Abstract
Purpose
It has been suggested that the use of computed tomography (CT) positive contrast agents has led to attenuation-induced artefacts on 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET/CT) systems. Consequently, centres may withhold the use of such agents. Whilst there is theoretical evidence to support the aforementioned claim, the clinical relevance of the induced artefacts has not been widely established. Moreover, the potential benefits of bowel enhancement on PET/CT have yet to be formally evaluated. We therefore prospectively examined PET/CT studies to assess whether the use of oral contrast medium induces clinically relevant artefacts and whether the use of these agents is diagnostically helpful.
Methods
Over a 2-month period, 18F-FDG PET/CT images were prospectively reviewed from 200 patients following Gastrografin administration 2 h prior to examination. Both a radiologist and a nuclear medicine physician reviewed the images for contrast medium-mediated clinically relevant artefacts. Artefacts were sought on the CT attenuation-corrected images and were compared with the appearance on non-attenuated-corrected images. The number of examinations in which the oral contrast aided image interpretation was also noted.
Results
There were no oral contrast medium-induced clinically significant artefacts. In 38 of the 200 patients, oral contrast aided image interpretation (owing to differentiation of mass/node from bowel, discrimination of intestinal wall from lumen or definition of the anatomy of a relevant site). In 33 of these 38 patients, the anatomical site of interest was the abdomen/pelvis.
Conclusion
The use of oral contrast medium in 18F-FDG PET studies should not be withheld as it improves image interpretation and does not produce clinically significant artefacts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Combined positron emission tomography (PET)/computed tomography (CT) has been readily available clinically only since 2001. PET/CT has two advantages over traditional PET systems. First, the patients are imaged sequentially with CT and PET on the same machine, so that the images are inherently spatially registered. Consequently, the functional data afforded by PET can be precisely localised anatomically. The second advantage of this system is the use of CT data for attenuation correction, which is significantly faster than in conventional PET systems.
The fusion of these two technologies has led to recognised advantages [1, 2]. Manoeuvres to improve the performance of this combined imaging modality have led to the use of CT contrast agents. However, it has been claimed that the use of positive contrast agents can lead to artefacts secondary to difficulties with CT attenuation correction of the PET examination [3–5]. As a result of these claims, some centres have developed special algorithms to correct for such attenuation artefacts [6]. Other centres have developed negative oral contrast medium regimens [7], whilst many institutions refrain from the use of oral contrast (e.g. none of the centres in the UK have been routinely using it) despite the potential benefits. The clinical relevance of oral contrast medium-induced artefacts has undergone only limited investigation. Moreover, the potential clinical benefits of bowel enhancement on PET/CT have not been widely investigated. This is surprising since new contrast regimens are still being developed for dedicated CT [8], where the benefits have been realised since the late 1970s [9]. It has been suggested that in the clinical setting, use of low concentration barium may minimise artefacts [4]. However, this work was performed retrospectively and without simultaneous analysis of the possible benefits of bowel enhancement [4]. In another clinical investigation, this time using dilute iodine-based oral contrast medium, there was no measurable increase in 18F-fluorodeoxyglucose (18F-FDG) standard uptake values (SUVs) in opacified versus non-opacified bowel [10]. As a consequence, it was suggested that, in the clinical setting, oral contrast medium does not cause artefacts. However, this is contrary to the experimental evidence [3, 5]. Furthermore, the study suffered from limited sample size (n=30) and, once again, the clinical benefits were not assessed [10].
Against this background, a prospective study was designed to evaluate the clinical implications of artefacts induced by oral contrast medium during 18F-FDG PET/CT examinations. In addition, the benefits of positive bowel enhancement were simultaneously evaluated.
Materials and methods
Images from 200 consecutive 18F-FDG PET/CT studies were prospectively examined at the time of reporting. In 46% the primary pathology was in the abdomen/pelvis. The anatomical sites of interest and the primary diagnoses in the study subjects are presented in Tables 1, 2 and 3. The primary diagnosis was based on histology in all patients. All the studies were simultaneously viewed by two reporters. These consisted of one of two radiologists, whilst the other was one of two nuclear medicine physicians. A third reader was available where there was disagreement in image interpretation.
