Abstract
Purpose
Scintigraphic localisation of parathyroid glands is often unsuccessful in patients with renal failure on chronic haemodialysis who have secondary hyperparathyroidism (HPT). The purpose of this study was to investigate the use of 11C-methionine PET/CT to detect hyperfunctioning parathyroid glands in patients with renal failure on chronic haemodialysis who had 99mTc-sestamibi-negative HPT.
Methods
11C-methionine PET/CT was performed in 18 patients (11 women and 7 men, aged 42–79 years; mean age 57.8 years) on haemodialysis for renal failure (2–14 years’ duration), with normo-, hypo- or hypercalcaemia and HPT not localised by either dual-tracer 99mTc-pertechnetate/99mTc-sestamibi subtraction scans or dual-phase 99mTc-sestamibi scans.
Results
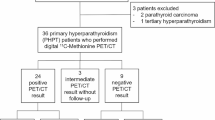
In three of ten patients with normo- or hypocalcaemic HPT there was increased 11C-methionine accumulation in one gland. Seven of eight patients with hypercalcaemic HPT showed increased uptake: in five of these patients increased 11C-methionine accumulation was present in one gland, while in two it was demonstrated in two glands. All patients also had high-resolution ultrasound of the neck and were treated with subtotal parathyroidectomy, leaving a remnant of the smallest of the four glands. Regardless of their size, all glands with abnormal 11C-methionine parathyroid uptake were removed, and all demonstrated parathyroid hyperplasia. All patients developed post-parathyroidectomy hypoparathyroidism and one patient with normocalcaemic HPT relapsed 8 months after surgery.
Conclusion
These data suggest that 11C-methionine PET/CT may be used to identify hyperfunctioning parathyroid glands in non-primary HPT, and especially hypercalcaemic HPT, when conventional 99mTc-sestamibi imaging is non-localising.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Parathyroid scintigraphy with methionine was first introduced almost 35 years ago using 75Se-selenomethionine [1–5]. The success of parathyroid localisation was variable, and methods were offered to enhance imaging by incorporating thyroid subtraction with 99mTc-pertechnetate and pharmacological manipulation of serum calcium levels [6]. 75Se-selenomethionine imaging was subsequently supplanted by more efficacious radiopharmaceuticals like 201Tl, 99mTc-sestamibi (MIBI), 99mTc-tetrofosmin and variations of MIBI or 99mTc-tetrofosmin imaging using thyroid subtraction with 123I or 99mTc or, more recently, dual-phase MIBI or 99mTc-tetrofosmin scans [7–10]. The availability of the positron-emitting radioisotope 11C has allowed a re-evaluation of methionine as a parathyroid imaging agent, taking advantage of the increased resolution afforded by positron emission tomography (PET), and 11C-methionine has been used to identify hyperfunctioning parathyroid tissues in primary, secondary and recurrent hyperparathyroidism [11–14]. In this study we investigated the value of 11C-methionine PET/CT in a small group of patients with renal failure on chronic dialysis who had normo-, hypo- or hypercalcaemic hyperparathyroidism and non-localising conventional parathyroid scintigraphy with either MIBI/99mTc subtraction or dual-phase MIBI imaging studies.
Materials and methods
Eighteen patients [11 women and 7 men, aged 42–79 years (mean 57.8 years)] with non-primary hyperparathyroidism were studied with 11C-methionine PET/CT scans. The study was approved by the Human Experimentation Committee (IRB), and each patient provided written informed consent prior to PET/CT. Ten patients had normo- or hypocalcaemic hyperparathyroidism and eight, hypercalcaemic hyperparathyroidism, the diagnosis being made on the basis of the relationship between serum calcium and parathyroid hormone levels (Table 1). All patients had been treated with haemodialysis for renal failure for 2–14 years.
