Abstract
Autoimmune hyperthyroidism may occur several months after radioiodine therapy (RIT) for functional thyroid autonomy. Exacerbation of pre-existing subclinical Graves’ disease (GD) has been held responsible for this phenomenon. Determination of TSH receptor antibody using solubilised porcine epithelial cell membranes is insensitive and may have failed to diagnose GD in these patients before RIT. Following the introduction of a more sensitive assay, using the human TSH receptor as an antigen, it has been expected that the incidence of radiation-induced GD after RIT for functional thyroid autonomy will be reduced. In a first group of 1,428 patients treated between November 1993 and March 1997 (group I) we used the porcine TRAb assay to exclude GD, while in a second group comprising 1,408 patients treated between January 2000 and December 2001 (group II), GD was excluded using the human TRAb assay. A matched control group of 231 patients was derived from group II. In group I a total of 15 (1.05%) patients developed obvious or suspected radiation-induced GD, while in group II 17 (1.2%) did so; the interval until development of GD was 8.4 and 8.8 months, respectively, after RIT. Serum anti-thyroid peroxidase levels before RIT were elevated in 36.4% of group I patients and 47.1% of group II patients, but in only 5.6% of the control group. Other non-specific signs of mild immunopathy of the thyroid were seen retrospectively in 73.3%, 64.7% and 16.0% of the patients in these three groups, respectively. In conclusion, the introduction of a high-sensitivity TRAb assay did not reduce the incidence of autoimmune hyperthyroidism occurring late after RIT for functional thyroid autonomy, but mild immunopathy of the thyroid is seen more frequently in these patients and seems to be a predisposing factor in the development of radiation-induced GD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In a small number of patients, autoimmune hyperthyroidism may occur several months after radioiodine therapy (RIT) for functional thyroid autonomy [1, 2, 3, 4, 5, 6, 7, 8]. Previous studies have indicated that this phenomenon is due to pre-existing subclinical Graves’ disease (GD) not detectable by laboratory tests used before 2000. The sensitivity of the first-generation TRAb assay using solubilised porcine epithelial cell membranes was as low as 80%, and thus a considerable number of patients with GD may have been overlooked. A new, second-generation TRAb bioassay, which uses the human TSH receptor as an antigen, shows a much higher diagnostic sensitivity of 95.3% [9] to 98.8% [10] for the detection of TRAbs in patients with GD. On the assumption that the hypothesis of pre-existing subclinical GD in those patients who develop autoimmune hyperthyroidism after RIT is correct, it has been expected that the introduction of the high-sensitivity TRAb assay, which has the highest diagnostic power to differentiate GD from thyroidal autonomy [7, 8, 11], will lead to a reduction in the incidence of radiation-induced GD after RIT for unifocal, multifocal or disseminated autonomy.
Materials and methods
Two groups of patients treated with radioiodine for functional thyroid autonomy were investigated retrospectively (Table 1). In both groups the TRAb assay was negative, and no endocrine ophthalmopathy was observed in any patient at the time of RIT. In group I, comprising 1,428 patients (1,148 women, 280 men, age 40–82 years, mean age 63 years), we used the solubilised porcine epithelial TRAb assay (TRAK) from November 1993 to March 1997. The radiation dose delivered to the thyroid in multifocal (MFA) or disseminated (DISA) autonomy was uniformly 150 Gy before January 1995, but it later varied from 150 to 300 Gy depending on the 99mTc-pertechnetate uptake in the thyroid under TSH suppression (TcTUs), which was used as a functional parameter of thyroid autonomy [12]. In unifocal autonomy (UFA), the radiation dose was 400 Gy. After the introduction of the new TRAb assay (TRAK human) in January 2000, 1,408 patients (group II: 1,199 women, 209 men, age 24–86 years, mean age 59.4 years) were treated with radioiodine for UFA, MFA or DISA. In these patients, the individual radiation dose was varied in a similar manner, and there was no significant difference in the mean radiation dose between the two groups (Table 1).
