Abstract
Purpose
The aim of this study was to evaluate the frequency of false-negative (FN) sentinel node procedures in patients with breast cancer and the subsequent clinical outcome in such patients.
Methods
A total of 325 breast cancer patients underwent sentinel lymph node biopsy at our institution between June 1998 and May 2004. A 2-day protocol was used to localise the sentinel node with the injection of 99mTc-nanocolloid. There were two phases in the study: the learning phase (105 patients) and the application phase (220 patients). In the learning phase, a complete lymphadenectomy was always performed. In the application phase, sentinel nodes were studied intraoperatively and lymphadenectomy was performed when considered warranted by the pathological intraoperative results.
Results
The median follow-up duration in the 220 patients studied during the application phase was 21.2 months (range 4–45 months). In this phase a total of 427 sentinel nodes were obtained (range 1–5 per patient, median 1.99), with 66 positive sentinel nodes in 56 patients (26%). The lymphadenectomies performed were also positive in 25% of cases (14 patients). We observed a total of two false-negative sentinel lymph node results (3.45%). One of them was found during the surgical excision of non-sentinel nodes, and the other presented as an axillary recurrence 17 months postoperatively (1.72% clinical false-negative rate). The latter patient died 1 year after the first recurrence.
Conclusion
After a median follow-up of 21.2 months we observed only one clinical recurrence among 220 patients. Our results indicate that adequate local control is achieved by application of the sentinel node protocol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sentinel lymph node (SLN) biopsy provides accurate staging information in women with breast cancer. Axillary metastasis is the most important prognostic indicator in women with early breast cancer, and SLN biopsy can provide information on axillary metastasis with a reduced morbidity compared with complete axillary lymphadenectomy. Several studies have documented SLN biopsy to be highly predictive of axillary nodal status, with a false-negative (FN) rate of less than 5% after an initial learning curve [1]. According to Nieweg and Estourgie [2], the definition of an FN result may be controversial, and depending on this definition, the rate could vary. One might use the original definition: tumour identification in any axillary lymph node at any time despite observation of a disease-free SLN at initial pathological evaluation. Alternatively, one might consider the procedure to yield an FN result only if an axillary recurrence develops during follow-up. With regard to this question, although the concept of SLN biopsy has been extensively validated in breast cancer, the safety of the technique will only be proven by examining the rate of axillary recurrence and overall survival after long-term follow-up [3]. The initial results seem promising, with minimal axillary recurrences at a median follow-up of 3 years [3, 4], although there are no reports with a longer follow-up. The aim of the present study was to provide new evidence about the safety of SLN biopsy in breast cancer patients and to evaluate our FN SLN procedures.
Materials and methods
Study group
The study group consisted of 325 consecutive patients with breast cancer treated between June 1998 and May 2004 in the Breast Pathology Unit at the Hospital Clinic of Barcelona. The pathological assessment of proven breast cancer was routinely done by core biopsy or fine-needle aspiration. The use of SLN biopsy was introduced in our institution in June 1998 in a learning phase study. During this learning phase, a complete lymphadenectomy was also performed; 105 patients were treated during this phase, until December 2000. After this learning period, we started the application phase. A total of 220 patients were treated in this phase until May 2004, with lymphadenectomy being performed only if intraoperative or delayed biopsy of the SLN was positive. Two surgeons performed nearly 90% of the procedures. Three other surgeons performed the remaining procedures.
SLN biopsy technique
The SLN biopsy technique was performed using a 2-day protocol. On the day prior to surgery, 74–111 MBq of 99mTc-nanocolloid (Nanocoll, Amersham, Saluggia, Italy) was injected into the lesion with a volume of 0.5 ml. This procedure was adopted since we and other authors [5, 6] observed that the SLN identification results achieved were better than those obtained with other approaches (subdermal and peritumoural injections). In cases of non-palpable breast cancer, the intratumoural injection was guided by ultrasound. We made four injections in the cases performed with peritumoural approach.
Scintigraphic images were obtained at 30 min and 2 h after injection, and later if necessary, using a gamma camera (Apex-4 HR, Elscint, Haifa, Israel). Anterior, oblique and lateral images were performed. The locations of a node or nodes were marked on the skin with indelible ink.
