common table. Proper registration of the two images is ensured by shared positional information of the table and patient for both the CT and the PET acquisition. Data obtained from the CT were used for low-noise attenuation correction of PET emission data and for fusion with attenuation-corrected PET images. PET images were reconstructed iteratively using ordered subset expectation maximisation software (OSEM). PET, CT and fused PET/CT images were available for review, displayed in axial, coronal and sagittal planes. The PET data were displayed as non-corrected and attenuation-corrected images and also in a rotating maximum intensity projection (MIP).
Analysis of data
Two nuclear medicine physicians and two skeletal radiologists reviewed all studies. PET/CT was used as a localising tool, to ensure that the same lesion was assessed on PET and on CT. Diagnosis of bone metastases was based on previously described criteria [12–14]. These included the presence of focal abnormal FDG uptake localised by PET/CT to bony structures, which showed osteoblastic, osteolytic or mixed lytic-sclerotic lesions on the CT component of the study, with or without associated intramedullary changes and/or soft tissue abnormalities. The diagnosis of bone metastases was histologically confirmed by biopsy in four patients with single bone lesions. In 56 patients, the diagnosis and the location of bone metastases were confirmed by additional imaging studies showing the same findings and/or evidence of progression or improvement of the skeletal metastatic involvement following treatment. These included follow-up PET/CT studies in 18 patients (eight with single lesions), separate diagnostic CT examinations in 16 patients (eight with single lesions), bone scintigraphy in 19 patients (11 with single lesions) and MRI in three patients (one with a single lesion). In 16 patients (four with single lesions) the diagnosis of bone metastases was based on matched findings in the present PET/CT study, intense focal FDG uptake on the PET component being associated with definite characteristic morphological changes of metastases on the CT component in the same location [14].
The presence or absence of each bone metastasis on PET and on CT was recorded. For the CT component, each bone lesion was further defined as showing osteolytic, osteoblastic or mixed lytic and blastic changes. For further CT data analysis, osteolytic and mixed (lytic and blastic) lesions were combined as a single group. The presence of malignant skeletal lesions on both PET and CT, or on only one of the study components, was recorded. The relationship between FDG avidity, lytic or sclerotic changes on CT, and previous treatment was recorded for each bone metastasis.
The chi-square test was used to assess the differences in the number of treated and untreated bone metastases detected on PET and CT. The same statistical analysis was performed for evaluation of the amount of lytic and blastic metastases showing FDG uptake in relationship to previous treatment. A p value <0.05 was considered significant. In addition, the same data analysis was separately performed in two subgroups of patients with breast cancer and lymphoma. These patients showed a sufficiently large number of bone lesions to permit comparative statistical assessment.
Results
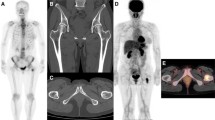
Two hundred and ninety-four bone metastases were diagnosed in 76 PET/CT studies, including 81 (28%) in previously untreated and 213 (72%) in patients previously treated with systemic anti-cancer therapy. FDG avidity was demonstrated in 174/294 bone metastases (59%) and CT detected 280 malignant bone lesions (95%) (Table 1). A concordant pattern of FDG uptake and structural changes on CT was seen in 160/294 bone metastases (54%) (Fig. 1). A discordant pattern was found in 120 lesions (41%) detected on CT and negative on PET, as well as in 14 lesions (5%) showing abnormal uptake on PET with no corresponding findings on CT (Figs. 2, 3).
A 40-year old woman with breast cancer (same patient as in Fig. 2) at 6 months following institution of chemotherapy. There is a sclerotic lesion in the posterior arch of the T8 vertebra on CT (right), with no FDG uptake as demonstrated on the PET/CT image (left)
Of the untreated metastases, 74/81 (91%) showed abnormal FDG uptake. CT identified 76 lesions (94%) (p NS), with sclerotic changes in 29 and lytic changes in 47 metastases. Combined assessment of both modalities showed a congruent pattern in 69 lesions (85%), with FDG uptake in 26 CT-sclerotic lesions and in 43 CT-lytic metastases. An incongruent pattern was found in 12 metastases (15%), with seven lesions seen only on CT (three blastic, four lytic) and five lesions showing only abnormal FDG uptake. Untreated bone metastases showed a predominant pattern of PET-positive CT-lytic lesions (53%). Eight of the 14 patients (57%) with multiple untreated bone metastases showed the same pattern of FDG avidity and structural changes on the CT component in all lesions.