Oral contrast medium protocol
This protocol was prospectively designed to investigate the possibility of artefacts or diagnostic benefit. All patients, including those with head/neck or thoracic pathologies, were given oral contrast medium. This reflects the fact that the abdomen and pelvis are routinely examined in all our 18F-FDG PET/CT patients, unlike in many dedicated CT examinations, where sometimes only the region of interest is interrogated.
Patients presenting to our department were given 8 ml of Gastrografin (sodium amidotrizoate 100 mg/ml, meglumine amidotrizoate 660 mg/ml, 370 mg I/ml, Schering Health Care, UK) in 500 ml of water by reception staff on arrival. 350 ml was consumed 30 min prior to 18F-FDG injection (in order to reduce the risk of oropharyngeal muscle uptake). The final 150 ml was given on the examination table.
PET/CT protocol
One hour prior to the examination, 370 MBq of 18F-FDG was injected intravenously. Using a dedicated combined GE Discovery LS PET/CT unit (GE Advance PET scanner and the GE Light-speed CT; MI, USA), whole-body and half-body examinations were performed with the patient supine with the arms held above the head (in those patients who could tolerate it). CT was performed using the four 3.75-mm detectors, a pitch of 1.5 and a 5-mm collimation. The CT exposure factors for all examinations were 140 kVp and 80 mA in 0.8 s. Maintaining patient position, a whole-body PET emission scan was performed and covered an area identical to that covered by CT (with five to six bed positions). All acquisitions were carried out in 2D mode, consisting of emission scans of 5 min per bed position. PET images were reconstructed using CT for attenuation correction by employing CT maps. Transaxial emission images of 3.9×3.9×4.25 mm (in plane matrix size 128×128) were reconstructed using ordered subsets expectation maximisation (OSEM) with two iterations and 28 subsets. The axial field of view was 148.75 mm, resulting in 35 slices per bed position.
Analysis
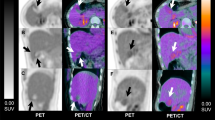
Images were viewed on a monitor in cine mode using Xeleris software. Attenuation- and non-attenuation-corrected images were examined at identical intensity thresholds. Both attenuation- and non-attenuation-corrected images were examined for the presence of significant oral contrast medium-induced artefacts. For the purpose of this study, we used the following definition for attenuation-corrected image artefacts: “An area of increased 18F-FDG activity on the CT attenuation-corrected image corresponding to the site of oral contrast medium, which was absent or noticeably reduced on non-attenuation-corrected images [3, 4] and which could have been confused with an 18F-FDG-avid lesion” (Figs. 1, 2). In other words, the artefact not only had to be identified, but also had to have a clinical impact on the case, i.e. it had to be clinically relevant.
PET/CT images from a patient with lung carcinoma showing 18F-FDG uptake in bowel containing oral contrast medium. The uptake is slightly higher on the attenuation-corrected (arrow) than on the non-attenuation-corrected (dashed arrow) image. However, the appearance should not be confused as metastatic disease
PET/CT images showing 18F-FDG uptake in bowel (arrow on the attenuation-corrected images and dashed arrow on the non-attenuation-corrected images). The uptake is present in bowel with no oral contrast (at the site of the arrows). It is recognised that such uptake is typically physiological [11], although enterocolitis may explain the prominent uptake in this case. It should be noted that the uptake is no less marked at sites with no contrast compared to sites with oral contrast medium. This is in contradiction to unpublished suggestions that oral contrast medium may cause local gut inflammation
Careful comparison was made of the anatomical relationship of luminal viscous and vertebral bodies and abdominal wall between CT and PET images to minimise the effect of peristalsis.
Since beam-hardening artefacts are known to occur on CT when the arms are positioned by the sides [12], the posture of the arms was noted, just in case it might potentiate the induction of artefacts by oral contrast medium. In addition, patients with their arms down, with partial tissuing of 18F-FDG activity in the antecubital fossa, were excluded.
Studies were also evaluated for the potential diagnostic benefit gained by the use of oral contrast medium. Reporters were asked to identify the advantage of oral contrast medium in the following situations (Table 4):
-
a)
Differentiation of intra-abdominal/pelvic nodes or masses from bowel
-
b)
Discrimination of bowel wall from lumen
-
c)
Definition of anatomy around 18F-FDG avid foci at anatomically difficult sites, including in patients who had undergone intestinal diversion
In order to assess the appropriateness of both the timing and the concentration of the oral contrast medium used in our protocol, the distal extent and concentration of bowel contrast were estimated.
