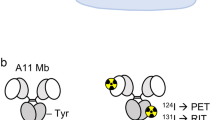
Abstract
Purpose
Lutetium-177 (177Lu) is a radionuclide of interest for radioimmunoimaging (RII) and radioimmunotherapy (RIT) on account of its short half-life (161 h) and the ability to emit both β and γ radiation. Single-chain Fv (scFv) constructs have shown advancement in cancer diagnosis and therapy due to the pharmacokinetics advantage and seem to be intriguing tools in oncology. The objective of this study was to evaluate the pharmacokinetics and biodistribution characteristics of the 177Lu-labeled tetravalent scFv of CC49 MAb and intact CC49 IgG in vivo.
Methods
Conjugation and labeling conditions of multivalent scFv with 177Lu were optimized without affecting integrity and immunoreactivity. For this purpose, multivalent scFv constructs {dimer, sc(Fv)2; tetramer, [sc(Fv)2]2} of the MAb CC49 were expressed as secretory proteins in Pichia pastoris. The purified scFv constructs and IgG form of CC49 were conjugated with a bifunctional chelating agent, ITCB-DTPA, and labeled with 177Lu. The comparative biodistribution, blood clearance, and tumor-targeting characteristics of 177Lu-labeled tetravalent [sc(Fv)2]2 construct of CC49 MAb and intact CC49 IgG were investigated in the athymic mice bearing LS-174T xenografts.
Results
Approximately, 90% of 177Lu incorporation was achieved using ITCB-DTPA chelator, and the labeled immunoconjugates maintained integrity and immunoreactivity. Blood clearance studies demonstrated an alpha half-life (t1/2α) of 177Lu-labeled [sc(Fv)2]2 and IgG of CC49 at 4.40 and 9.50 min and a beta half-life (t1/2β) at 375 and 2,193 min, respectively. At 8 h post administration, the percent of the injected dose accumulated/gram (%ID/g) of the LS-174T tumor was 6.4±1.3 and 8.9±0.6 for 177Lu-labeled [sc(Fv)2]2 and IgG of CC49, respectively, in the absence of l-lysine. The corresponding values were 8.0±0.6 and 8.4±1.2 in the presence of l-lysine. Renal accumulation of [sc(Fv)2]2 was significantly (p<0.005) reduced in the presence of l-lysine.
Conclusion
The results of this study demonstrate that the ITCB-DTPA conjugation and 177Lu-labeling of scFvs are feasible without influencing the antibody characteristics. 177Lu-labeled [sc(Fv)2]2 showed faster clearance and equivalent tumor uptake at 8 h compared with its IgG form, with a markedly reduced renal uptake in the presence of l-lysine. Therefore, 177Lu-labeled [sc(Fv)2]2 may be a potential radiopharmaceutical for the treatment of cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The binding of monoclonal antibodies (MAb) to tumor-associated antigens (TAA) offers a powerful approach to cancer therapy in view of their exquisite specificity and targeting capability, coupled with the delivery of cytocidal agents (i.e., radionuclides, enzymes, genes, drugs, and cytotoxins) [1–6]. The successful application of radioimmunotherapy (RIT)/radioimmunoimaging (RII) is mainly contingent upon TAA, a specific antibody with favorable pharmacokinetics, radionuclide characteristics, and the method of antibody labeling [1–4, 7]. Intact mouse monoclonal antibodies have certain limitations in cancer treatment due to their long persistence in the blood pool. Further, only a limited quantity is delivered to tumors because of poor diffusion and human anti-mouse antibody (HAMA) responses (when multiple doses are required for therapy) [7–9]. These problems associated with the use of intact murine antibodies have directed the efforts of various groups toward the improvement of antibody molecules using recombinant DNA technology. As a result, several genetically engineered single-chain Fv (scFv) constructs of antibody molecules were developed with reduced immunogenicity, more desirable size, and preferential pharmacokinetics for RII/RIT applications [2, 7, 8, 10–12].
Investigators have previously constructed and characterized the monovalent, divalent, and multivalent scFv of CC49 and other monoclonal antibodies [2, 7–9, 11, 12]. The radioiodinated scFvs of different MAbs disappeared very rapidly from the blood pool and provided a homogeneous tumor penetration. Yet these scFvs showed a significantly lower percent of injected dose in tumors due to short plasma half-lives and lower binding affinity [11, 12]. Similar to radioiodinated scFv, 177Lu-labeled monovalent scFv showed a high renal uptake with low tumor localization [13, 14]. While monovalent scFv (Mr∼30,000) has proven to be an ideal reagent for diagnostic applications on account of its excellent tumor penetration and low background, a low tumor to normal tissue ratio limits the use of monovalent scFv for RIT applications [8, 12, 15, 16]. The scFv constructs with increasing valency have exhibited a marked gain in the functional affinity attributable to multiple interactions within a single antigen–antibody complex, resulting in improved biodistribution and desired pharmacokinetics [8, 15, 17–20].