In previously treated patients 100/213 lesions (47%) showed abnormal FDG uptake and 204 lesions (96%) were seen on CT (p<0.001), with sclerotic changes in 134 and lytic changes in 70 metastases. Combined assessment of both modalities showed a congruent pattern in 91 lesions (43%), with FDG uptake in 39 CT-sclerotic and in 52 CT-lytic lesions. An incongruent pattern was found in 122 metastases (57%), with 113 lesions positive only on CT (blastic 95, lytic 18) and nine showing only increased FDG uptake. Treated bone metastases showed a predominant pattern of FDG-negative CT-sclerotic lesions (45%). Eighteen of the 26 patients (69%) with multiple treated bone metastases showed the same pattern of FDG avidity and structural changes on the CT component in all lesions.
There was a statistically significant difference (p<0.001) between the FDG avidity of skeletal metastases in untreated and previously treated patients. There was no statistically significant difference between the number of bone metastases diagnosed on CT in patients evaluated before and after treatment. There was a statistically significant difference between the number of blastic (29/81 vs 134/213, p<0.001) and lytic (47/81 vs 70/213, p<0.001) lesions before and after treatment, between the number of PET- and CT-positive lesions in untreated (69/81) and previously treated patients (91/213, p<0.001), and between the number of PET-negative, CT-positive lesions (7/81 vs 114/213, p<0.001). The data summarising the number of bone metastases positive or negative on PET and CT in relationship to previously administered treatment for the whole study group and for the selected subgroups of patients are presented in Tables 1 and 2.
Discussion
The degree of FDG uptake in bone metastases may be related to the morphological appearance of the metastases or be dependent on the primary tumour type. While sclerotic metastases may be negative on FDG-PET, it is unknown whether these findings reflect differences in the metabolic activity of individual cancer lesions or are due to the presence of a smaller volume of malignant cells in osteoblastic lesions [9]. FDG-PET has been shown to perform poorly for the diagnosis of bone metastases in prostate cancer, with a sensitivity as low as 18% in a study population including mostly previously treated patients [15]. In a selected population of untreated patients a sensitivity of 65% has been reported [16]. In contrast, in lymphoma, FDG-PET has been shown to have a higher sensitivity than routine bone scintigraphy [17].
The main purpose of present study was to assess whether there is a relationship between a history of previous anti-cancer treatment and the presence of increased FDG uptake on PET reflecting altered skeletal metabolism in bone metastases and, if so, to what extent these findings could be related to differences in morphological features on CT. Since about one-third of bone metastases assessed in the present study were identified in patients who had not received previous treatment, it was also possible to compare the different PET and CT patterns in patients with and without a history of previous treatment.
The present results indicate that PET and CT have a similar detectability rate for bone metastases in untreated patients of 91% and 94% respectively. In patients who had previously received therapy, however, the detection rate of bone metastases on CT was 96% as compared to 47% on FDG-PET. These findings were demonstrated for the whole study group, as well as when two homogeneous subgroups of patients with single malignancies, breast cancer and lymphoma involving the bone, were analysed and compared, and therefore appear to be consistent. The clinical relevance of these findings is as yet unknown. They potentially represent a direct effect of successful therapy on bone metastases, leading to the transformation of lytic, metabolically active disease into sclerotic, metabolically inactive metastases. This hypothesis, however, requires further assessment in a more homogeneous study population, with long-term follow-up.
Cook et al. evaluated 23 patients with previously treated metastatic breast cancer, the majority of whom had progressive disease [9]. Sclerotic lesions were either not visualised by FDG-PET or had lower SUVs when compared with mixed or lytic lesions, and patients showing osteoblastic metastases had a better prognosis [9]. In the present series only 40% of sclerotic bone metastases showed FDG uptake as compared with 81% of lytic lesions. The majority of lesions in the untreated group (53%) were lytic and FDG avid, while in patients who had been previously treated, 45% of bone metastases were sclerotic on CT and negative on PET.
The limitations of our study are related mainly to the inability to assess any potential relationship between the presence of FDG uptake and the CT pattern separately for each histological tumour type, and to the time elapsed since previous treatment. This inability is due to the cross-sectional nature and the heterogeneity of the patient population in this initial series. Also, more extensive data on patient outcome would have been of value in an attempt to further understand the clinical significance of congruent or discordant PET and CT patterns in previously treated and untreated patients.
