Abstract
Purpose
Percutaneous transluminal coronary angioplasty (PTCA) is one of the main therapy options for patients with coronary artery disease (CAD), resulting in an improvement in myocardial perfusion and exercise capacity. Nevertheless, studies have also demonstrated a positive effect of regular exercise training on myocardial perfusion and maximum exercise capacity. The aim of this study was to evaluate changes in myocardial stress perfusion after 1 year of exercise training in comparison with the effects of PTCA in patients with CAD.
Methods
In 66 male patients with angiographically confirmed significant coronary artery stenosis in one target vessel, myocardial perfusion scintigraphy was performed at baseline and 12 months after randomisation into either a physical exercise group or a PTCA group. Circumferential count rate profiles in 16 wall segments were classified according to their relative count rate and localisation within or outside the area supplied by the stenosed vessel.
Results
Ischaemic segments showed a significant improvement in myocardial count rate within the target area after 12 months in both the PTCA and the training group (PTCA group: from 76.8±4.9% to 86.6±10.9%, p=0.03; training group: from 74.0±7.3% to 83.7±10.8%, p<0.01). Outside the target area only the training group showed a significant improvement (from 77.7±4.4% to 91.7±4.8%, p<0.01).
Conclusion
Our data indicate a significant improvement in stress myocardial perfusion in the training group after 12 months. The ischaemia is reduced not only in the target region of the leading stenosis but also in other ischaemic myocardial areas. In contrast, after PTCA stress perfusion improves only in the initially ischaemic parts of the target area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary revascularisation by percutaneous techniques is widely used in the treatment of patients with stable coronary artery disease (CAD). Studies comparing percutaneous transluminal coronary angioplasty (PTCA) with medical treatment suggested that patients who underwent revascularisation had an improvement in their quality of life, exercise performance or both [1–3]. However, the effect of percutaneous intervention as compared with medical treatment on the incidence of ischaemic events and the need for subsequent revascularisation was less certain. Lipid-lowering treatment has been shown to significantly reduce the incidence of cardiovascular events, overall mortality and the need for revascularisation [4, 5]. Furthermore, in low-risk patients with stable CAD, aggressive lipid-lowering therapy is at least as effective as angioplasty and usual care in reducing the incidence of ischaemic events [6]. A positive effect on lipoprotein levels, stress-induced myocardial ischaemia, maximum exercise capacity and myocardial perfusion could be achieved by a low-fat diet and regular physical exercise [7–9].
The aim of this study was to evaluate the change in myocardial stress perfusion after 1 year of exercise training in comparison with the effects of PTCA in patients with stable CAD.
Materials and methods
Eighty-six male patients aged ≤70 years with stable CAD and one angiographically confirmed coronary artery stenosis of ≥75% by visual assessment that was amenable to percutaneous coronary intervention were randomly assigned to a training group or a PTCA group. Patients with an indication for coronary bypass surgery, unstable CAD, insulin-dependent diabetes mellitus, hypertension resistant to therapy, significant organic heart defect or high-grade arrhythmia and patients with previous PTCA or bypass surgery were excluded from the study. Inclusion criteria were male gender, willingness to participate in the study for at least 12 months, good angiographic documentation of at least one coronary artery stenosis amenable to PTCA/stent implantation and the ability to exercise daily. After an introductory session during which the patients were familiarised with the aims of the study, the randomisation process and alternative therapeutic approaches, their written consent was obtained. Sealed envelopes were used to randomise the patients between the groups.
Patients assigned to the training group stayed in hospital for the first 2 weeks and exercised on a bicycle ergometer six times per day for 10 min each under close supervision. Individual anti-anginal medical treatment (including lipid-lowering drugs) was provided. After discharge the patients were asked to exercise daily on a provided bicycle ergometer for a minimum of 20 min. The patient’s target heart rate was determined as 75% of the individual symptom-limited heart rate. In addition there were weekly supervised exercise sessions.
The patients assigned to the revascularisation group underwent a standard PTCA with stent implantation followed by usual care.
Myocardial perfusion scintigraphy was performed at baseline and after 12 months.