On the day of the surgical procedure, methylene blue in a volume of 1 ml was injected into the tumour. Intraoperative detection of the SLN was performed with a gamma ray detection probe (Navigator, USSC/Tyco Healthcare, USA). In some of the patients we used both tracers (radiocolloid and blue dye) to identify the SLN. Also, commencing in 2003 we performed palpation of the wound to localise suspicious nodes.
During lymphoscintigraphy we considered there to be an SLN if the hot spot had an afferent lymphatic channel or it was the first one seen in a sequential pattern or it was the only one depicted. If two hot spots were seen on the first image, both of them were considered to be SLNs. Using the blue dye, a node was identified as an SLN if it was stained blue or if a blue lymphatic channel coming from the tumour reached the lymphatic node (although it was not blue).
A complete axillary lymphadenectomy was performed if no SLN was identified during surgery. These cases were defined as “not found” but not as FNs because no SLN was removed.
Histopathological examination
During the learning phase, a complete lymphadenectomy was performed after SLN identification. The histopathological study consisted of formalin fixation followed by paraffin sectioning and embedding. Three microsections were stained with haematoxylin and eosin (H&E). Negative cases underwent further immunohistochemistry (IHC) analysis with anti-Cam 5.2 cytokeratin (monoclonal, 1/100, Becton-Dickinson, San José, CA).
During the application phase, the fresh tissue containing the SLN (one or more fragments) was submitted for intraoperative pathological examination by cytological imprinting and frozen section. All nodal structures isolated were serially cross-sectioned at 2-mm intervals perpendicular to the longitudinal axis. In cases in which the size of the SLN was less than 5 mm, bisection along the longest axis was considered acceptable. Cytological imprinting was then performed from alternative cut surfaces on a glass slide (all imprints from each node usually on a single glass slide). The whole material was then submitted to frozen section. Two consecutive 4-μm-thick sections, each containing all tissue surfaces, were cut and intraoperatively evaluated by the pathologist. When the pathologist found a metastatic node, a complete lymphadenectomy was performed at the same time.
For definitive evaluation, SLNs were fixed in neutral buffered formalin, embedded in paraffin blocks and routinely processed for H&E examination. In our department, two or three 4-μm-thick sections are usually discarded to obtain an adequate slide. One or more slides (depending on the number of isolated SLNs), including all tissue surfaces, were H&E stained.
When the pathologist did not identify tumour cells, IHC with anti-Cam 5.2 cytokeratin (monoclonal, 1/100, Becton-Dickinson) was performed, which increases the sensitivity of detection of micrometastasis.
False-negative procedures
An SLN procedure was considered FN if the pathological evaluation did not demonstrate tumour cells in the SLN, while one or more non-SLNs were tumour-positive. During the learning phase these cases were detected by axillary lymphadenectomy. Once the technique was accepted for inclusion in a routine clinical procedure, the FN cases were detected on the basis of the definitive delayed pathological specimen or as a clinical recurrence during follow-up.
Follow-up
After completion of surgery and adjuvant therapy, all patients were followed up at the Breast Pathology Unit with clinical examination at 3- to 4-month intervals and mammography at 6- to 12-month intervals.
Results
Learning phase
During the learning phase, 105 patients were treated. The median age of the patients was 56.8 years (range 23–88). Median tumour size was 1.74 cm (range 0.3–4.5 cm). Twenty-six mastectomies were performed (24.7%; 26/105).
There were nine negative lymphoscintigraphies (8.6%). An SLN was not found in ten patients (9.6%), including those with a negative lymphoscintigraphy. Blue dye was used in 80 patients (76%). We obtained 139 SLNs with a median of 1.47 (range 1–3). There were 31 positive SLNs in 24 of the 95 patients with identification of an SLN (25.2%). Among these 24 patients, only one positive node was found in 12 (50%). There were two FN cases (2/26), giving a percentage of 7.6%; both were detected in the lymphadenectomy sample performed in the first 50 cases.