The implications of a multi-modality assessment of bone metastases using combined patterns derived from anatomical (CT) and metabolic (FDG-PET) imaging techniques need to be further clarified. Following the present initial observation of a significant difference in FDG uptake and morphological changes in bone metastases related to a history of treatment, appropriately designed prospective longitudinal trials correlated with clinical outcome in the individual patient need to be performed.
Discrepant FDG avidity on PET in bone metastases of malignant tumours such as lung and prostate cancer [10] suggest that other factors, such as tumour-specific metabolic rates, may play a significant role in the degree of FDG avidity of individual bone metastases, in addition to their structural pattern. The conclusion from a previous study suggesting that only FDG-positive lesions represented “active” bone disease [11] raises the question as to whether FDG-negative bone lesions that are detected by PET/CT are of clinical significance. If proven to be of value by further studies, FDG-PET may become the tool to differentiate between clinically significant and “burnt-out” bone metastases detected with high-resolution CT, using hybrid PET/CT imaging.
In conclusion, the results of the present study show a similar detectability rate for untreated bone metastases with both PET (91%) and CT (94%). In previously treated patients, CT identified a significantly higher number of bone metastases (96%) as compared with PET (47%), with a predominance of PET-negative, CT-positive osteoblastic lesions.
References
Lonneux M, Borbath II, Berliere M, Kirkove C, Pauwels S. The place of whole-body PET FDG for the diagnosis of distant recurrence of breast cancer. Clin Positron Imaging 2000;3:45–49
Ohta M, Tokuda Y, Suzuki Y, Kubota M, Makuuchi H, Tajima T, et al. Whole body PET for the evaluation of bony metastases in patients with breast cancer: comparison with 99Tcm-MDP bone scintigraphy. Nucl Med Comm 2001;22:875–879
Yang SN, Liang JA, Lin FJ, Kao CH, Lin CC, Lee CC. Comparing whole body 18F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol 2002;128:325–328
Moon DH, Maddahi J, Silverman DH, Glaspy JA, Phelps ME, Hoh CK. Accuracy of whole-body fluorine-18-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med 1998;39:431–435
Gallowitsch HJ, Kresnik E, Gasser J, Kumnig G, Igerc I, Mikosch P, et al. F-18 fluorodeoxyglucose positron-emission tomography in the diagnosis of tumor recurrence and metastases in the follow-up of patients with breast carcinoma: a comparison to conventional imaging. Invest Radiol 2003;38:250–256
Fogelman I. Skeletal uptake of diphosphonate: a review. Eur J Nucl Med 1980;5:473–476
Cook GJ, Fogelman I. The role of nuclear medicine in monitoring treatment in skeletal malignancy. Semin Nucl Med 2001;31:206–211
Warburg O. The metabolism of tumors.New York: Smith; 1931; p 129–169
Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol 1998;16:3375–3379
Garcia JR, Simo M, Perez G, Soler M, Lopez S, Setoain X, et al. 99mTc-MDP bone scintigraphy and 18F-FDG positron emission tomography in lung and prostate cancer patients: different affinity between lytic and sclerotic bone metastases. Eur J Nucl Med Mol Imaging. 2003;30:1714
Morris MJ, Akhurst T, Osman I, Nunez R, Macapinlac H, Siedlecki K, et al. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology 2002;59:913–918
Nakai T, Okuyama C, Kubota T, Yamada K, Ushijima Y, Taniike K, et al. Pitfalls of FDG-PET for the diagnosis of osteoblastic bone metastases in patients with breast cancer. Eur J Nucl Med Mol Imaging 2005;32:1253–1258
Nakamoto Y, Cohade C, Tatsumi M, Hammoud D, Wahl RL. CT appearance of bone metastases detected with FDG PET as part of the same PET/CT examination. Radiology 2005;237:627–634
Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of view SPECT, 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med 2006;47:287–297
Yeh SD, Imbriaco M, Larson SM, Garza D, Zhang JJ, Kalaigian H, et al. Detection of bony metastases of androgen-independent prostate cancer by PET-FDG. Nucl Med Biol 1996;23:693–697
Shreve PD, Grossman HB, Gross MD, Wahl RL. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology 1996;199:751–756
Moog F, Kotzerke J, Reske SN. FDG PET can replace bone scintigraphy in primary staging of malignant lymphoma. J Nucl Med 1999;40:1407–1413
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Israel, O., Goldberg, A., Nachtigal, A. et al. FDG-PET and CT patterns of bone metastases and their relationship to previously administered anti-cancer therapy. Eur J Nucl Med Mol Imaging 33, 1280–1284 (2006). https://doi.org/10.1007/s00259-006-0141-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0141-3