SUVs were calculated as the average of values measured in a 1-cm-diameter circular region of interest and were corrected for patient body weight.
Results
No clinically significant oral contrast medium-induced artefact was seen in any of the images. However, some degree of discrepancy between attenuation- and non-attenuation-corrected images was frequently observed. Variable gastrointestinal uptake of 18F-FDG (SUV 1.1–4.5} was seen in all studies.
Oral contrast was thought diagnostically helpful in 38 (19%) patients. In all these cases it helped to anatomically localise foci of abnormal FDG activity (Figs. 3, 4). The types of benefit included differentiation of a node/mass from adjacent bowel, discrimination of bowel wall from lumen and improved delineation of anatomy at the site of interest, e.g. in the region of the pancreatic bed and in patients with surgical diversion, sub-diaphragmatic lymphoma, oesophageal carcinoma or peritoneal spread of ovarian carcinoma (Table 4). In six patients the use of oral contrast helped differentiate primary tumours (all pancreatic) from bowel (usually duodenum, as shown in Fig. 3). In two patients the contrast helped to differentiate what appeared to be unsuspected additional pathology from bowel, e.g. in a patient with lung cancer an FDG-avid pelvic mass was differentiated from adjacent loops of small bowel. In the other 30 patients the abnormal FDG foci were identified in abdominal lymph nodes or intraperitoneal deposits appeared to represent metastases. These structures were distinguished from adjacent bowel due to the presence of positive contrast medium in the intestine (Fig. 4). In 33 of the 38 patients, the primary site of interest was the abdomen/pelvis. In the other five patients, two studies were performed for the thorax and two for the head and neck. In the other case there was no specific site of interest as the indication was to identify an occult primary lesion (Table 3). Of the 91 patients in whom the primary site of interest was the abdomen/pelvis, 36% benefited from oral contrast medium (Table 3). The number of patients in whom Gastrografin administration assisted in the primary diagnosis is shown in Table 1.
Axial PET/CT at the level of the head of the pancreas in a patient with known pancreatic carcinoma, illustrating the anatomical advantage gained by the use of oral contrast medium. On the CT image, the soft tissue attenuation (density) pancreatic head is identified by the white dashed arrow. A stent is seen in the head of the pancreas (white arrowhead). The pancreatic head is defined separately from the higher attenuation duodenum (because of the oral contrast medium) posterolaterally (white solid arrow). On the attenuation-corrected PET image a focus of FDG activity is seen in the region of the pancreatic bed (black dashed arrow). This focus is centred on the pancreatic head when viewed on the fusion image (black solid arrow). Without the use of bowel opacification it would be difficult to separate duodenum from the pancreatic head, thus making it hard to ascertain the organ of abnormality
Axial PET/CT of the pelvis in a patient with lymphoma. The CT image shows loops of opacified small bowel (black arrow) separated from adjacent soft tissue masses (white arrow). The PET images show two foci of increased FDG activity (black dashed arrows). The fused images demonstrate that the foci of FDG activity (white dashed arrows) are centred in the non-opacified soft tissue masses. Use of oral contrast medium increased the diagnostic certainty that the foci represent nodal disease rather than bowel activity
The concentration of oral contrast was too dilute (enhanced bowel could not be confidently differentiated from non-enhanced bowel) in eight patients. The contrast medium reached the mid-small bowel in all patients and extended to the colon in 42 (21%).
Finally, there was no inter-observer variation and no studies were read by the third reader. There were no tissued injections in the “arms down” position.
Discussion
Our study shows that, using our protocol, no oral contrast medium-induced artefacts were identified that could have been confused clinically with disease in any of the 200 patients. In fact, use of oral contrast medium improved the diagnosis in nearly a fifth of studies. Accordingly, the use of oral positive contrast medium should be encouraged, not withheld.