The procedure of radiometal labeling of the proteins is more complex than the labeling with radioiodine, since radiometal labeling requires conjugation of a bifunctional chelating agent (i.e., DOTA, DTPA) to the proteins [13, 14, 21–24]. One of the potential problems in using the radiometal immunoconjugates, especially with small molecules like scFv, is the high renal uptake, which limits the use of the engineered products for RII and RIT application [13, 14]. Preclinical studies showed that intravenous (i.v.) and intraperitoneal (i.p.) infusion of l-lysine and arginine block negatively charged sites on the surface of renal tubule and thereby prevent binding and subsequent absorption of radiolabeled Fab or scFv fragments [25, 26]. Among the radiometal nuclides, 177Lu is an interesting isotope due to its moderately short half-life (161 h) and the ability to emit both beta and gamma radiation [β497 (maximum) and 133 keV (average) and γ208 (maximum) and 113 keV (average)] [14, 21, 27]. Unlike 90Y, 177Lu has an imagible gamma and this property allows one to track the radioimmunoconjugates during therapy procedures by using external gamma scintigraphy [14]. Several preclinical and clinical studies have been performed with 177Lu-labeled Ig for the treatment of a variety of cancers [14, 27–29].
In the present study, we compared the biodistribution and pharmacokinetic properties of (S)-1-p-isothiocyanatobenzyl-diethylenetriamine penta-acetic acid (ITCB-DTPA or SCN-Bz-DTPA)-conjugated and 177Lu-labeled tetravalent scFv and intact CC49 IgG that recognizes tumor-associated glycoprotein-72 (TAG-72), which is expressed by a majority of human adenocarcinomas and is absent in most normal tissues [30]. Additionally, we performed these studies in the presence of an excess of l-lysine for reducing the nonspecific kidney uptake of the radionuclide. The 177Lu-labeled multivalent construct of CC49 scFv {[sc(Fv)2]2} demonstrated higher tumor localization and a marked reduction in the renal uptake compared with the DOTA-conjugated monomeric scFv [14].
Materials and methods
Purification and characterization of scFvs
The divalent CC49 scFv gene (VL-linker-VH-linker-VL-linker-VH-linker) was constructed (Fig. 1a), cloned and expressed in P. pastoris as described previously [7, 31]. The purified protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to check the integrity, and by solid-phase competition enzyme linked immunosorbent assay (ELISA) for immunoreactivity. Due to the spontaneous noncovalent association of divalent scFv, tetravalent scFv (Fig. 1b) and aggregates were also produced by the cultured yeast cells. Tetravalent and divalent scFvs were separated from aggregates and breakdown products by size exclusion chromatography described previously [7, 31].
Schematic representation of multivalent single-chain Fv constructs of the CC49 expressed in P. pastoris. A scheme for the construction of scFv is shown in a. The high level of expression of divalent scFv results in the formation of a noncovalent stable tetravalent scFv (b). VL and VH are the variable light and heavy chain domains of MAb CC49, respectively. L designates the 205C linker that is composed of 25 amino acids (LSADDAKKDAAKKDDAKKDDAKKDL), and the His is a purification tag (H-H-H-H-H-H). Noncovalent bonds are denoted by the dotted lines
SDS-PAGE analyses
Recombinant protein products were evaluated by SDS-PAGE according to the method described by Laemmli [32], with or without reduction by β-mercaptoethanol. Protein bands were visualized by staining the gels with Coomassie Blue R-250. 177Lu-labeled immunoconjugates were visualized by autoradiography using Kodak film (Rochester, NY) and a DuPont (Wilmington, DE) Lightning-Plus intensifying screen. Films were exposed to gel for 1–2 h at −70°C and then developed.
Preparation of ITCB-DTPA-sc(Fv)2, ITCB-DTPA-[sc(Fv)2]2 and ITCB-DTPA-IgG immunoconjugates
All the reagents used for the preparation of ITCB-DTPA immunoconjugates were treated with Chelex-100 resin (Bio-Rad Laboratories, CA) to eliminate metal ions. Antibody buffer (PBS, pH 7.4) was exchanged with sodium carbonate buffer (0.05 M, pH 8.3) by using Centricon 30 (Millipore Corporation, MA, USA). Finally, antibodies were diluted at 5 mg/ml (33 μM) in sodium carbonate buffer pH 8.3 and then 33 μl aqueous solution (5 mM) of ITCB-DTPA was added. The reaction was allowed to proceed for 2 h at room temperature. The unbound or free ITCB-DTPA was removed from the mixture and the sodium carbonate buffer was exchanged by PBS (pH 7.4) by ultra-filtration using Centricon 30 concentrator. Finally the immunoconjugates were diluted at 10 mg/ml, transferred to metal-free tubes in small aliquots, and stored at −20°C for later use.