The first case was a 39-year-old patient with a palpable left supra-areolar tumour of 2.8 cm. It was a moderately differentiated ductal carcinoma. Lymphoscintigraphy with peritumoural injection showed an isolated axillary hot spot that was also localised with blue dye on the following day in the operating theatre, first thing in the morning. This SLN was negative but we found one positive node among 12 at lymphadenectomy, this being a 4-mm metastasis. Conservative surgery was performed followed by chemotherapy, radiotherapy and tamoxifen. The patient is disease free 65 months later.
The second case was very similar. This patient was also 39 years old, and had a palpable left supra-areolar tumour of 2.5 cm. It was a grade III ductal carcinoma. Lymphoscintigraphy with peritumoural injection showed an isolated axillary hot spot that was also localised with blue dye on the following day in the operating theatre, first thing in the morning. This SLN was negative but we found another positive node among 19 at lymphadenectomy, this being a 15-mm macroscopic metastasis. A mastectomy was performed followed by chemotherapy. Two years afterwards, the patient developed a contralateral breast tumour. A mastectomy was performed, again followed by chemotherapy. The patient is disease free 59 months after the first tumour. In this case, the injection of 99mTc-nanocolloid was peritumoural and not intratumoural. At that time we did not perform palpation of the wound during surgery to feel for enlarged nodes because routine lymphadenectomy was always performed. One could hypothesise that such a metastasis would be palpable and would now be detected independently of the radioactivity and blue dye depiction. Lymph flow obstruction could have been the reason for this FN case.
Tables 1 and 2 show the results of this learning phase.
Application phase
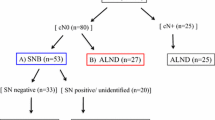
During the application phase, 220 patients were treated. The median age of the patients was 60.4 years (range 30–89). Median tumour size was 1.74 cm (range 0.3–3.6). Ten mastectomies were performed (4.5%; 10/220). The median follow-up duration in these patients was 21.2 months (range 4–45).
There were seven negative lymphoscintigraphies (3.2%). We did not find an SLN in six patients (2.7%), including five patients with negative lymphoscintigraphy and another in whom a surgeon not used to the SLN technique was operating. In this phase the blue dye was used in only 92 patients (41.8%). We obtained 427 SLNs with a median of 1.99 (range 1–5). There were 66 positive SLNs in 56 patients (26%). Among the positive SLN patients, in 42 there was only one positive node (75%). There were two FN cases (2/58), giving a rate of 3.45%. One case was detected because of the removal of palpable non-SLNs and the other because of a clinical axillary recurrence (1.72% clinical FN rate).
The first case was a 65-year-old patient with a palpable left tumour of 2 cm. It was a moderately differentiated ductal carcinoma. Lymphoscintigraphy with intratumoural injection showed three axillary hot spots that were also localised with blue dye on the following day in the operating theatre, first thing in the morning. These SLNs were negative but the palpation of the wound included in the protocol at that time localised two more nodes that were studied postoperatively. The pathologist found a metastasis in each node, with a maximum size of 3 mm. Subsequent lymphadenectomy identified a further positive node among the nine excised, this also being a 3-mm metastasis. Conservative surgery was performed followed by radiotherapy and tamoxifen. The patient refused chemotherapy. She is disease free 14 months later. We have not found any possible mistake in this case and it reinforces the necessity of wound palpation.
The second case was a 61-year-old patient with a non-palpable left tumour of 1.5 cm. It was a grade II ductal carcinoma. Lymphoscintigraphy with intratumoural injection showed two axillary hot spots. The operation was performed first thing the following morning. Blue dye was not used in this case. These SLNs were negative (H&E and IHC). Hormonal receptors were negative and p53 was positive. Conservative surgery was performed followed by chemotherapy and radiotherapy. Nineteen months later the patient had an axillary recurrence with lung metastasis. She was treated with radiotherapy and different regimens of chemotherapy and finally died 11 months after the recurrence. It is difficult to find an explanation for this clinical FN case. We did not use blue dye but we had no problems in localising the SLN. The original SLNs were reviewed and, again, no metastasis was found.