Initial parathyroid imaging was performed in two centres (the S. Orsola-Malpighi Hospital, Bologna, Italy and the S. Maria della Misericordia Hospital, Rovigo, Italy), using dual-tracer 99mTc-pertechnetate/99mTc-sestamibi (MIBI) subtraction scanning in ten patients and dual-phase 99mTc-sestamibi imaging in eight cases, employing previously described methods [15]. High-resolution ultrasound of the neck was also performed with 7.5- to 10-MHz transducers, and parathyroid glands were identified as hypoechoic areas located near the thyroid gland that had well-defined margins and were distinct from thyroid tissue. PET was performed with a dedicated PET/CT tomograph (Discovery 4ST, GE Corporation, Milwaukee, WI, USA) 10 min after the intravenous injection of 370–444 MBq (10–12 mCi) of 11C-methionine. Images of the neck and thorax were obtained in three consecutive bed positions, each of 4-min duration, from the base of the skull to the diaphragm. The standardised uptake value (SUV) was calculated using the region of interest (ROI, 3×3 pixels, matrix 128×128) method and was corrected for weight and height.
L-[S-methyl-11C]-methionine (11C-methionine) was synthesised according to the solid phase method, as described by Pascali et al. [16] Briefly, 11CO2 produced by a PET-trace cyclotron (GE Medical Systems, Milwaukee, WI, USA) was converted into 11CH3I by the conventional LiAlH4 /HI reaction. 11CH3I was used for the methylation of L-homocysteine thiolactone (freshly dissolved in 0.5 M NaOH in EtOH/H2O 50/50) directly placed on solid phase support (tC18 Plus SepPak, Waters, Milford, MA, USA). L-[S-methyl-11C]-methionine was eluted with 2.5 ml of 0.05 M NaH2PO4 in the collecting vial containing 4.2 ml of saline and 3.3 ml of the same eluting buffer, sterilised by passage through a 0.22-μm filter and collected in a sterilised vial with a final volume of 10 ml. The duration of synthesis was 14 min and the radiochemical yield was 72%. Evaluation of radiochemical purity was done using radio-HPLC equipped with a reversed-phase column, and the concentration of organic solvents was assessed by gas chromatography. Endotoxin content was measured by a conventional limulus amoebocyte lysate (LAL) method (Cambrex Bioscience, East Rutherford, NJ, USA).
PET/CT interpretations were performed by three nuclear medicine physicians (S.F., C.N., D.R.) with extensive experience in both parathyroid and PET imaging; scan interpretations were done independently and there were no discrepancies amongst the readers, i.e. they agreed on all the pathological glands that were seen.
Intact parathyroid hormone was measured by the immunochemiluminescent method (Liason, Byk Gulden, Italy; normal range 10–54 pg/ml). The normal range of serum calcium levels was considered to be 8.4–10.5 mg/dl.
Results
Serum calcium levels were normal or low (mean 8.9 mg/dl, range 7.8–10.1 mg/dl) and parathyroid hormone levels were elevated (mean 705 pg/ml, range 290–1,900 pg/ml) in 10 of 18 patients, while in 8 of 18 patients both calcium and parathyroid hormone levels were elevated (mean 11.8 mg/dl, range 11.0–12.6 mg/dl, and 306 pg/ml, range 124–478 pg/ml, respectively) (Table 1).
Among the 18 patients, high-resolution ultrasound identified two enlarged parathyroid glands in 11, three glands in six and four enlarged glands in one (Table 1). In ten of the 18 patients (55.5%), PET/CT demonstrated abnormal accumulation of 11C-methionine (one parathyroid gland in eight patients and two glands in two patients) (Table 1). Mean SUV(parathyroid tissue/cervical soft tissue) was 3.3 (range 2.1–4.9) and mean SUV(parathyroid tissue/thyroid tissue) was 2.5 (range 1.5–3.6). There was no significant difference in parathyroid gland 11C-methionine SUVs between the group with normal or low calcium levels and the group with hypercalcaemic hyperparathyroidism. It is of note that in patients with normo- or hypocalcaemic hyperparathyroidism, 11C-methionine PET/CT identified one abnormal gland in only three of ten patients (30%), while in the group with hypercalcaemic hyperparathyroidism, seven of eight patients (87.5%) had abnormal 11C-methionine PET/CT (p<0.01 by χ2 test). Of the latter seven patients, five had one abnormal gland (Figs. 1, 2) and two, two abnormal glands on PET/CT. The significant difference observed in the rate of detection of abnormal parathyroid glands at 11C-methionine PET/CT in the group of normo- or hypocalcaemic patients versus the group of hypercalcaemic patients might be explained by the fact that in hypercalcaemic patients, that is patients more likely to be affected by tertiary hyperparathyroidism, autonomous parathyroid glands could be biologically more active in trapping 11C-methionine. Further, in four of the ten patients (40%) with abnormal 11C-methionine PET/CT scans (all of whom had hypercalcaemic hyperparathyroidism), the largest parathyroid gland seen on high-resolution ultrasound did not demonstrate the highest 11C-methionine avidity. Comparisons of SUV with serum calcium and parathyroid hormone levels and parathyroid gland weight by regression analysis did not reveal statistically significant differences. No patient had co-existing thyroid nodules in the present series.