Although there was no evidence of clinically significant oral contrast medium-induced artefacts, there were regular examples where oral contrast in bowel had a higher 18F-FDG uptake on attenuation-corrected images than on non-attenuation-corrected images (Fig. 1). This would tend to support the findings of previous investigators [4]. However, as Fig. 1 shows, this type of increased uptake should not confuse a reader (of any experience) into reporting the presence of 18F-FDG-avid disease. Increased 18F-FDG activity was seen regularly in the bowel containing Gastrografin. The presence of such activity in the bowel is well recognised as physiological [11], and the intensity of 18F-FDG uptake was shown to be similar on attenuation- and non-attenuation-corrected images, thus confirming the lack of attenuation-induced artefact (Fig. 2). Our study design would have benefited from a control group, so that each patient would have had a set of non-contrast-enhanced images and a set of contrast-enhanced ones. However, it would be ethically and practically difficult to perform two CT studies on each patient.
The theoretical basis for contrast medium-induced artefact is the attenuation correction mechanism for the PET images. To correct for the differing energies and therefore different attenuation of PET and CT photons, most systems use a simple segmentation and scaling algorithm [13]. In this methodology, data are segmented into bone and non-bone components, with different scaling of attenuation of these two components used to correct the attenuation at CT energies to those at PET energies. This method, which assumes a constant ratio of attenuation at PET and CT energies for the bone and non-bone components, works well with non-contrast CT studies [14]. In studies where contrast agents are used, the attenuation of the agent is considerably different than soft tissue at CT energies, whereas at PET energies, there is practically no difference in attenuation [15]. This results in a ratio of attenuation at the two energies that fits neither the bone nor the non-bone model. The result is seen as an overestimation of attenuation at 511-keV energies in areas of contrast accumulation, and therefore an overestimation of the emission activity. For quantitative purposes, the resulting increase in SUV has been found to be less than the known reproducibility error from such measurements [15].
In our study, the choice of oral contrast medium concentration appears to have been appropriate, as there was a paucity of artefacts with only a few studies where contrast appeared too dilute. As far as choice of bowel-enhancing agent is concerned, the iodine-based compound had the desired effect: there was no evidence that it induced clinically relevant artefacts, whilst it was of diagnostic benefit in many studies. Barium-based compounds, so long as they are diluted, appear to be similarly appropriate [4]. The timing of administration of oral contrast medium used in our study also appeared correct, since contrast medium extended into the mid small intestine in all patients and entered the colon in 22% of individuals. Perhaps we could have achieved better distal bowel enhancement by increasing the volume of contrast used.
The use of oral contrast medium in dedicated CT has been developed since the late 1970s [8]. A similar evolution can be expected in PET/CT. For example, in our study oral contrast medium was only given on the day of examination. In the future, it may be worth investigating the use of earlier administration of contrast agents in patients with colonic disease, so as to improve large bowel visualisation in keeping with stand-alone CT examinations [16]. Our study clearly showed the use of oral contrast medium to be beneficial in a third of patients in whom the site of disease was the abdomen/pelvis. Given the lack of significant artefacts, there is no reason to withhold oral contrast in these patients. In our study, and partially in keeping with recognised CT experience, the benefits of oral contrast medium were most marked in those patients with pancreatic carcinoma, sub-diaphragmatic lymphoma, oesophageal carcinoma with disease in the gastrohepatic ligament/gastro-oesophageal region, or peritoneal spread of ovarian carcinoma [8, 17–19]. The benefit of oral contrast medium in the region of the pancreatic bed was particularly helpful. This is an anatomically busy region with close relationships of the duodenum with the pancreatic head/body, superior mesenteric vessels, para-aortic lymph nodes and left renal vein. Thus opacification of the duodenum (as well as other segments of nearby small intestine) can help distinguish bowel loops from these important adjacent structures (Table 4, Figs. 3, 4). In patients in whom the site of suspected disease was not in the abdomen, the benefit of oral contrast medium was limited, and thus its use may not be justified in such cases. This may be especially true for head and neck malignancy, where not only is abdominal disease unlikely but there is the potential for use of oral contrast medium to cause increased cervical muscle uptake [4].
Conclusion
-
1.
The use of oral contrast medium in 18F-FDG PET/CT scanning improves image interpretation and does not produce clinically significant artefacts.
-
2.
Maximum benefit from oral contrast medium use is noted where the anatomical site of interest is the abdomen/pelvis; thus its use in such patients should be strongly encouraged and not withheld.
-
3.