The ethical committee for human studies at the University of Leipzig approved the investigational protocol
Scintigraphic protocol
Stress and rest myocardial perfusion scintigraphy was performed using a 2-day protocol. Exercise consisted of a symptom-limited maximal upright treadmill test in all patients. Upon reaching the highest attainable workload, 400 MBq 99mTc-methoxyisobutylisonitrile (MIBI) or alternatively 400 MBq 99mTc-tetrofosmin was administered intravenously and exercise was continued for another 1–2 min at the same level. β-Blockers, nitrates and other anti-anginal medications were discontinued 24 h before the test. Exercise was terminated only when patients experienced progressive anginal chest pain or exhaustion, or when pathological ECG changes were observed. Maximum pressure–frequency product (double product) was calculated from maximal, simultaneously recorded heart rate and systolic blood pressure during exercise. This variable has been shown to correlate reliably with myocardial oxygen consumption (MVO2) over a wide range of exercise levels [10]. Since the exercise level affects the results of single-photon emission computed tomography (SPECT) imaging [11], we sought to achieve at least the same double product in the follow-up scintigraphy, thus guaranteeing the same exercise capacity. After 15–20 min of recovery, a fatty meal was provided to accelerate hepatobiliary clearance of the 99mTc-MIBI. One hour after injection, tomographic images were obtained.
In the rest protocol all individual anti-anginal medication was taken. In addition, sublingual nitrogen was given before injection of the radiopharmaceutical.
Data acquisition
For details of the data acquisition and quantification, see Kluge et al. [12]. Briefly, transmission and emission data were acquired using a standard protocol and a dual-head camera with detectors positioned at an angle of 90° (ADAC Laboratories, Vertex). For reconstruction and reorientation, a visually controlled software program (ADAC Laboratories, Autospect Plus, Dual Vantage Version 4.2) was used. A polar plot was generated and circumferential count rates were calculated by a standard software program (ADAC Laboratories, Polar Plot Generation Version 4.2).
The wall segments were allocated to the territories of the main coronary arteries: left anterior descending artery (LAD) = anterolateral, anterior, anteroseptal and midseptal wall regions; left circumflex artery (LCx) = midlateral and posterolateral regions; right coronary artery (RCA) = inferior, inferoseptal regions. The individual supply type, according to coronary angiography, was considered. To guarantee anatomical matching between the baseline and the follow-up acquisition, the junction of the right ventricle was used as an anatomical landmark. All four acquisitions were quantified to an individual reference region (=100%), which was obtained by averaging the maximum counts in all normal segments outside the area supplied by the stenosed artery.
According to Ficaro et al. [13], the relative count rates of segments with perfusion defects were classified as: fully reversible (<82% of max. under stress, >82% of max. under rest), partly reversible (<82% of max. under stress, more than 7% improvement under rest) or fixed (<82% of max. under stress, less than 7% improvement under rest). For each patient, segments were analysed and compared with baseline individually. Thereby, two main regions were investigated: segments supplied by the coronary artery with the angiographically confirmed significant stenosis and segments with perfusion defects outside this target area.
Statistics
After verifying normal distribution of data within the group, two-tailed Student’s t test for paired values was used for significance testing between baseline and follow-up and two-tailed Student’s t test for non-paired values was used for significance testing between the groups. If data were not normally distributed, Wilcoxon’s test or the Mann-Whitney U test was used. The significance level was set at p<0.05.
Results
We conducted a large exercise study, which focussed mainly on clinical symptoms, angina-free exercise capacity, cost-effectiveness and frequency of a combined clinical end point (death from a cardiac cause, stroke, bypass surgery and acute myocardial infarction). The results have been published recently [14]. In the course of this large exercise training study including 101 patients, the first 85 patients received myocardial scintigraphy. In this paper, we focus on the scintigraphic results. Of the 85 patients (43 in the training group and 42 in the PTCA group), 66 (36 in the training group and 30 in the PTCA group) were available for final analysis. Five acquisitions were lost due to a hard drive crash. Nine patients (seven in the PTCA and two in the training group) had to undergo a (second) PTCA or bypass surgery due to relevant angina. In one patient PTCA was not successful, and four patients could not participate in a follow-up ergometer stress test owing to previous ischaemic stroke, severe joint disorder or acute tachyarrhythmia.
There was no significant difference between the two groups with respect to age, body mass index, ejection fraction, previous acute myocardial infarction, systolic and diastolic blood pressure, heart rate at rest, total cholesterol, high-density lipoproteins, low-density lipoproteins or smoking. Both groups were comparable with regard to medical therapy (ACE inhibitors/AT1-receptor antagonists, β-HMG-CoA reductase inhibitors, β-receptor antagonists and acetylsalicylic acid), which remained unchanged during follow-up. There was no significant difference between the two groups with respect to the baseline parameters of the stenosed target vessel. In the training group, the reference diameter of the stenosed vessel was 2.85±0.56 mm, the minimal luminal diameter was 0.60±0.37 mm and the relative stenosis diameter was 73.9±11.1%, while in the PTCA group the corresponding values were 2.74±0.28 mm, 0.52±0.24 mm and 80.8±8.7%. There was no significant difference between the two groups for reference diameter (p=0.822), minimal luminal diameter (p=0.511) or relative stenosis (p=0.723) (Mann-Whitney U test).