Labeling procedures
177Lu was produced by the neutron irradiation of isotopically enriched 176Lu2O3 (Oak Ridge National Laboratory, Oak Ridge, TN) at the University of Missouri Research Reactor. The appropriate volume of the immunoconjugates (1 mg) was thawed, allowed to come to room temperature, and transferred to the reaction tubes. An equal volume (50 μl) of sodium acetate (0.6 M) buffer, pH 5.3 and sodium citrate (0.06 M) buffer, pH 5.5 was added to each reaction tube. The desired amount of 177Lu radioactivity (1 mCi) was added to the reaction mixture by using metal-free tubes and pipette tips, and was incubated at room temperature for 2 h. The reaction mixture was loaded on the top of a BSA-blocked Sephadex-G25 column and the hot fractions were pooled. The total protein content was determined in order to calculate the specific activity of the labeled immunoconjugates. Instant thin-layer chromatography (ITLC) was performed to check the labeling efficiency.
HPLC analyses
Gel filtration on high-performance liquid chromatography (HPLC) was performed to analyze the integrity of the radiolabeled immunoconjugates (CC49 IgG and scFvs). Radiolabeled immunoconjugates were diluted in PBS and injected onto TSK G2000SW and TSK G3000SW (Toso Haas, Tokyo, Japan), connected in series using 67 mM sodium phosphate buffer (pH 6.8), 0.1 M KCl as the mobile phase. The elution was monitored by an in-line UV detector at 280 nm, and the radioactivity of each of these eluates was determined in a Packard Minaxi Auto-Gamma 5000 gamma counter (Meriden, CT).
Binding analyses
The immunoreactivity of the radiolabeled immunoconjugates was determined by a solid phase ELISA using BSM (Sigma Chemical Co., St Louis, MO) as the antigen. Test samples were incubated for 2 h at room temperature in threefold serial dilutions with 6 ng of biotinylated CC49 IgG followed by incubation with alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch Lab, West Grove, PA) for 1 h at room temperature. The p-nitrophenyl phosphate was used as the substrate, and absorbance was read at 410 nm using a Dynatech MR 5000 automatic 96-well microtiter reader (Chantilly, VA). The quality control analysis of radiolabeled immunoconjugates was performed using RIA, where BSM or BSA (positive and negative controls, respectively) was attached to a solid-phase matrix (Reacti-Gel HW-65F; Pierce Chemical). Radiolabeled immunoconjugates were allowed to bind to the beads for 1 h at room temperature. The bound and unbound fractions were counted in a gamma scintillation counter, and the total percentage of bound and unbound activity was calculated.
Biodistribution and pharmacokinetic studies
Female athymic mice (nu/nu; 4–6 weeks old) were used for the in vivo biodistribution and pharmacokinetic studies (Charles River, Wilmington, MA). LS-174T cells, a human colon carcinoma cell line, were implanted s.c. (1×107), under a IACUC-approved protocol, and the mice were used 12 days (tumor volume, 200–300 mm3) after the injection of cells. Biodistribution studies were performed following an i.v. injection (given via the tail vein) of 185 kBq (5 μCi) 177Lu-labeled [sc(Fv)2]2 and IgG of CC49 MAb in the absence and presence of l-lysine. l-Lysine was purchased from Sigma Chemicals Co. (St Louis, MO) and dissolved in PBS, pH 7.4 at a concentration of 160 mg/ml. Animals were injected with l-lysine (250 μl) via the i.p. route several times as described by others [25]. Animals were sacrificed at designated time points in groups (n=5), and the tumor, blood, and major organs were removed, weighed, and counted in a gamma scintillation counter to determine the %ID/g for each labeled protein. The blood clearance studies were performed in non-tumor-bearing female athymic mice (nu/nu; 4–6 weeks old) as described previously [7]. Blood samples were drawn from the tail vein at various time points following the injection of 370 kBq (10 μCi) of the 177Lu-labeled tetravalent scFv and IgG of CC49 MAb. The half-lives (t1/2α and β) were calculated using the WinNonlin computer program (Pharsight Corporation, CA) for kinetic analysis. The data was fitted into a bi-exponential equation of a bolus injection as an experimental model. The p values were analyzed using the SSPS Window’s two-sample Student’s t test for showing differences between means.
Results
Expression and purification of tetravalent scFv
The divalent sc(Fv)2 (∼60 kDa) of CC49 MAb was expressed as a secreted soluble protein in P. pastoris, in which, upon expression, it has a tendency to form stable tetravalent scFv {[sc(Fv)2]2; ∼120 kDa} by noncovalent interactions as described previously[7, 31]. Purification of scFv was performed using the hexahistidine tag attached to the COOH-terminal of the construct (Fig. 1a). The purity and integrity of the scFv were analyzed by SDS-PAGE under reducing and nonreducing conditions. Both divalent and tetravalent scFv migrated as a single ∼60-kDa protein band under reducing as well as under nonreducing conditions (Fig. 2a). CC49 IgG was also run in parallel and showed ∼50-kDa and ∼24-kDa bands corresponding to heavy and light chains, respectively, under reducing conditions. Under nonreducing conditions, the intact IgG migrated at ∼150 kDa (Fig. 2a). The tetravalent form of scFv also migrated at ∼60 kDa, indicating that the two polypeptide chains of [sc(Fv)2]2 were noncovalently linked (Fig. 1b).