All patients were examined in our department before RIT. A gamma camera (medium field) (MIE, Hamburg, Germany) was used for scintigraphy of the thyroid, images being obtained 20 min after intravenous administration of 80 MBq 99mTc-pertechnetate. Sonography of the thyroid was performed with a 5- and 7.5-MHz linear transducer to evaluate thyroid volume, nodules and echogenicity before and after RIT (Sonoline Versa/Pro, Siemens, Erlangen, Germany). Thyroid volume was calculated employing the ellipsoid formula: volume = height × depth × width × π/6. The latter constant was replaced by a factor of 0.479, according to Brunn et al. [13]. The average inaccuracy is claimed to be 16%, and the maximum error, 32% [13]. The gamma camera and sonographic device used were identical in the two groups. We also determined in vitro parameters: TRAb (group I: normal range <9 IU/l, borderline 9–14 IU/l, inter-assay variation coefficient in the borderline zone 13.1%, TRAK; group II: normal range <1.5 IU/l, borderline 1.5–2 IU/l, variation coefficient in the borderline zone 12.7%, TRAK human), anti-thyroid peroxidase (anti-TPO) [in-house normal range <60 IU/ml, Anti-TPO (group I), Anti-TPOn (group II)] (all BRAHMS, Berlin, Germany), TSH (normal range 0.23–4.2 mIU/l, thyrotropin), serum free triiodthyroxine (fT3, normal range 2.8–7.1 pmol/l) and serum free thyroxine (fT4, normal range 11.8–24.6 pmol/l) (all Roche, Mannheim, Germany). Laboratory kits for TSH and anti-TPO changed during the trial, but the reference ranges were nearly identical in both groups. From November 1993 to November 1994, TPO-Abs were not routinely determined. In this period, one elevated value was adopted from the referring physican (patient 7 in group I). All patients underwent at least one control examination at a mean of 9.5 (4–13) months after RIT. Nearly half of these control examinations were performed by the referring physicians. To make the results of the control examinations comparable to those of the initial examinations, a control group of 231 patients, all examined in our department, was derived from group II, matched for gender (179 woman, 52 men), age (mean 63.1 years, range 24–87) and time interval between RIT and control examination (mean 8.0 months, range 4–17). All patients believed to have radiation-induced GD were examined or re-examined in our department.
Radiation-induced GD was defined primarily by a positive TRAb titre and in group I also by the development of endocrine ophthalmopathy after RIT. Deterioration in metabolism after RIT, even when only transient, was obligatory in these patients. Secondary criteria, also implicating radiation-induced GD, were deterioration of metabolism combined with an increase in anti-TPO titres and/or sonographic hypoechogenicity (only group I) or euthyroidism combined with an increase in TRAb titres above the normal range (only group II). Since not all patients were controlled primarily in our department and since not all referring physicians used the TRAb human assay from the beginning, the true number of patients with euthyroidism and elevated TRAb (human) titres after RIT in group II might have been higher than shown here (maximum error 10%).
Results
Obvious radiation-induced GD was defined as a combination of newly developed hyperthyroidism and positive TRAb titre or, alternatively, endocrine ophthalmopathy (= minimum number of affected patients). Additionally (= maximum number of affected patients), secondary criteria suspicious for radiation-induced GD were newly developed hyperthyroidism and immunopathy or hypoechogenicity (only group I) or euthyroidism and positive TRAb titre (only group II). More details regarding the minimum and maximum criteria are shown in Table 2.
Using a low-sensitivity assay for exclusion of TSH receptor antibodies, a minimum of 12 (0.84%) and a maximum of 15 (1.05%) of the 1,428 patients from group I treated for functional thyroid autonomy developed radiation-induced GD at a mean of 8.4 (4–13) months after RIT (Table 2). When GD was excluded by using the human TRAK assay in all patients treated with radioiodine for functional thyroid autonomy (group II), a minimum of 13 (0.92%) and a maximum of 17 (1.2%) of 1,408 patients developed radiation-induced GD at a mean of 8.8 (6–13) months later.
Thyroid function after RIT and further therapy
All of the patients who developed radiation-induced GD showed TSH suppression before RIT. Two of the 15 patients (maximum) in group I and one patient in the control group with radiation-induced GD needed thyroxine to achieve TSH suppression before RIT. Deterioration in metabolism after RIT was seen in all of the aforementioned 15 patients in group I, but was only transient in three. These three patients entered remission without any therapy within 6 months after RIT. Nine of the remaining 12 patients received a second and two a third RIT; three patients needed antithyroid drugs. Among the 17 patients (maximum) in group II with radiation-induced GD, TSH, fT3 and fT4 levels remained normal in four; the other 13 showed suppressed TSH and higher levels of fT3 and fT4 than before RIT. Ten of these 13 patients received a second, and one a third RIT. Antithyroidal treatment was recommended for the remaining three patients.