The distribution of significant stenoses was similar in the two groups: the target lesion was located in the LAD in six patients in the training group and in three patients in the PTCA group, the RCA was affected in 13 patients in the training group and in eight patients in the PTCA group and the LCX was the target vessel in 17 patients in the training group and 19 patients in the PTCA group.
There was no significant difference between the groups regarding the Gensini score [15] (PTCA group: 14±11; training group: 17±16; p=0.928; Mann-Whitney U test).
As mentioned previously, the individual workload was quantified by using the double product to gain a reproducible parameter for maximum exercise capacity. In the follow-up study, exercise was truncated at the previously achieved double product, thus guaranteeing the same value of individual stress at both times. Double product was 2.38±0.70 at baseline versus 2.45±0.58 at follow-up (p=0.561) in the training group and 2.27±0.62 at baseline versus 2.42±0.68 at follow-up (p=0.447) in the PTCA group (Wilcoxon’s test). Inter-group testing showed no significant difference between the two groups [p=0.708 (baseline) and p=0.750 (follow-up); Mann-Whitney U test]. However, the patients in the training group achieved a significantly higher exercise capacity, measured by the power in Watts, to gain their baseline double product: 133±28 at baseline versus 163±32 at follow-up (p<0.01; Wilcoxon’s test) (Table 1). This change was also significant if compared with the PTCA group (p<0.01; Mann-Whitney U test).
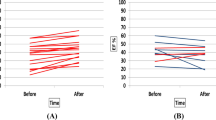
Comparing baseline and follow-up data, segments with fully reversible perfusion defects within the target area showed a significant increase in relative activity (Figs. 1, 2), from 76.8±4.9% to 86.6±10.9% (p=0.03; Student’s t test for paired values) in the PTCA group and from 74.0±7.3% to 83.7±10.8% (p<0.01; Student’s t test for paired values) in the training group. There was no significant difference between the groups (p=0.793; Student’s t test for non-paired values). Regarding fully reversible perfusion defects outside the target area, there was a significant increase in the training group, from 77.4±4.4% to 91.7±4.8% (p<0.01; Student’s t test for paired values), but not in the PTCA group (non-significant increase from 75.9±5.5% to 83.0±16.2%; p=0.194; Student’s t test for paired values) (Fig. 1). The difference between the two groups was significant (p=0.002; Student’s t test for non-paired values).
a, b Inside the target area: changes in relative count rates in segments with fully reversible perfusion defects in the training group (a) and the PTCA group (b). c, d Outside the target area: changes in relative count rates in segments with fully reversible perfusion defects in the training group (c) and the PTCA group (d)
In addition to fully reversible perfusion defects, some segments with perfusion defects under stress showed only partial remission under rest; these segments were classified as segments with partly reversible perfusion defects. Taking these segments into account, and thus comparing follow-up and baseline data of all segments with partly reversible as well as fully reversible perfusion defects within the target area, significant increases in relative activity were observed in both groups: from 65.1±10.5% to 77.8±11.5% in the PTCA group and from 68.1±9.8% to 76.6±10.1% in the training group (p<0.01 in both groups; Student’s t test for paired values) (Table 2). There was no significant difference between the groups (p=0.918; Student’s t test for non-paired values). Outside the target area these segments showed a significant increase in the training group, from 72.5±11.9% to 87.9±10.5% (p<0.01; Student’s t test for paired values) but not in the PTCA group (non-significant increase from 66.2±17.2% to 74.3±24.2%; p=0.064; Student’s t test for paired values) (Table 2). There was a significant difference between the groups outside the target area (p=0.026; Student’s t test for non-paired values).
After PTCA, segments with fixed perfusion defects indicating scars showed a small but significant improvement in relative count rate within the target area, from 69.3±9.7% to 73.8±12.3% (p=0.032; Wilcoxon’s test); by contrast, there was no significant change within the target area for the exercise training group (non-significant increase from 71.6±11.1% to 72.8±8.6%; p=0.493; Wilcoxon’s test) (Table 2). Outside the target area, no significant changes in such segments could be documented in either group [training group: from 75.2±8.4% to 79.6±13.9% (p=0.198; Wilcoxon’s test); PTCA group: from 74.0±10.8% to 75.3±14.3% (p=0.345; Wilcoxon’s test)] (Table 2). Inter-group testing showed no significant differences either within or outside the target area (p=0.052 and 0.254 respectively; Mann-Whitney U test).