The SDS-PAGE analysis of purified and 177Lu-labeled IgG, and [sc(Fv)2]2 constructs of the MAb CC49 under reducing (+) and nonreducing (−) conditions. a Coomassie Blue R-250 stained gel of the ITCB-DTPA-conjugated unlabeled immunoconjugates. b Autoradiography image of the 177Lu-labeled ITCB-DTPA-conjugated immunoconjugates
Lu labeling and quality control of divalent scFv-ITCB-DTPA, tetravalent scFv-ITCB-DTPA, and intact IgG-ITCB-DTPA conjugated constructs
The specific activity of 100–120 kBq/μg (2.7–3.2 μCi/μg) was observed for the different ITCB-DTPA-conjugated 177Lu-labeled immunoconjugates. 177Lu radiolabeling of the sc(Fv)2, [sc(Fv)2]2, and IgG of CC49 immunoconjugates was analyzed by ITLC, SDS-PAGE, and HPLC. After ITLC analysis, 177Lu-labeled immunoconjugates were further analyzed by SDS-PAGE and HPLC to check the integrity of the labeled products. Electrophoresis of the labeled products under reducing and nonreducing conditions and autoradiography showed the ∼60-kDa protein bands of sc(Fv)2 and [sc(Fv)2]2 (Fig. 2b). The CC49 IgG immunoconjugate showed ∼50-kDa and 24-kDa protein bands under the reducing condition, corresponding to the heavy and the light chain, respectively. Under the nonreducing condition, CC49 IgG showed a major ∼150-kDa protein band (whole IgG) and minor ∼50-kDa and ∼24-kDa (corresponding to the heavy and the light chain, respectively) protein bands (Fig. 2b). In HPLC analysis, 177Lu-labeled sc(Fv)2, [sc(Fv)2]2, and IgG immunoconjugates eluted at Mr ∼60 kDa, 120 kDa, and 150 kDa, respectively, as single peaks (Fig. 3). SDS-PAGE and HPLC analyses confirmed that ITCB-DTPA conjugation and 177Lu-labeling procedures did not affect the integrity of the proteins. In order to check whether or not the ITCB-DTPA conjugation and/or 177Lu labeling affected the immunoreactivity, solid-phase RIA was performed. In solid-phase RIA, specific binding for [sc(Fv)2]2 and IgG of CC49 was ∼75.30% and 79.55%, respectively (Table 1).
Pharmacokinetics studies of ITCB-DTPA-conjugated 177Lu-labeled immunoconjugates
Since our previous study [7] demonstrated that the tetravalent scFv, with higher avidity and prolonged pharmacokinetics in blood, meets the prerequisites of an optimum tumor-targeting reagent in radionuclide-mediated therapy and diagnosis, we focused on 177Lu-labeled tetravalent scFv in the present study. 177Lu-labeled CC49 IgG was used for comparison purposes.
Pharmacokinetic studies were performed to determine the blood clearance of 177Lu-labeled tetravalent scFv forms of CC49 MAb and intact CC49 IgG. The data were analyzed using a bi-exponential model for analyzing α phase t1/2 (the clearance of immunoconjugates from the blood to the extravascular space) and β phase t1/2 (principally the clearance of immunoconjugates from blood to the nonextravascular space or out of the body) values. The half-life alpha (t1/2α) values for [sc(Fv)2]2 and intact IgG were 4.4 min and 9.5 min (0.073 h and 0.158 h), respectively (Fig. 4). In addition, the [sc(Fv)2]2 showed a beta half-life (t1/2β) value of 375.0 min (6.25 h) while the corresponding value for IgG was 2,193.0 min (36.55 h). Thus, 177Lu-labeled [sc(Fv)2]2 showed markedly lower t1/2α (approximately 2.1 times lower) and t1/2β (approximately 5.8 times lower) values compared with the intact IgG. ITCB-DTPA-conjugated and 177Lu-labeled [sc(Fv)2]2, and intact IgG immunoconjugates exhibited a different blood clearance pattern compared with the previously used 125I- and 131I-labeled [sc(Fv)2]2 and IgG [7].
Biodistribution studies in the presence/absence of l-lysine
The radioiodinated tetravalent scFv showed in vivo and in vitro stability and better tumor localization compared with the divalent scFv in our previous study [7]; therefore, we performed the biodistribution studies with 177Lu-labeled tetravalent scFv construct in the present study. Intact CC49 IgG was also used in these experiments for the comparison.