TRAb
The magnitude of the increase in TRAb after RIT did not show any correlation with the TRAb titres in serum before RIT in either group. Prior to RIT, the TRAb levels were within the normal range in 14 of 15 affected patients in group I and in 16 of 17 affected patients in group II (normal ranges <9 IU/l for group I and <1.5 IU/l for group II). Only one patient of each group was in the borderline zone, i.e. patient 10 with 9.5 IU/l in group I and patient 13 with 1.8 IU/l in group II (Figs. 1, 2).
In patients 11, 12, 13, 14 and 15 of group I, TRAb remained within the normal range or borderline zone after RIT. Two patients of this subgroup (11, 13) developed endocrine ophthalmopathy, while patients 12, 14 and 15 showed deterioration of metabolism combined with an increase in anti-TPO (Fig. 3). In patients 10, 13 and 14, the increase in TRAb titres was only moderate and not significant but was accompanied by development of endocrine ophthalmopathy (10, 13) or severe hyperthyroidism (14). In the latter patient, fT4 increased from 20 to 38 pmol/l, and anti-TPO from 3 to 552 IU/ml.
Anti-TPO
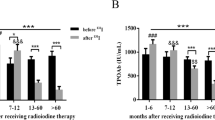
Elevated serum anti-TPO titres before RIT were seen in four (36.4%) of 11 affected patients in group I and in eight (47.1%) of 17 affected patients in group II, but in only 13 (5.6%) of 231 patients in the control group (Table 3). After RIT, the anti-TPO titres seen in the same groups increased slightly in two of ten patients in group I and in eight of 17 patients in group II, increased intensively in five (group I) or six patients (group II) and returned to normal in only one of 15 patients in group I (from 4,000 to 26 IU/ml, patient 1; Fig. 3) and two of 17 patients (patients 1 and 7) in group II (Fig. 4). The mean increase in TPO-Ab titres after RIT is shown in Fig. 5. In group I, the titres increased by a factor of nearly 2, and in group II by a factor of 7.4. In the control group the absolute increase in anti-TPO titres was low, but the relative increase high (by a factor of 9.6).
Endocrine ophthalmopathy
There was no direct suggestion of endocrine ophthalmopathy in either group before RIT. After RIT, 5 of the 15 patients (2, 9, 10, 11, 13, Fig. 1) in group I developed first signs of endocrine ophthalmopathy, in all cases including exophthalmos, and in one case diplopia as well. In group II, ten of the 17 patients developed non-specific symptoms such as burning or temporary swelling of the eyelids (Table 2).
Thyroid volume and ultrasound signs
The thyroid volume was reduced after RIT in all groups (Fig. 6). The mean absolute reduction in thyroid volume after RIT was significantly lower in the control group (8.6±14 ml) than in groups I and II (21.2±23.1 and 15.6±17.4, respectively, P<0.01). Corresponding to this, the relative thyroid volume reduction was significantly higher in groups I and II (46.7% and 44.6%) than in the control group (27.5%, P<0.03).
Other results of thyroid ultrasound examination before RIT are shown in Table 3. Diffuse hypoechogenicity of the thyroid as a non-specific sign of immunopathy was seen more frequently before RIT in patients with radiation-induced GD. However, the number of patients with nodules or inhomogeneity did not differ significantly between groups I and II and the control group. Considering all the available signs of immunopathy (hypoechogenicity, elevated anti-TPO Ab, TRAb in the borderline zone), the percentage of patients with immunopathy before RIT ranged from 64.7% to 73.3% among patients with radiation-induced GD, but was only 16% in the control group (Table 3).
Discussion
Development of autoimmune hyperthyroidism in patients treated for functional thyroid autonomy by radioiodine has been described in case reports [1, 5, 6] or in larger patient populations at a rate of 1–5% [2, 3, 4, 7, 8]. Before the introduction of a high-sensitivity TRAb assay, it was claimed that radiation-induced GD is related to exacerbation of pre-existing subclinical GD and that the previously used TRAb assay was insensitive and may have failed to diagnose GD in these patients before RIT [4, 8, 11]. Indeed, a significant number (22%) of patients formerly classified as having DISA were disclosed to have GD when a high-sensitivity TRAb assay (TRAK human) was used [11]. Moreover, using this TRAb assay to exclude GD before RIT, TRAbs were detectable after RIT in 36% of patients with multinodular goitre showing diffuse or even patchy 99mTc uptake, but not in patients with localised 99mTc uptake in multiple discrete nodules of varying size [8]. These findings are thought to provide more evidence of pre-existing GD in patients with homogeneous (DISA) or patchy 99mTc uptake [7]. Furthermore, 27% of these patients developed TPO-Abs after RIT.