If disease is suspected outside the peritoneal cavity, the use of oral contrast medium should probably be restricted.
References
Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 2003;348:2500–7
Wahl RL. Why nearly all PET of abdominal and pelvic cancers will be performed as PET/CT. J Nucl Med 2004;45(Suppl 1):82–95S
Antoch G, Freudenberg LS, Egelhof T, Stattaus J, Jentzen W, Debatin JF, et al. Focal tracer uptake: a potential artefact in contrast-enhanced dual-modality PET/CT scans. J Nucl Med 2002;43:1339–42
Cohade C, Osman M, Nakamoto Y, Marshall LT, Links JM, Fishman EK, et al. Initial experience with oral contrast in PET/CT: phantom and clinical studies. J Nucl Med 2003;44(3):412–6
Antoch G, Jentzen W, Freudenberg LS. Effect of oral contrast agents on computed tomography-based positron emission tomography attenuation correction in dual-modality positron emission tomography/computed tomography imaging. Invest Radiol 2003;38(12):784–9
Nehmeh SA, Erdi YE, Kalaigian H. Correction for oral contrast artefacts in CT attenuation-corrected PET images obtained by combined PET/CT. J Nucl Med 2003;44:1940–4
Antoch G, Kuehl H, Kanja J, Lauenstein TC, Schneemann H, Hauth E, et al. Dual-modality PET/CT scanning with negative oral contrast agent to avoid artefacts: introduction and evaluation. Radiology 2004;230:879–85
Moss AA, Kressel HY, Korobkin M, Goldberg HI, Rohlfing BM, Brasch RC, et al. The effect of gastrografin and glucagon on CT scanning of the pancreas: a blind control trial. Radiology 1979;126:711–4
Giuliano V, Giuliano C, Pinto F, Scaglione M. Rapid CT scan visualisation of the appendix and early acute non-perforated appendicitis using an improved oral contrast method. Emerg Radiol 2004;10:235–7
Dizendorf EV, Treyer V, Von Schulthess GK, Hany TF. Application of oral contrast media in coregistered positron emission tomography-CT. Am J Roentgenol 2002;179 2:477–81
Engel H, Steinert H, Buck A, Berthold T, Huch Boni RA, von Schulthess GK, et al. Whole-body PET: physiological and artefactual fluorodeoxyglucose accumulation. J Nucl Med 1996;37:441–6
Dendy PP, Heaton B. Diagnostic imaging with radioactive materials. In: Dendy PP, Heaton B, editors. Physics for diagnostic radiology. 2nd ed. London: IoP Medical Sciences Series; 1999. p. 163–86
Kinahan PE, Townsend DW, Beyer T, Sashin D. Attenuation correction for a combined 3D PET/CT scanner. Med Phys 1998;25(10):2046–53
Burger C, Goerres G, Schoenes S, Buck A, Lonn AH, Von Schulthess GK. PET attenuation coefficients from CT images: experimental evaluation of the transformation of CT into PET 511-keV attenuation coefficients. Eur J Nucl Med Mol Imaging 2002;29:922–7
Dizendorf E, Hany TF, Buck A, Von Schulthess GK, Burger C. Cause and magnitude of the error induced by oral CT contrast agent in CT-based attenuation correction of PET emission studies. J Nucl Med 2003;44:732–8
Fletcher JG, Johnson CD, Welch TJ, MacCarty RL, Ahlquist DA, Reed JE, et al. Optimization of CT colonography technique: prospective trial in 180 patients. Radiology 2000;216:704–11
Einstein DM, Singer AA, Chilcote WA, Desai RK. Abdominal lymphadenopathy: spectrum of CT findings. Radiographics 1991;11:457–72
Cayea PD, Seltzer SE. A new barium paste for computed tomography of the esophagus. J Comput Assist Tomogr 1985;9:214–6
Thoeni RF, Blankenberg F. Pancreatic imaging. Computed tomography and magnetic resonance imaging. Radiol Clin North Am 1993;31:1085–113
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Groves, A.M., Kayani, I., Dickson, J.C. et al. Oral contrast medium in PET/CT: should you or shouldn’t you?. Eur J Nucl Med Mol Imaging 32, 1160–1166 (2005). https://doi.org/10.1007/s00259-005-1833-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-1833-9