At 8 h post administration, the percent of the injected dose accumulated/g (%ID/g) of LS-174T colon carcinoma tumors was 6.4±1.3 and 8.9±0.6 for 177Lu-labeled [sc(Fv)2]2 and IgG, respectively (Table 2). 177Lu-labeled IgG showed higher tumor uptake compared with the tetravalent scFv, but its accumulation was also higher in the blood, heart, and lungs at all time points (Table 2), which reduced the radiolocalization index (RI; %ID/g of tumor divided by %ID/g of normal tissue) (Fig. 5). In contrast, 177Lu-labeled tetravalent scFv showed a low tumor uptake with very low accumulation in the blood, heart, and lungs compared with the IgG (Table 2). 177Lu-labeled tetravalent scFv showed higher accumulation in the liver, spleen, and kidneys compared with the IgG (Table 2), which also reduced its RI index in these organs (Fig. 5). As compared with the DOTA-conjugated monovalent scFv (241.1%ID/g) at 1 h [14], the tetravalent scFv showed considerably (approximately 5 times) lower (50.3%ID/g) kidney uptake (Table 2). This value, however, was significantly (p<0.005) higher than that observed for IgG (Table 2).
Radiolocalization index (RI, ratio of %ID/g of tumor divided by %ID/g of normal tissues) of the ITCB-DTPA-conjugated 177Lu-labeled tetravalent scFv and IgG of the CC49 MAb at different time points in the nude mice bearing LS-174T colon carcinoma xenografts in the absence (−) and presence (+) of l-lysine. 177Lu-labeled tetravalent scFv construct showed better tumor to blood ratios compared with the CC49 MAb at 24 and 48 h (11 vs 0.6 and 20 vs 2.6) in the absence and (4 vs 1.4 and 25.8 vs 5.0) presence of lysine. The presence of lysine also resulted in a significant increase (p<0.005) in the RI in the kidneys at 24 h (8.8-fold) and 48 h (12.8-fold)
Since 177Lu-labeled tetravalent scFv showed very low retention in the blood, heart, and lungs, with very high renal uptake compared to the IgG, we also performed biodistribution experiments in the presence of l-lysine, which is known to prevent the nonspecific binding and subsequent absorption of radiometal conjugates in the kidneys [25, 26]. The percent injected dose accumulated/g (%ID/g) colon carcinoma tumors for 177Lu-labeled [sc(Fv)2]2 and IgG was 8.0±0.6 and 8.4±1.2, respectively, at 8 h in the presence of l-lysine (Table 3). The tetravalent scFv showed better tumor localization with a maximum (12.9±4.5%ID/g) value at 48 h (Table 3) compared with the tumor localization values observed in the absence of l-lysine, with no or minimal retention in blood. A threefold increase in the tumor uptake value was observed at 48 h in the presence of l-lysine compared with the absence of lysine. In addition, an approximately four-fold gain in the tumor localization of [sc(Fv)2]2 was observed when compared with the previously used DOTA-conjugated and 177Lu-labeled monovalent scFv [14]. 177Lu-labeled IgG showed a higher tumor uptake compared with the tetravalent scFv in the presence of l-lysine, but its accumulation again was also higher in the blood at all time points (Table 3), which lowered its RI values (Fig. 5). In the presence of l-lysine, however, 177Lu-labeled [sc(Fv)2]2 and IgG showed comparable accumulation in the liver, heart, and lungs (Table 3). A comparative RI (tumor to normal tissue ratio) of 177Lu-labeled tetravalent scFv and CC49 IgG is shown in the absence and presence of lysine (Fig. 5).
Renal accumulation of 177Lu-labeled [sc(Fv)2]2 was significantly (p<0.005) lower compared with the renal accumulation values of [sc(Fv)2]2 in the absence of l-lysine. In addition, there was no significant (p>0.005) difference in the renal uptake values of [sc(Fv)2]2 and IgG (Table 3) in the presence of l-lysine. In contrast, a significant (p>0.005) difference in renal uptake values of IgG (Tables 2, 3) was observed when comparing the presence and absence of l-lysine. A comparative RI of 177Lu-labeled tetravalent scFv and CC49 IgG in the kidneys is shown in the presence and absence of l-lysine (Fig. 5).
At 8 h post administration, the tetravalent construct of scFv demonstrated tumor localization and renal uptake comparable to those observed with intact IgG, in the presence of l-lysine, with faster clearance from the blood pool and more retention in the liver for the metabolic/catabolic process at later time points (Table 3, Fig. 5). The 177Lu-labeled tetravalent construct, however, showed an increase in the kidney uptake in the absence/presence of l-lysine, compared with the previously used iodinated tetravalent construct of CC49 IgG [7]. These results also suggested that the metal-chelated tetravalent scFv construct followed a metabolic pattern very different than that of the iodinated tetravalent scFv construct, most likely due to the retention of the metal by the organs metabolizing scFv.
Discussion
When performing RIT it has remained a challenge to optimize the therapeutic index by improving the tumor localization while reducing the uptake by normal tissues [8, 33, 34]. This is the first report to utilize the 177Lu-labeled, engineered tetravalent scFv form of the MAb CC49 for pharmacokinetic and biodistribution studies. One of the potential problems in using the radiometal immunoconjugate, especially with small molecules like scFv, is high renal uptake, which limits the use of radiometals like 177Lu-labeled engineered scFv products for RII and RIT applications [14]. In this study, we also focused our efforts on dealing with the problem of renal uptake of the engineered scFv construct.