Induction of or deterioration in thyroidal immunopathy as a result of RIT seems to be a general pathophysiological mechanism. In a majority (55–85%) of patients with proven GD, RIT induces an increase in thyroid-stimulating immunoglobulins in the circulation over a period of several months, with a gradual return to original levels by 1 year [14]. This was also seen in our patients. In group I, TPO-Abs were increased in twice as many patients after RIT than before RIT, while in the control group nearly three times as many showed increased TPO-Abs after RIT than did so before it. From these data it is obvious that pre-existing autoimmune thyroiditis, as well as deterioration of immunopathy, is more frequent in these patients. Nygaard et al. [2] concluded that the presence of TPO-Abs before RIT seems to be a marker of an increased risk of later development of TRAb-associated hyperthyroidism as well as hypothyroidism. Wallaschofski [8] came to the conclusion that only patients with toxic multinodular goitre and scintigraphic diffuse-patchy distribution of the radioisotope and high TPO-Abs before RIT show an increased risk of developing TRAbs and suspected side-effects such as relapse of hyperthyroidism or worsening of thyroid-associated ophthalmopathy. In our study, however, patients with scintigraphically localised 99mTc uptake in multiple hot nodules were also included. Moreover, no signs of autoimmune thyroiditis were seen before RIT in more than half of the patients and, as shown here, exclusion of pre-existing subclinical GD by use of a high-sensitive TRAb assay is not able to reduce the number of patients affected. It may be concluded that GD after RIT of functional thyroid autonomy is a rare condition which may be related to spontaneous development of GD in most cases [15]. From a clinical point of view, our patients believed to have radiation-induced GD did not differ from those with usual GD. On the other hand, the constant time interval between RIT and the occurrence of GD suggests a possible link between the two.
It may be postulated that exacerbation of immunological phenomena in the thyroid is caused by primary immunosuppression of the thyroid by radiation followed by a secondary rebound effect generating immunostimulation. A similar pathogenesis is observed in postpartum autoimmune thyroid disease, which is induced by a rebound in autoimmunity secondary to increased immune tolerance during pregnancy [16, 17]. Lymphocytic infiltration of the thyroid, recognisable by a hypoechoic ultrasound pattern [18, 19, 20], is held responsible for most of the TRAb generation within the body, since TRAb titres have been found to be increased in the venous blood drained from the thyroid [21]. Lymphocytes are extremely radiosensitive and RIT is able to significantly lower the TRAb titres within 48 h after radioiodine administration (Groth P, Schuemichen C, personal communication, 2003). But later autoimmune thyroid disorders may be caused not only by temporary depletion of lymphocytes from the thyroid but also by radiation-induced alteration of lymphocytes, as proved experimentally in mice [22].
On the other hand, the occurrence of GD has been observed after percutaneous ethanol injection in autonomous adenoma [23, 24], following spontaneous regression of a toxic adenoma [25], following thyroid surgical resection of hyperfunctioning autonomous adenoma or parathyroidectomy [26, 27] and even after subacute thyroiditis [28]. All these conditions are consistent with a massive release of thyroid materials from follicular cells, including TSH-receptor antigenic components, which may trigger an autoimmune response to the TSH receptor [23]. This hypothesis is supported by the fact that the loss of thyroid volume after RIT was more pronounced in our patients with radiation-induced GD than in the control group. From the data of this study, it is clear that pre-existing subclinical GD is not a prerequisite for this trigger mechanism, but this may be the case for pre-existing autoimmune thyroiditis. In our patients with radiation-induced GD, TPO-Abs could be detected in 36–47%. Although the more sensitive and specific TPO-Ab immunoassays have replaced insensitive antimicrosomal antibody tests, there is increasing evidence from studies correlating thyroid ultrasound abnormalities with the presence of TPO-Abs in serum that the current TPO-Ab assays are only qualitative markers of thyroid autoimmunity and may be negative in some patients with occult disease [18, 19, 29]. Thyroglobulin (Tg)-Abs were not routinely assessed in our patients, but seem to be positive in only a small number of individuals with thyroid autoimmunity [30]. On the other hand, in a population with mild and moderate iodine deficiency, the prevalence rates of TPO-Abs and Tg-Abs were similar (13.1% vs 13.0%) [31]. Thus, determination of Tg-Abs may also contribute to recognition of patients at risk.