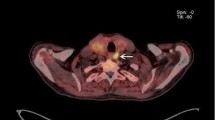
Right inferior parathyroid gland depicted by 11C-methionine (patient 6, hypercalcaemic hyperparathyroidism; Table 1). Upper left, CT scan; upper right, 11C-methionine PET scan [SUV(parathyroid tissue/cervical soft tissue)=2.2]; lower left, 11C-methionine PET/CT fusion image; lower right, right sagittal 11C-methionine PET scan of the neck
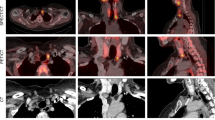
Left superior parathyroid gland depicted by 11C-methionine (patient 1, hypercalcaemic hyperparathyroidism; Table 1). Upper left, CT scan; upper right, 11C-methionine PET scan [SUV(parathyroid tissue/cervical soft tissue)=4.9]; lower left, 11C-methionine PET/CT fusion image
All patients were treated with subtotal parathyroidectomy. In the majority of patients the remnant parathyroid tissue was from the smallest of the parathyroid glands. All 11C-methionine-accumulating glands were resected, regardless of their size. Pathological evaluation of the resected parathyroid glands was consistent with glandular hyperplasia and there were no differences in the pathological findings between 11C-methionine-avid parathyroid glands and non-11C-methionine-avid glands.
Postoperative hypoparathyroidism was seen in all patients. Serum calcium and parathyroid hormone measurements were obtained at 1 month and then at 3-monthly intervals after surgery. Follow-up ranged from 3 to 13 months, with a median of 6 months. One case of recurrent hyperparathyroidism was seen in a woman with normocalcaemic hyperparathyroidism (patient 18, Table 1) 8 months after subtotal parathyroidectomy.
Discussion
Contemporary modalities for the localisation of parathyroid glands in patients with hyperparathyroidism provide high-resolution anatomical and functional maps of abnormal hormone secretion. When used in tandem, neck ultrasound and MIBI scintigraphy using various imaging techniques (thyroid subtraction or dual-phase imaging) have been shown to offer an efficacious approach for localisation of offending glands, for preoperative planning and for intra-operative decision making [15, 17–20]. Although controversy remains over the use of scintigraphy in the initial evaluation of primary hyperparathyroidism and even more so over its use for localisation of multiglandular hyperplasia in the context of renal failure, radionuclide imaging is recommended for recurrent disease or disease located outside of the neck [19–24].
The retention of MIBI by parathyroid tissue appears to be related to the predominant cell type (chief versus oxyphil), the density of mitochondria, the presence of P-glycoprotein and other multi-drug resistance proteins and, of course, the inherent spatial resolution of gamma cameras, which limits scintigraphic identification to glands that are generally larger than 500 mg in primary hyperparathyroidism [21]. In addition, the cell cycle may play a role in imaging, especially in secondary hyperparathyroidism, where parathyroid cells are more likely to be in a G0 or non-growth phase while autonomous parathyroid tissues such as adenomas tend to be in a growth phase (G2+S) [21]. MIBI has been used to depict abnormal parathyroid response to vitamin D therapy in patients with renal failure on chronic haemodialysis as a means to identify glands refractory to therapy with selective parathyroidectomy aimed at preserving parathyroid function [22].
Other tracers have been used with some success for parathyroid localisation, such as 18F-fluorodeoxyglucose (FDG), which depicts the process of glucose accumulation and phosphorylation. However, when using FDG, the specificity is lower owing to the normal accumulation of the tracer by the thyroid and other benign and malignant processes in the neck [25, 26].