We conjugated the ITCB-DTPA, a bifunctional chelating agent, to the antibodies prior to the labeling procedure and optimized the 177Lu labeling. 177Lu-labeled IgG, divalent, and tetravalent scFv constructs of the MAb CC49 showed >90% l77Lu incorporation with a specific activity of 100–120 kBq/μg (2.7–3.2 μCi/μg). SDS-PAGE analysis and autoradiographic studies of the 177Lu-labeled products showed a single band without any major degradation products (Fig. 2b). In addition, HPLC analysis also demonstrated single peaks of the labeled products in the respective areas (Fig. 3). 177Lu-labeled IgG and the tetravalent scFv construct showed good immunoreactivity (Table 1). Therefore, 177Lu labeling with ITCB-DTPA-conjugated proteins appears to be a reliable and reproducible method and may be useful for other radiometal-labeling procedures.
Biodistribution studies with 177Lu-labeled IgG and [sc(Fv)2]2 construct of CC49 were performed in the presence and absence of l-lysine. l-Lysine is a cationic amino acid that is known to prevent kidney uptake of the radiometal-labeled immunoconjugates [25, 26, 33]. Excessive renal uptake of the 177Lu-labeled immunoconjugates, especially low molecular weight engineered scFv constructs, has limited the use of engineered immunoconjugates for RII and RIT [14]. In a previous study, the DOTA-conjugated and 177Lu-labeled monovalent scFv of CC49 exhibited about 241%ID/g in the kidneys at 1 h [14]. This study also aimed to improve the biodistribution characteristics of 177Lu-labeled scFv immunoconjugates by using multivalent forms of scFv with desirable pharmacokinetic properties, a different bifunctional chelating agent (ITCB-DTPA), and i.p. administration of cationic amino acid l-lysine. 177Lu-labeled intact CC49 IgG was used for the comparison in this study. Intact CC49 IgG exhibited a better tumor uptake with very slow blood clearance (t1/2α and t1/2β = 9.5 and 2,193.0 min, respectively), in the presence or absence of lysine. In contrast, the tetravalent scFv construct showed rapid clearance from the blood pool, and alpha (t1/2α) and beta half-life (t1/2β) values were observed at 4.40 min and 375 min, respectively (Fig. 4). In addition to this, the engineered tetravalent scFv construct demonstrated comparable tumor localization to IgG at 8 h post injection (Table 2). Moreover, the renal uptake of the [sc(Fv)2]2 construct was considerably lower (50.3%ID/g) (Table 2) compared with the previously used DOTA-conjugated 177Lu-labeled monovalent scFv (241%ID/g) at 1 h [14], in the absence of lysine. In the presence of lysine [sc(Fv)2]2 demonstrated only 7.2%ID/g renal uptake at 1 h, which is 33-fold lower compared with the 241%ID/g of DOTA-conjugated monovalent scFv [14] and very close (only 1.5 times more) to the value for intact CC49 IgG (Table 3).
At 8 h, [sc(Fv)2]2 demonstrated less accumulation in the liver (13%ID/g) in the presence of l-lysine than without lysine (22.6%ID/g). These results suggest that tetravalent scFv exhibited a different metabolic/catabolic pattern in the presence of lysine, while in the absence of lysine [sc(Fv)2]2 was primarily excreted or metabolized by the kidney. It is also evident that the renal uptake of the engineered constructs can be regulated using these strategies (i.e., using different multimeric forms of scFv and lysine administration before and after the labeled immunoconjugate injection). The results of this study with the tetravalent scFv are encouraging because this immunoconjugate demonstrated good tumor localization with limited kidney uptake compared with the previously used DOTA-conjugated monomeric scFv [14]. This study also indicated that the ITCB-DTPA-conjugated and 177Lu-labeled immunoconjugates followed entirely different metabolic patterns in the presence and absence of lysine. The metabolic pattern of 177Lu-labeled engineered tetravalent immunoconjugates also showed a very different metabolic pattern as compared to radioiodinated immunoconjugates [7].