In conclusion, based on findings like ultrasound abnormalities with a diffuse hypoechoic pattern, positive TPO-Ab and/or borderline TRAb titres, it could be inferred that a majority of the patients with radiation-induced GD showed evidence of pre-existing thyroid autoimmunity. This percentage was much lower in the control group. In patients with functional thyroid autonomy and immunopathy, the risk of developing radiation-induced GD is increased by a factor of nearly 10, thus reaching a magnitude of 10% absolute risk. Whether this warrants therapeutic consequences in these patients, such as the use of adjuvant prednisolone, remains to be considered.
References
Chiovato L, Santini F, Vitti P, Bendinelli G, Pinchera A. Appearance of thyroid stimulating antibody and Graves’ disease after radioiodine therapy for toxic nodular goitre. J Clin Endocrinol 1994; 40:803–806.
Nygaard B, Knudsen JH, Hegedues L, Veje A, Hansen JEM. Thyrotropin receptor antibodies and Graves’ disease, a side effect of131I treatment in patients with non toxic-goiter. J Clin Endocrinol Metab 1997; 82:2926–2930.
Hirsch C, Spyra J, Langhammer R, Laubenbacher C, Senekowitsch-Schmidtke R, Schwaiger M. Zum Auftreten einer Immunhyperthyreose nach Radioiodtherapie von Schilddrüsenautonomien. Med Klin 1997; 92:130–137.
Dunkelmann S, Rudolph F, Prillwitz A, Groth P, Schuemichen C. Paradoxical effects of radioiodine therapy in functional thyroid autonomy and mild immunothyropathy. Nuklearmedizin 1998; 37:17–22.
Nygaard B, Faber J, Veje A, Hegedus L, Hansen JM. Transition of nodular toxic goiter to autoimmune hyperthyroidism triggered by131I therapy. Thyroid 1999; 9:477–481.
Soule J, Mayfield R. Graves’ disease after131I therapy for toxic nodule. Thyroid 2001; 10:91–92.
Wallaschofski H, Orda C, Georgi P, Paschke R. Distinction between autoimmune and non-autoimmune hyperthyroidism by determination of TSH-receptor antibodies in patients with the initial diagnosis of toxic multinodular goiter. Horm Metab Res 2001; 33:504–507.
Wallaschofski H, Muller D, Georgi P, Paschke R. Induction of TSH-receptor antibodies in patients with toxic multinodular goitre by radioiodine treatment. Horm Metab Res 2002; 34:36–39.
Costagliola S, Morgenthaler NG, Hoermann R, Badenhoop K, Struck J, Freitag D, Poertl S, Weglohmer W, Hollidt JM, Quadbeck B, Dumont JE, Schumm-Draeger PM, Bergmann A, Mann K, Vassart G, Usadel KH. Second generation assay for thyrotropin receptor antibodies has superior diagnostic sensitivity for Graves’ disease. J Clin Endocrinol Metab 1999; 84:90–97.
Pedersen I, Knudsen N, Perrild H, Ovesen L, Laurberg P. TSH-receptor antibody measurement for differentiation of hyperthyroidism in Graves’ disease and multinodular toxic goitre: a comparison of two competitive binding assays. Clin Endocrinol (Oxf) 2001; 55:381–390.
Meller J, Jauho A, Hüfner M, Gratz S, Becker W. Disseminated thyroid autonomy or Graves’ disease: reevaluation by a second generation TSH receptor antibody assay. Thyroid 2000; 10:1073–1079.
Dunkelmann S, Endlicher D, Prillwitz A, Rudolph F, Groth P, Schuemichen C. Results of TcTUs-optimized radioiodine therapy of multifocal and disseminated functional thyroid autonomy. Nuklearmedizin 1999; 38:131–139.
Brunn J, Block U, Ruf G, Bos I, Kunze WP, Scriba PC. Volumetry of the lobe of the thyroid gland by means of realtime sonography. Dtsch Med Wochenschr 1981; 106:1338–1340.