Discussion
Our results show primarily that a learning phase is always needed. During the learning phase we were not able to find an SLN in 9.6% of patients. Furthermore, we had an FN rate of 7.6%. During the application phase we reduced the rate of “not found” SLNs to 2.7% and the FN rate to 3.45%. A review of learning phase studies showed a wide variety of FN rates between 0% and 40% [7], although some authors have established that an acceptable rate of FNs would be about 5% with an identification rate above 90% [1]. After the learning phase we were able to meet both standards. In a recently published randomised trial [8] comparing routine axillary dissection and SLN biopsy, the rate of FNs in the axillary dissection group was 8.8%, similar to our results in the learning phase. Another study, again with complete lymphadenectomy, reported an FN rate of 18% [9]. In each of the aforementioned studies, the accepted definition of FN rate was applied (number of patients with metastasis to non-SLNs/total number of patients with axillary metastasis × 100). We therefore concluded that our learning phase was successful and we decided to start the application phase [10].
We now consider further the two FN cases which occurred during the learning phase. These cases could have been avoided in some circumstances. In the first case we performed peritumoural injections instead of intratumoural injection. In our experience, intratumoural injection is superior to peritumoural injection, in accordance with the findings of Valdés-Olmos and Van der Ent [5, 6, 11], though others disagree [1, 12]. Another possible reason for these FNs could have been the size of the tumours: both were larger than 2 cm, and some authors [9, 13, 14] have found that a tumour size greater than 2 cm is correlated with metastasis in a non-SLN.
It has been postulated that grossly positive nodes could block lymphatic channels, thereby preventing the passage of mapping agents that are channelled to a pathologically negative node [15]. One of the FN patients had a macrometastasis of 15 mm, with the other having a 4-mm metastasis. Macrometastasis could have been the reason for the FN. Also, with the current practice of intraoperative palpation of the biopsy wound we would probably have detected this macrometastasis. According to Nieweg and Estourgie [2], if palpation of the biopsy wound at the time of surgery is part of the standard procedure, the FN rate will be reduced.
During the application phase we had a 3.45% FN rate and a clinical FN rate of 1.72%, accepting that we detected one case by palpation of the wound. The clinical purpose of identifying metastatic disease in a less invasive manner was achieved.
One weakness in the progress from the learning phase to the application phase was the reduction in blue dye use from 76% to 41.8%. Although the employment of blue dye does not dramatically increase the rate of SLN detection, most authors agree with the use of both methods (radiocolloid and blue dye) to enhance the detection rate [16, 17]. In the clinical FN patient, blue dye was not injected, and it is possible that this would have been helpful. We are firmly trying to improve this step of the protocol.
Our clinical FN rate is similar to that reported in other series [4, 18]. Chung et al. [4] reported a clinical FN rate of 1.4% in 206 patients with negative SLNs and a median follow-up of 26 months. Roumen et al. [18] found a clinical FN rate of 1% in 100 patients and a median follow-up of 24 months, and Giuliano et al. [19] achieved a 0% clinical FN rate, though in only 67 patients. The low rate of axillary recurrence when SLN biopsy is performed is comparable to the rate of about 1% recurrence obtained after formal axillary dissection or radiotherapy [20, 21].
Our series represents one of the largest published, although a potential criticism of our study is the short follow-up period of 21 months. It is possible that more regional recurrences would be detected with a longer follow-up, although Veronesi et al. [8] did not find any axillary metastases among 167 SLN-negative patients after a median follow-up of 46 months.
It can be concluded that SLN biopsy is a safe procedure, although the prescribed technique should be followed carefully and a learning phase is required. We recommend the use of a combined method (radiocolloid and blue dye) to identify the SLN, with palpation of the biopsy wound to identify enlarged suspicious nodes. Adopting these procedures, we obtained a low clinical recurrence rate of 1.72% with a follow-up period of 21 months, although it is recognised that more future recurrences are possible.
References
Allweis TM, Badriyyah M, Bar Ad V, Cohen T, Freund HR. Current controversies in sentinel lymph node biopsy for breast cancer. Breast 2003;12:163–71.
Nieweg OE, Estourgie SH. What is a sentinel node and what is a false-negative sentinel node?. Ann Surg Oncol 2004;11(3):169S–73S.
Torrenga H, Meijer S, Fabry H, van der Sijp J. Sentinel node biopsy in breast cancer patients: triple technique as a routine procedure. Ann Surg Oncol 2004;11(3):231S–5S.