An alternative to conventional single-photon imaging agents like MIBI and FDG is methionine, an agent that had demonstrated some value for parathyroid imaging when labelled with 75Se, and can now be labelled with an alternative, positron-emitting isotope, 11C [11–14]. The mechanisms of accumulation of methionine, a neutral amino acid, by parathyroid tissues are transmembrane amino acid transport, protein synthesis and methionine donor transmethylation [11, 14]. Previous studies in hyperparathyroidism demonstrated that accumulation of 11C-methionine correlated with serum parathyroid hormone and calcium levels and measurements of SUV, and it was estimated that the lower limit of detection of parathyroid adenoma by 11C-methionine PET is ∼200 mg [12, 14]. Further, these reports noted that, in comparison with adenomas, parathyroid hyperplasia exhibited a lower SUV and other measurements of methionine transport. Lower accumulation of 11C-methionine in hyperplastic glands and the small number of cases in our series, as compared with other studies, may account for the lack of a statistically significant relationship between SUV and parameters of parathyroid hyperfunction and the weight of the resected glands [13, 14]. Although the number of cases studied with 11C-methionine PET to date is small, its efficacy in localising hyperfunctioning glands in hyperparathyroidism appears superior to that of MIBI [12–14, 23]. Unfortunately, 11C has a short half-life (∼20 min), necessitating close proximity to a source of isotope and the synthetic capacity to produce 11C-methionine; this limits its availability to only a few centres.
An interesting aspect of the present study is the use of a hybrid PET/CT tomograph to evaluate the potential role of 11C-methionine in visualising MIBI-negative enlarged parathyroid glands in patients with secondary and tertiary hyperparathyroidism. Very recently, Biggs and Hain [27] reported a similar study, though with a PET scanner, in which the role of 11C-methionine in visualising MIBI-negative or equivocal parathyroid adenomas in patients with primary hyperparathyroidism was assessed. These authors found 11C-methionine PET to have a sensitivity of 83%, a specificity of 100% and an accuracy of 88% in a group of 51 patients evaluated retrospectively. The authors concluded that 11C-methionine PET scanning is valuable in patients with primary hyperparathyroidism in whom conventional imaging techniques have failed to localise the adenoma before proceeding to surgery, or in whom surgery has been performed but has failed to correct the hyperparathyroidism.
In the present, admittedly small series, CT appeared to add little to the functional localisation information derived from the pattern of 11C-methionine uptake on PET imaging alone. CT has also appeared to be of limited supplementary value in studies using combined MIBI SPECT/CT, although SPECT itself has been found to offer some increase in resolution over planar studies in primary and recurrent hyperparathyroidism [28, 29]. The reasons for the lack of benefit from additional CT information are obscure, since high-resolution ultrasound was successful in identifying multiple glandular abnormalities in these patients. It is possible that no substantial incremental benefit is to be derived from direct anatomical correlation when hyperfunctioning parathyroid glands are small, as in most cases (this is especially true of hyperplastic glands), and when a radiopharmaceutical with high specificity is deployed to evaluate abnormal function in a very small, circumscribed region. However, the presence of thyroid pathology, i.e. multinodular goitre, has an effect upon the efficacy of MIBI imaging in hyperparathyroidism since thyroid adenomas and carcinomas will accumulate MIBI, resulting in a reduction in specificity in the search for parathyroid disease. In this context, high-resolution CT should have some value in distinguishing parathyroid from non-parathyroid MIBI-accumulating lesions. This is less apparent for 11C-methionine PET/CT, but larger studies in patients with underlying thyroid pathology will be needed to answer this question. Our data confirm the results of others that 11C-methionine PET can be used to identify hyperactive parathyroid glands in patients with non-primary hyperparathyroidism not detected by conventional single-photon imaging techniques and that autonomously functioning glands are more readily depicted than functional hyperplasia [13, 14]. Further, we noted discordance between the size of some parathyroid glands seen on high-resolution ultrasound and their functional abnormality depicted by 11C-methionine PET, which was also reflected in the absence of a relationship between the SUV and serum calcium and parathyroid hormone. While the numbers of patients in this series are small, the data offer interesting insights into the possibility of using 11C-methionine accumulation to identify the most functionally abnormal parathyroid glands in patients with non-primary hyperparathyroidism and perhaps those glands that are becoming or are already autonomous. This knowledge would be most useful in predicting the course of development in the natural history of secondary hyperparathyroidism and, more importantly, in choosing the optimal time for selective and limited surgical intervention.