DeGroot LJ. Radioiodine and immune system. Thyroid 1997; 7:259–264.
Regalbuto C, Salamone S, Scollo C, Vigneri R, Pezzino V. Appearance of anti TSH-receptor antibodies and clinical Graves’ disease after radioiodine therapy for hyperfunctioning thyroid adenoma. J Endocrinol Invest 1999; 22:147–150.
Davies TF. The thyroid immunology of the postpartum period. Thyroid 1999; 9:675–684.
Ando T, Davies TF. Clinical review 160: postpartum autoimmune thyroid disease: the potential role of fetal microchimerism. J Clin Endocrinol Metab 2003; 88:2965–2971.
Pedersen OM, Bennedbaek FN, Hoir-Madsen M, Jacobsen BB, Hegedus L. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid 2000; 10:251–259.
Hansen D, Bennedbaek FN, Hoir-Madson M, Hegedus L, Jacobsen BB. A prospective study of thyroid function, morphology and autoimmunity in young patients with type 1 diabetes. Eur J Endocrinol 2003; 148:245–251.
Weetmann AP. Autoimmune thyroid disease: propagation and progression. Eur J Endocrinol 2003; 148:1–9.
Sugenoya A, Kobayashi S, Kasuga Y, Masuda H, Fujimori M, Komatsu M, Takahashi S, Yokoyama S, Shimizu T, Yamada T. Evidence of intrathyroidal accumulation of TSH receptor antibody in Graves’ disease. Acta Endocrinol 1992; 126:416–418.
Sakaguchi S, Ermak TH, Toda M, Berg LJ, Ho W, Fazekas de St Groth B, Perterson PA, Sakaguchi N, Davis MM. Induction of autoimmune disease in mice by germline alteration of the T cell receptor gene expression. J Immunol 1994; 152:1471–1484.
Monzani F, Del Guerra P, Caraccio N, Casolaro A, Lippolis PV, Goletti O. Appearance of Graves’ disease after percutaneous ethanol injection for the treatment of hyperfunctioning thyroid adenoma. J Endocrinol Invest 1997; 20:294–298.
Verde G. Graves’ disease after percutaneous ethanol injection for the treatment of autonomous thyroid adenoma. J Endocrinol Invest 1998; 21:334–335.
Orsolon P, Lupi A, De Antoni Migliorati G, Vianello Dri A. Appearance of Graves’ disease following regression of autonomously functioning thyroid nodules. Two case reports. Minerva Endocrinol 1998; 23:53–56.
Wallfish PG, Caplan D, Rosen IB. Postparathyroidectomy transient thyrotoxicosis. J Clin Endocrinol Metab 1992; 75:224–227.
Freeman JS, Ertel NH, McA’Nulty JA, Khan MY. Graves’ disease following resection of an autonomous solitary thyroid adenoma. J Med Soc N Y 1983; 80:444–446.
Bartalena L, Bogazzi F, Pecori F, Martino E. Graves’ disease occurring after subacute thyroiditis: report of a case and review of the literature. Thyroid 1996; 6:345–348.
Bergoglio LM, Vilchez PE, Fatemi S, Spencer CA. TPOAb assay limitations may be responsible for the skew in the TSH upper reference limit. Proceedings of the Latin American Thyroid Society, Cordoba, Argentina 2003; A:123.
Hollowell JG, Staehling NW, Hannon WH, Flanders WD, Gunter EW, Spencer CA, Bravermann LE. Serum thyrotropin, thyroxine and thyroid antibodies in the United States population (1988-1994): NHANES II. J Clin Endocrinol Metab 2002; 87:489–499.
Pedersen IB, Knudsen N, Jorgensen T, Perrild H, Ovesen L, Laurberg P. Thyroid peroxidase and thyroglobin autoantibodies in a large survey of populations with mild and moderate iodine deficiency. Clin Endocrinol (Oxf) 2002; 58:36–42.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dunkelmann, S., Wolf, R., Koch, A. et al. Incidence of radiation-induced Graves’ disease in patients treated with radioiodine for thyroid autonomy before and after introduction of a high-sensitivity TSH receptor antibody assay. Eur J Nucl Med Mol Imaging 31, 1428–1434 (2004). https://doi.org/10.1007/s00259-004-1519-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1519-8