When baseline and follow-up data were compared with regard to extent of perfusion defects, i.e. number of segments with perfusion defects, the training group showed a significant reduction in the extent of the area with perfusion defect both inside the target area, from 154 to 134 segments (p<0.05; Wilcoxon’s test), and outside the target area, from 59 to 44 segments (p<0.05; Wilcoxon’s test). In contrast, the PTCA group showed a significant reduction only inside the target area, from 131 to 113 segments (p<0.05; Wilcoxon’s test); outside the target area no significant change was found (reduction from 54 to 51 segments) (Table 3).
Discussion
The significant improvement in myocardial perfusion (from 76.8±4.9% to 86.6±10.9%; p=0.03) caused by PTCA within the area supplied by the stenosed vessel, as indicated by our data, is easily explained and has been reported by previous observers [16]. Outside this target area a non-significant increase in myocardial perfusion (from 75.9±5.5% to 83.0±16.2%; p=0.194) was observed, possibly due to better perfusion in the vicinity of the target vessel after PTCA..
Regarding the training group, our data indicated a highly significant increase in myocardial perfusion in ischaemic areas after 1 year of daily exercise training, which was not limited to the area perfused by the coronary artery with the leading stenosis (increase from 74.0±7.3% to 83.7±10.8%; p<0.01) but rather was also documented in other myocardial areas (increase from 77.7±4.4% to 91.7±4.8%; p<0.01).
Several clinical and haemodynamic investigations have shown that continuous physical exercise can improve quality of life parameters and exercise capacity in patients with CAD [17–20]. Due to the strictly aerobic pattern of cardiac metabolism, and the near-maximal oxygen extraction, even under basal conditions, an increase in peak maximal oxygen uptake is induced by changes in coronary blood flow.
Multiple mechanisms have been proposed to explain the enhanced myocardial perfusion in patients with CAD under a regime of regular exercise training. Initially a net regression of epicardial coronary stenoses due to exercise training was hypothesised. In a randomised trial in which a low-fat diet and regular physical exercise were implemented in the intervention group, Ornish et al. [21] proved a reduction in relative stenosis diameter from 40±17% to 38±17% in the intervention group after 12 months, whereas the control group showed an increase in relative stenosis diameter from 43±16% to 46±19%; inter-group testing showed the difference to be significant. These results could be confirmed by Hambrecht et al. [22] in subjects undertaking an exercise workload of more than 2,200 kcal/week, requiring a daily exercise period of 4–5 h. Still, most studies have failed to document a net regression of coronary lesions, even with the addition of lipid-lowering strategies to exercise training interventions [23]. Moreover, a decrease in the incidence of myocardial ischaemia was observed in patients with progression of stenotic lesions [8]. This unexpected finding implies that improvement in myocardial perfusion may be achieved independently of changes in coronary lesions.
Other possible mechanisms causing an enhancement in myocardial perfusion during exercise training could be the recruitment of coronary collateral vessels or an improvement in blood rheology. Regarding collateral vessel formation, evidence from studies in animals suggests that long-term intensive exercise leads to an improvement in coronary collateralisation [24, 25]. However, angiographic studies performed at rest in patients with CAD did not substantiate this hypothesis [26]. Nevertheless, a post-training increase in the collateral score could be proven by Belardinelli et al. [27] in a subgroup of trained patients, the increase being correlated with improvements in both thallium uptake and systolic wall thickening scores. This might indicate a linkage between training-induced effects on collateral circulation and both perfusion and contractility of dysfunctional myocardial segments.
Ernst [28] investigated the hypothesis that exercise training improves blood rheology. He showed that blood viscosity can be reduced and blood flow improved by exercise training in healthy subjects and in patients with peripheral vascular disease. However, in patients with CAD and impaired left ventricular function, exercise training did not have any significant effect on blood viscosity [29].
Recent studies have indicated an improvement in coronary artery endothelial function due to regular physical exercise [30, 31], indicating that improved endothelium-dependent vasodilatation may play a key role in the improvement of myocardial perfusion due to regular physical exercise.