Another limitation of our study is that each patient was scanned only once with 11C-methionine PET, thus providing only a single “snapshot” of parathyroid function in what is obviously a continuum from normal to hyperplasia to autonomy, the time course of which may differ between individual patients and individual parathyroid glands. Despite the shortcomings of the present study, 11C-methionine uptake in a parathyroid gland appears to predict hyperfunction in hypercalcaemic hyperparathyroidism and in some patients with normo- or hypocalcaemic hyperthyroidism, as it does in primary hyperparathyroidism. Our results demonstrate that size may not be the best determinant of dysfunction and perhaps autonomy and suggest that 11C-methionine PET may provide additional information in the evaluation of patients with non-99mTc-MIBI-avid parathyroid disease.
In conclusion, the data of the present study suggest that 11C-methionine PET/CT may be used to identify hyperfunctioning parathyroid glands in non-primary hyperparathyroidism, and especially hypercalcaemic hyperparathyroidism, when conventional 99mTc-sestamibi imaging is non-localising.
References
Potchen EJ, Wilson RE, Dealy JB Jr. External parathyroid scanning with Se75 selenomethionine. Ann Surg 1965;162:492–504.
Sack H, Petry R, Duwell HJ. Darstellung eines Nebenschilddrusenadenoms mit Selen-methionin und der Szintillationskamera. Dtsch Med Wochenschr 1965;90:2353–4.
Grebe SF. Szintigraphische Darstellung eines Parathyreoideaadenoms mit 75Se-methionin. Med Klin 1967;62:672–4.
Piret L, Beckers C, DeVisscher M. Interet de la gammagraphy a la 75Se-selenomethionine dans la mise en evidence pre-operatoire de tumeurs parathyroidiennes. Revue Medicale de Liege (Suppl) 1968;1:225.
Conte N, Ziliotto D, Scandellari C. Localizzazione scintigrafica delle neoplasie paratiroidee con seleniometionina-Se-75. Acta Isot (Padova) 1965;5:337–45.
Askhar FS, Naya JL, Smith EM. Parathyroid scanning with 75Se-selenomethionine and glucagon stimulation. J Nucl Med 1971;12:751–3.
Winzelberg GG, Hydovitz JD. Radionuclide imaging of parathyroid tumors: historical perspectives and newer techniques. Semin Nucl Med 1985;15:161–70.
O’Doherty MJ, Kettle AG. Parathyroid imaging: preoperative localization. Nucl Med Commun 2003;24:125–31.
Sekiyama K, Akaakura K, Mikami K, Mizoguchi K-I, Tobe T, Nakano K, et al. Usefulness of diagnostic imaging in primary hyperparathyroidism. Int J Urol 2003;10:7–11.
Clark PB, Case D, Watson NE, Perrier ND, Morton K. Enhanced scintigraphic protocol required for optimal preoperative localization before targeted minimally invasive parathyroidectomy. Clin Nucl Med 2003;28:955–60.
Cook GJ, Wong JC, Smellie WJ, Young AE, Maisey MN, Fogelman I. [11C]methionine positron emission tomography for patients with persistent or recurrent hyperparathyroidism after surgery. Eur J Endocrinol 1998;139:195–7.
Sundin A, Johansson C, Hellman P, Bergstrom M, Ahlstrom H, Jacobson GB, et al. PET and parathyroid L-[carbon-11] methionine accumulation in hyperparathyroidism. J Nucl Med 1996;37:1766–70.
Hellman P, Ahlstrom H, Bergstrom M, Sundin A, Langstrom B, Westerberg G, et al. Positron emission tomography with 11C-methionine in hyperparathyroidism. Surgery 1994;116:974–81.