In biodistribution studies, at 8 h post injection the tumor localization of 177Lu-labeled tetravalent scFv was about fourfold higher compared with the previously used DOTA-conjugated monomeric scFv [14]. As CC49 recognizes the sialyl-Tn epitope, a unique disaccharide present in multiple copies in TAG-72 molecule, an additional increase in valency by generating tetravalent scFv without significantly compromising the pharmacokinetic advantage inherent to the scFv should provide a better reagent for RIT applications. Generation and characterization of tetravalent scFv formed by noncovalent interactions of covalent dimers has been described previously [7, 31]. Such noncovalent association of scFv to yield multimers has also been reported by several other investigators [16–20]. The multimers have been shown to exhibit stable thermodynamic characteristics rather than being associated with a simple equilibrium. Once purified, tetravalent scFv was found to be stable and did not dissociate upon dilution. We have recently evaluated the stability of divalent and tetravalent scFvs under in vivo conditions and these molecules appear to maintain their integrity over the time course used in the present study (unpublished data). Intrinsic affinity and antibody valency are known to contribute to the overall antigen binding and subsequent immunoreactivity of the antibodies [16–20, 35, 36]. Due to this, scFv multimers (divalent and tetravalent scFv) have demonstrated a significant increase in binding affinity in vitro as well as in vivo compared with the monovalent form of scFv. This increase in functional affinity is probably due to the presence of multiple antigen–antibody interaction sites. Thus, the [sc(Fv)2]2 of CC49 has better potential for RIT compared with the monovalent and divalent scFvs. However, the monovalent and divalent scFvs may be better reagents for imaging purposes.
References
Chester KA, Hawkins RE. Clinical issues in antibody design. Trends Biotechnol 1995;13:294–300.
Chester KA, Mayer A, Bhatia J, Robson L, Spencer DI, Cooke SP, et al. Recombinant anti-carcinoembryonic antigen antibodies for targeting cancer. Cancer Chemother Pharmacol 2000;46(Suppl):S8–12.
Colcher D, Zalutsky M, Kaplan W, Kufe D, Austin F, Schlom J. Radiolocalization of human mammary tumors in athymic mice by a monoclonal antibody. Cancer Res 1983;43:736–42.
Colcher D, Esteban J, Mornex F. Use of monoclonal antibodies as radiopharmaceuticals for the localization of human carcinoma xenografts in athymic mice. Methods Enzymol 1986;121:802–16.
Colcher D, Bird R, Roselli M, Hardman KD, Johnson S, Pope S, et al. In vivo tumor targeting of a recombinant single-chain antigen-binding protein. J Natl Cancer Inst 1990;82:1191–7.
Meredith RF, LoBuglio AF, Spencer EB. Recent progress in radioimmunotherapy for cancer. Oncology (Huntingt) 1997;11:979–84 (see also page 987).
Goel A, Colcher D, Baranowska-Kortylewicz J, Augustine S, Booth BJ, Pavlinkova G, Batra SK. Genetically engineered tetravalent single-chain Fv of the pancarcinoma monoclonal antibody CC49: improved biodistribution and potential for therapeutic application. Cancer Res 2000;60:6964–71.
Batra SK, Jain M, Wittel UA, Chauhan SC, Colcher D. Pharmacokinetics and biodistribution of genetically engineered antibodies. Curr Opin Biotechnol 2002;13:603–8.
Blanco I, Kawatsu R, Harrison K, Leichner P, Augustine S, Baranowska-Kortylewicz J, et al. Antiidiotypic response against murine monoclonal antibodies reactive with tumor-associated antigen TAG-72. J Clin Immunol 1997;17:96–106.
Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, et al. Single-chain antigen-binding proteins. Science 1988;242:423–6.
Colcher D, Milenic D, Roselli M, Raubitschek A, Yarranton G, King D, et al. Characterization and biodistribution of recombinant and recombinant/chimeric constructs of monoclonal antibody B72.3. Cancer Res 1989;49:1738–45.
Colcher D, Goel A, Pavlinkova G, Beresford G, Booth B, Batra SK. Effects of genetic engineering on the pharmacokinetics of antibodies. Q J Nucl Med 1999;43:132–9.
Schott ME, Milenic DE, Yokota T, Whitlow M, Wood JF, Fordyce WA, et al. Differential metabolic patterns of iodinated versus radiometal chelated anticarcinoma single-chain Fv molecules. Cancer Res 1992;52:6413–7.
Schott ME, Schlom J, Siler K, Milenic DE, Eggensperger D, Colcher D, et al. Biodistribution and preclinical radioimmunotherapy studies using radiolanthanide-labeled immunoconjugates. Cancer 1994;73:993–8.
Pavlinkova G, Booth BJ, Batra SK, Colcher D. Radioimmunotherapy of human colon cancer xenografts using a dimeric single-chain Fv antibody construct. Clin Cancer Res 1999;5:2613–9.
Pavlinkova G, Beresford GW, Booth BJ, Batra SK, Colcher D. Pharmacokinetics and biodistribution of engineered single-chain antibody constructs of MAb CC49 in colon carcinoma xenografts. J Nucl Med 1999;40:1536–46.
Pack P, Kujau M, Schroeckh V, Knupfer U, Wenderoth R, Riesenberg D, Pluckthun A. Improved bivalent miniantibodies, with identical avidity as whole antibodies, produced by high cell density fermentation of Escherichia coli. Biotechnology (N Y) 1993;11:1271–7.
Pack P, Muller K, Zahn R, Pluckthun A. Tetravalent miniantibodies with high avidity assembling in Escherichia coli. J Mol Biol 1995;246:28–34.
Power BE Hudson PJ. Synthesis of high avidity antibody fragments (scFv multimers) for cancer imaging. J Immunol Methods 2000;242:193–204.