The present study revealed that segments with fixed perfusion defects showed a significant improvement in myocardial perfusion after revascularisation, from 69.3±9.7% to 73.8±12.3% (p=0.032), and similar results have been reported by other investigators [32–35]. However, classifying regions with mild to moderate perfusion defects as complete or incomplete scars without metabolic imaging with 18F-fluorodeoxyglucose is difficult. vom Dahl et al. [36] showed a more pronounced recovery of myocardial regions displaying mild to moderate hypoperfusion with intact metabolism compared to those regions with hypoperfusion and reduced metabolism. In the training group, segments with fixed perfusion defects showed an improvement comparable to that in the PTCA group; however, the significance level was not achieved in the training group.
Conclusion
Since improvement in myocardial perfusion is not restricted to the area supplied by the mainly stenosed vessel but also occurs in other hypoperfused myocardial areas, our data indicate that physical exercise might be a valuable therapeutic alternative to PTCA in carefully selected patients with stable CAD who show a low risk profile.
References
Hueb WA, Bellotti G, De Oliveira SA, Arie S, de Albuquerque CP, Jatene AD, et al. The medicine, angioplasty or surgery study (MASS): a prospective, randomized trial of medical therapy, balloon angioplasty or bypass surgery for single proximal left anterior descending artery stenosis. J Am Coll Cardiol 1995;26:1600–5.
Parisi AF, Folland ED, Hartingan P. A comparison of angioplasty with medical therapy in the treatment of single-vessel coronary artery disease. Veterans Affairs ACME Investigators. N Engl J Med 1992;326:10–6.
Strauss WE, Fortin T, Hartigan P, Folland ED, Parisi AF. A comparison of quality of life scores in patients with angina pectoris after angioplasty compared with after medical therapy. Outcomes of a randomised clinical trial. The Veterans Affair Study of Angioplasty Compared to Medical Therapy Investigators. Circulation 1995;92:1710–9.
Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–9.
Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial Investigators. N Engl J Med 1996;335:1001–9.
Pitt B, Waters D, Brown WV, van Boven AJ, Schwartz L, Title LM, et al. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. N Engl J Med 1999;341:70–6.
Schuler G, Schlierf G, Wirth A, Mautner HP, Scheurlen H, Thumm M, et al. Low-fat diet and regular, supervised physical exercise in patients with symptomatic coronary artery disease: reduction of stress-induced myocardial ischemia. Circulation 1988;77:172–81.
Schuler G, Hambrecht R, Schlierf G, Grunze M, Methfessel S, Hauer K, et al. Myocardial perfusion and regression of coronary artery disease in patients on a regimen of intensive physical exercise and low fat diet. J Am Coll Cardiol 1992;19:34–42.
Schuler G, Hambrecht R, Schlierf G, Niebaurer J, Hauer K, Newmann J, et al. Regular physical exercise and low-fat diet. Effects on progression of coronary artery disease. Circulation 1992;86:1–11.
Gobel FL, Norstrom LA, Nelson RR, Jorgensen CR, Wang Y. The rate–pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation 1978;57:549–56.
Iskandrian AS, Heo J, Kong B, Lyons E. Effect of exercise level on the ability of thallium-201 tomographic imaging in detecting coronary artery disease: analysis of 461 patients. J Am Coll Cardiol 1989;14:1477–86.
Kluge R, Sattler B, Seese A, Knapp WH. Attenuation correction by simultaneous emission–transmission myocardial single-photon emission tomography using a technetium-99m-labelled radiotracer: impact on diagnostic accuracy. Eur J Nucl Med 1997;24:1107–14.
Ficaro EP, Fessler JA, Shreve PD, Kritzman JN, Rose PA, Corbett JR. Simultaneous transmission/emission myocardial perfusion tomography: diagnostic accuracy of attenuation-corrected 99mTc-sestamibi single-photon emission computed tomography. Circulation 1996;93:463–73.
Hambrecht R, Walther C, Mobius-Winkler S, Gielen S, Linke A, Conradi K, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation 2004;109:1371–8.
Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51:606.
Hirzel HO, Nuesch K, Gruentzig AR, Luetolf UM. Short and long term changes in myocardial perfusion after percutaneous transluminal coronary angioplasty assessed by thallium-201 exercise scintigraphy. Circulation 1981;63:1001–7.
Beniamini Y, Rubenstein JJ, Zaichkowsky LD, Crim MC. Effects of high-intensity strength training on quality-of-life parameters in cardiac rehabilitation patients. Am J Cardiol 1997;80:841–6.
Clausen JP, Larsen OA, Trap-Jensen J. Physical training in the management of coronary artery disease. Circulation 1969;40:143–54.