Otto D, Boerner AR, Hoffman M, Brunkhorst T, Meyer GJ, Petrich T, et al. Pre-operative localization of hyperfunctional parathyroid tissue with 11C-methionine PET. Eur J Nucl Med Mol Imaging 2004;31:1405–12.
Rubello D, Pelizzo MR, Boni G, Schiavo R, Vagelli L, Villa G, et al. Radioguided surgery of primary hyperparathyroidism using the low-dose 99mTc-sestamibi protocol: multiinstitutional experience from the Italian Study Group on Radioguided Surgery and Immunoscintigraphy (GISCRIS). J Nucl Med 2005;46:220–6.
Pascali C, Bogni A, Iwata R, Decise D, Crippa F, Bombardieri E. High efficiency preparation of L-[S-methyl-11C]-methionine by on-column 11C methylation on C18 Sep-Pak. J Labelled Cpd Radiopharm 1999;42:715–24.
Rubello D, Pelizzo MR, Gross MD, Fig LM, Shapiro B, Mariani G. Controversies on minimally invasive procedures for radio-guided surgery of parathyroid tumours. Minerva Endocrinol 2004;29:189–93.
Van der Wall H, Carmalt H, Fogelman I. 99mTc-sestamibi and minimally invasive radioguided surgery for primary hyperparathyroidism. J Nucl Med 2005;46:198–99.
Gotthardt M, Lohmann B, Behr TM, Bauhofer A, Franzius C, Schipper ML, et al. Clinical value of parathyroid scintigraphy with technetium-99m methoxyisobutylisonitrile: discrepancies in clinical data and a systematic metaanalysis of the literature. World J Surg 2004;28:100–7.
Ryan JA, Faye TL. Maximizing outcomes while minimizing exploration in hyperparathyroidism using localization tests. Arch Surg 2004;139:838–43
Biertho L, Kim C, Wu H-S, Unger P, Inabnet WB. Relationship between sestamibi uptake, parathyroid hormone assay, and nuclear morphology in primary hyperparathyroidism. J Am Coll Surg 2004;199:229–33.
Kakuta T, Suzuki Y, Tadaki F, Tanaka R, Sakai H, Kurokawa K, et al. Long-term prognosis of parathyroid function for chronic dialysis patients after minimally invasive radioguided parathyroidectomy (MIRP). Nephrol Dial Transplant 2003;18 (Suppl 3):iii71–75.
Fuster D, Ybarra J, Torregrosa JV, Setoain X, Martín F, Otrega ML, et al. Double-phase parathyroid 99mTc-sestamibi scintigraphy in chronic hemodialysis patients: correlation with biochemical markers of parathyroid function. Nucl Med Commun 2003;24:85–90.
Pons F, Torregrosa JV, Vidal-Sicart S. Preoperative parathyroid gland localization with technetium-99m sestamibi in secondary hyperparathyroidism. Eur J Nucl Med 1997;24:1494–8.
Newmann DR, Esselstyn Jr. CB, MacIntyre WJ, Chen EQ, Go RT, Licata AA. Regional body FDG-PET in post-operative recurrent hyperparathyroidism. J Comput Assist Tomogr 1997;21:25–8.
Pacak K, Eisenhofer G, Goldstein DS. Functional imaging of endocrine tumors: role of positron emission tomography. Endocr Rev 2004;25:568–80.
Beggs AD, Hain SF. Localization of parathyroid adenomas using 11C-methionine position emission tomography. Nucl Med Commun 2005;26:133–6.
Sfakianakis GN, Irvin GL, Foss J, Foss J, Mallin W, Georgiou M, et al. Efficient parathyroidectomy guided by SPECT-MIBI and hormonal measurements. J Nucl Med 1996;37:798–804.
Moka D, Voth E, Dietlein M, Larena-Avellaneda A, Schicha H. Technetium 99m-MIBI-SPECT: a high sensitive diagnostic tool for localization of parathyroid adenomas. Surgery 2000;128:29–35.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rubello, D., Fanti, S., Nanni, C. et al. 11C-methionine PET/CT in 99mTc-sestamibi-negative hyperparathyroidism in patients with renal failure on chronic haemodialysis. Eur J Nucl Med Mol Imaging 33, 453–459 (2006). https://doi.org/10.1007/s00259-005-0008-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-0008-z