Chung MA, Steinhoff MM, Cady B. Clinical axillary recurrence in breast cancer patients after a negative sentinel node biopsy. Am J Surg 2002;184:310–4.
Ortega M, Vidal-Sicart S, Zanón G, Pahisa J, Santamaría G, Velasco M, et al. Comparative study of the different radiotracer administration route to locate the sentinel node in breast cancer. Rev Esp Med Nucl 2004;23:153–61.
Valdés-Olmos RA, Jansen L, Hoefnagel CA, Nieweg OE, Muller SH, Rutgers EJ, et al. Evaluation of mammary lymphoscintigraphy by a single intratumoural injection for sentinel node identification. J Nucl Med 2000;41:1500–6.
Nieweg OE, Jansen L, Valdés-Olmos RA, Rutgers EJ, Peterse JL, Hoefnagel KA, et al. Lymphatic mapping and sentinel node biopsy in breast cancer. Eur J Nucl Med 1999;26:S11–6.
Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003;349:546–53.
Sachdev U, Murphy K, Derzie A, Jaffer S, Bleiweiss IJ, Brower S. Predictors of nonsentinel nodes mestastasis in breast cancer patients. Am J Surg 2002;183:213–7.
Zanón G, Vidal-Sicart S, Ortega M, Pahisa J, Velasco M, Fernández PL, et al. Ganglio centinela en el carcinoma de mama. Estudio de 175 casos. Prog Obstet Gynecol 2002;45:280–6.
Van der Ent FW, Kengen RA, Vande Pol HA, Povel JA, Stroeken HJ, Hoofwijk AG, et al. Halsted revisited: internal mammary sentinel node biopsy in breast cancer. Ann Surg 2001;234(1):79–84.
Moreno A, Román JM, Ruiz J, González A, Delgado R, Cabrera MN, et al. Controversias en la biopsia del ganglio centinela de la mama. Rev Oncol 2004;6(3):122–9.
Reynolds C, Mick R, Donohue JH, Grant CS, Farley DR, Callans LS, et al. Sentinel node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer?. J Clin Oncol 1999;17:1720–6.
Weiser RW, Montgomery LL, Tan LK, Susnik B, Leung DY, Bergen PI, et al. Lymphovascular invasion enhances the prediction of nonsentinel node metastases in breast cancer patients with positive sentinel nodes. Ann Surg Oncol 2001;8:45–9.
Quan ML, McCready D, Temple WJ, McKinnon JG. Biology of lymphatic metastases in breast cancer: lessons learned from sentinel node biopsy. Ann Surg Oncol 2002;9(5):467–71.
Derossis AM, Fey J, Yeung H, Yeh SD, Heerdt AS, Petrek J, et al. A trend analysis of the relative value of blue dye and isotope localization in 2000 consecutive cases of sentinel node biopsy for breast cancer. J Am Coll Surg 2001;193(5):473–8.
Fraile M, Solá M, Vidal-Sicart S, Solsona J, Martín J. Revisión sistemática de la literatura científica sobre la técnica del ganglio centinela en el cáncer de mama. Rev de Senología y Patología Mamaria 2003;16:16–30.
Roumen RM, Kuijt GP, Liem IH, van Beek MW. Treatment of 100 patients with sentinel node-negative breast cancer without further axillary dissection. Br J Surg 2001;88:1639–43.
Giuliano AE, Haigh PI, Brennan MB, Hansen NM, Kelley MC, Ye W, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel-node-negative breast cancer. J Clin Oncol 2000;18:2553–9.
Chua B, Ung O, Boyages J. Competing considerations in regional nodal treatment for early breast cancer. Breast J 2002;8:15–22.
Newman LA, Hunt KK, Buchholz T, Kuerer HM, Vlastos G, Mirza N, et al. Presentation, management and outcome of axillary recurrence from breast cancer. Am J Surg 2000;180:252–6.
Acknowledgements
We thank Dr. Jonathan Rogerson for the English revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanjuàn, A., Vidal-Sicart, S., Zanón, G. et al. Clinical axillary recurrence after sentinel node biopsy in breast cancer: a follow-up study of 220 patients. Eur J Nucl Med Mol Imaging 32, 932–936 (2005). https://doi.org/10.1007/s00259-005-1763-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-1763-6