Power BE, Kortt AA, Hudson PJ. Generation of recombinant multimeric antibody fragments for tumor diagnosis and therapy. Methods Mol Biol 2003;207:335–50.
Knox SJ, Goris ML, Tempero M, Weiden PL, Gentner L, Breitz H, et al. Phase II trial of yttrium-90-DOTA-biotin pretargeted by NR-LU-10 antibody/streptavidin in patients with metastatic colon cancer. Clin Cancer Res 2000;6:406–14.
Leichner PK, Akabani G, Colcher D, Harrison KA, Hawkins WG, Eckblade M, et al. Patient-specific dosimetry of indium-111- and yttrium-90-labeled monoclonal antibody CC49. J Nucl Med 1997;38:512–6.
Milenic DE, Roselli M, Mirzadeh S, Pippin CG, Gansow OA, Colcher D, et al. In vivo evaluation of bismuth-labeled monoclonal antibody comparing DTPA-derived bifunctional chelates. Cancer Biother Radiopharm 2001;16:133–46.
Roselli M, Schlom J, Gansow OA, Brechbiel MW, Mirzadeh S, Pippin CG, et al. Comparative biodistribution studies of DTPA-derivative bifunctional chelates for radiometal labeled monoclonal antibodies. Int J Rad Appl Instrum B 1991;18:389–94.
Behr TM, Sharkey RM, Juweid ME, Blumenthal RD, Dunn RM, Griffiths GL, et al. Reduction of the renal uptake of radiolabeled monoclonal antibody fragments by cationic amino acids and their derivatives. Cancer Res 1995;55:3825–34.
Behr TM, Becker WS, Sharkey RM, Juweid ME, Dunn RM, Bair HJ, et al. Reduction of renal uptake of monoclonal antibody fragments by amino acid infusion. J Nucl Med 1996;37:829–33.
Schlom J, Siler K, Milenic DE, Eggensperger D, Colcher D, Miller LS, et al. Monoclonal antibody-based therapy of a human tumor xenograft with a 177lutetium-labeled immunoconjugate. Cancer Res 1991;51:2889–96.
Alvarez RD, Partridge EE, Khazaeli MB, Plott G, Austin M, Kilgore L, et al. Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: a phase I/II study. Gynecol Oncol 1997;65:94–101.
Mulligan T, Carrasquillo JA, Chung Y, Milenic DE, Schlom J, Feuerstein I, et al. Phase I study of intravenous Lu-labeled CC49 murine monoclonal antibody in patients with advanced adenocarcinoma. Clin Cancer Res 1995;1:1447–54.
Thor A, Ohuchi N, Szpak CA, Johnston WW, Schlom J. Distribution of oncofetal antigen tumor-associated glycoprotein-72 defined by monoclonal antibody B72.3. Cancer Res 1986;46:3118–24.
Goel A, Beresford GW, Colcher D, Pavlinkova G, Booth BJ, Baranowska-Kortylewicz J, Batra SK. Divalent forms of CC49 single-chain antibody constructs in Pichia pastoris: expression, purification, and characterization. J Biochem (Tokyo) 2000;127:829–36.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5.
Gansow OA, Brechbiel MW, Mirzadeh S, Colcher D, Roselli M. Chelates and antibodies: current methods and new directions. Cancer Treat Res 1990;51:153–71.
Guadagni F, Roselli M, Ferroni P, Amato T, Colcher D, Greiner JW, Schlom J. Clinical evaluation of the new tumor marker TAG-72. Anticancer Res 1991;11:1389–94.
Santos AD, Kashmiri SV, Hand PH, Schlom J, Padlan EA. Generation and characterization of a single gene-encoded single-chain-tetravalent antitumor antibody. Clin Cancer Res 1999;5:s3118–23.
Wu AM, Chen W, Raubitschek A, Williams LE, Neumaier M, Fischer R, et al. Tumor localization of anti-CEA single-chain Fvs: improved targeting by non-covalent dimers. Immunotechnology 1996;2:21–36.
Acknowledgments
The authors thank Barbara J.M. Booth and Brandon Henley for their technical support. We also thank Dr. Z.P. Kortylewicz, Department of Radiation Oncology, UNMC, for the synthesis and generous supply of ITCB-DTPA, and Ms. Kristi L.W. Berger (Eppley Institute) for editorial assistance. The CC49 scFv construct was a generous gift from Dr. Jeff Schlom of the Laboratory of Tumor Immunology at the National Cancer Institute, NIH, and the Dow Chemical Company. This work was supported by a grant from the United States Department of Energy (DE-FG0295ER62024).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chauhan, S.C., Jain, M., Moore, E.D. et al. Pharmacokinetics and biodistribution of 177Lu-labeled multivalent single-chain Fv construct of the pancarcinoma monoclonal antibody CC49. Eur J Nucl Med Mol Imaging 32, 264–273 (2005). https://doi.org/10.1007/s00259-004-1664-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1664-0