Froelicher V, Jensen D, Genter F, Sullivan M, McKirnan MD, Witztum K, et al. A randomized trial of exercise training in patients with coronary heart disease. JAMA 1984;252:1291–7.
Kennedy CC, Spiekerman RE, Lindsay MI Jr, Mankin HT, Frye RL, McCallister BD. One-year graduated exercise program for men with angina pectoris. Evaluation by physiologic studies and coronary arteriography. Mayo Clin Proc 1976;51:231–6.
Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, et al. Intensive lifestyle changes for reversal coronary heart disease. JAMA 1998:280:2001–7.
Hambrecht R, Niebauer J, Marburger C, Grunze M, Kalberer B, Hauer K, et al. Various intensities of leisure time physical activity in patients with coronary artery disease. Effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J Am Coll Cardiol 1993;22:468–77.
Haskell WL, Alderman EL, Fair JM, Maron DJ, Mackey SF, Superko HR, et al. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP). 1994;89:975–90.
Cohen MV, Yipintsoi T, Scheuer T. Coronary collateral stimulation by exercise in dogs with stenotic coronary arteries. J Appl Physiol 1982;52:664–71.
Scheel KW, Ingram LA, Wilson JL. Effects of exercise on the coronary and collateral vasculature of beagles with and without coronary occlusion. Circ Res 1981;48:523–30.
Fergusson RJ, Petitclerc R, Choquette G, Chaniotis L, Gauthier L, Huot R, et al. Effects of physical training on treadmill exercise capacity, collateral circulation and progression of coronary artery disease. Am J Cardiol 1974;34:764–9.
Belardinelli R, Georgiou D, Ginzton L, Cianci G, Purcaro A. Effects of moderate exercise training on thallium uptake and contractile response to low-dose dobutamine of dysfunctional myocardium in patients with ischemic cardiomyopathy. Circulation 1998;97:553–61.
Ernst E. Influence of regular physical activity on blood rheology. Eur Heart J 1987;Suppl. G:59–62.
Reinhart WH, Dziekan G, Goebbels U, Myers J, Dubach P. Influence of exercise training on blood viscosity in patients with coronary artery disease and impaired left ventricular function. Am Heart J 1998;135:379–82.
Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med 2000;342:454–60.
Hosokawa S, Hiasa Y, Takahashi T, Itoh S. Effect of regular exercise on coronary endothelial function in patients with recent myocardial infarction. Circ J 2003;67:221–4.
Altehoefer C, vom Dahl J, Messmer BJ, Hanrath P, Bull U. Fate of the resisting perfusion defect as assessed with technetium-99m-methoxy-isobutyl-isonitrile single-photon emission computed tomography after successful revascularisation in patients with healed myocardial infarction. Am J Cardiol 1996;77:88–92.
Liu P, Kiess MC, Okada RD, Block PC, Strauss HW, Pohost GM, et al. The persistent defect on exercise thallium imaging and its fate after myocardial revascularisation: does it present scar or ischemia? Am Heart J 1985;110:996–1001.
Lucignani G, Paolini G, Landoni C, Zuccari M, Paganelli G, Galli L, et al. Presurgical identification of hibernating myocardium by combined use of technetium-99m-hexakis-2-methoxyisobutylisonitrile single photon emission tomography and fluorine-18-fluoro-2-deoxy-d-glucose positron emission tomography in patients with coronary artery disease. Eur J Nucl Med 1992;19:874–81.
Raff W, Sialer G, von Segesser L, Pfeiffer A, Turina M, Schulthess GK. Perioperative myocardial perfusion scintigraphy at rest with technetium-99m-methoxyisobutylisonitrile before and after coronary bypass operations. Eur J Nucl Med 1991;18:99–105.
vom Dahl J, Altehoefer C, Sheehan FH, Buechin P, Uebis R, Messmer BJ, et al. Recovery of regional left ventricular dysfunction after coronary revascularization. Impact of myocardial viability assessed by nuclear imaging and vessel patency at follow-up angiography. J Am Coll Cardiol 1996;28:948–58.
Acknowledgement
We would like to thank Amersham Buchler GmbH & Co. KG, General Electric Healthcare for the financial support in the publication of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kendziorra, K., Walther, C., Foerster, M. et al. Changes in myocardial perfusion due to physical exercise in patients with stable coronary artery disease. Eur J Nucl Med Mol Imaging 32, 813–819 (2005). https://doi.org/10.1007/s00259-005-1768-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-1768-1