Abstract
Electrocardiographic (ECG) gated single-photon emission tomography (SPET) allows for simultaneous assessment of myocardial perfusion and left ventricular (LV) function. Presently 8-frame per cardiac cycle ECG gating of SPET images is standard. The aim of this study was to compare the effect of 8-frame and 16-frame gated SPET on measurements of LV volumes and to evaluate the effects of the presence of myocardial perfusion defects and of radiotracer dose administered on the calculation of LV volumes. A total of 86 patients underwent technetium-99m SPET myocardial perfusion imaging using 16-frame per cardiac cycle acquisition. Eight-frame gated SPET images were generated by summation of contiguous frames. Left ventricular end-diastolic volume (EDV), end-systolic volume (ESV) and ejection fraction (EF) were calculated from the 16-frame and 8-frame data sets. The patients were divided into groups according to the administered dose of the radiotracer and the size of the perfusion defect. Results. Sixteen frame per cardiac cycle acquisition resulted in significantly larger EDV (122±72 ml vs 115±68 ml, P<0.0001), smaller ESV (64±58.6 ml vs 67.6±59.5 ml, P<0.0001), and higher LVEF (55.3%±18% vs 49%±17.4%, P<0.0001) as compared to 8-frame SPET imaging. This effect was seen regardless of whether a high or a low dose was administered and whether or not significant perfusion defects were present. This study shows that EDV, ESV and LVEF determined by 16-frame gated SPET are significantly different from those determined by 8-frame gated SPET. The radiotracer dose and perfusion defects do not affect estimation of LV parameters by 16-frame gated SPET.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
State of the art single-photon emission tomography (SPET) imaging provides combined information about myocardial perfusion and function, from the same image data. This became feasible after the introduction of technetium-99m labeled agents and acquisition with electrocardiographic (ECG) gating. Numerous studies have validated the use of ECG-gated SPET for the assessment of global and regional left ventricular function [1, 2, 3, 4, 5, 6, 7]. Both geometry-based [1, 2, 3] and count-based [5, 6] methods have been developed for computation of the left ventricular ejection fraction (LVEF) and for assessment of wall motion and thickening [8, 9, 10]. More recently, estimation of LV volumes by gated SPET has also been validated against magnetic resonance imaging (MRI) [11, 12, 13], thermodilution [14, 15], ventriculography [11, 15, 16], and echocardiography [17]. In general, LV volumes obtained from 8-frame gated SPET imaging have been noted to be smaller than those obtained by standard techniques [12, 16, 18].
For gated SPET imaging, image data are acquired in synchrony with the ECG signal using a specific number of intervals, from R wave to R wave. Counts accumulated during each of these intervals generate individual images. Each individual image is subsequently reconstructed into a tomographic set. At present, using commercially available software it is standard to acquire 8 frames per cardiac cycle. Using standard imaging times, there is an inverse relationship between the temporal resolution (i.e., number of frames per cardiac cycle) of gated images and the count density in each gated frame. State of the art multi-headed cameras have made it possible to obtain high-count density images without prolonging the acquisition time, which in turn makes the acquisition of more frames per cardiac cycle attractive. Theoretically the acquisition of 16 frames instead of 8 frames per cycle should improve the temporal resolution of the cardiac contraction and consequently allow more accurate determination of LV systolic function. However, the lower count density in each frame with 16-frame acquisition, as compared to 8-frame acquisition, may cause inferior image quality and suboptimal LV edge definition.
Two previous studies have shown systematic underestimation of LVEF when measured by 8-frame gated SPET as opposed to 16-frame gated SPET [1, 19]. One study comparing assessment of LV function by gated SPET and by first-pass ventriculography found that even though LVEF was higher with gated SPET, volumes were smaller [16]. Another study showed that gated SPET resulted in smaller end-diastolic volumes (EDV) and larger end-systolic volumes (ESV), when compared with thermodilution [14]. Hence the effect of 16-frame acquisition on LV volume calculation remains unclear. The aim of this study was to compare the effect of 8-frame and 16-frame gated SPET imaging on measurements of LV volumes and in addition to evaluate the effects of radiopharmaceutical dose and myocardial perfusion defects on estimation of LV volumes.
Materials and methods
Patient data
Data from 86 patients who underwent 99mTc SPET myocardial perfusion imaging for various indications at the Yale University Cardiovascular Nuclear Imaging Laboratory were used in this study. Of the 86 patients, 53 were males and 33 females with a mean age of 61.7±13.5 (mean±SD) years, ranging from 29 to 92 years.
Imaging protocol
Gated SPET images were acquired using either a 1-day protocol (either stress first or rest first) or a 2-day protocol. Only stress studies were used in this study since only stress studies were acquired in the 16-frame/cycle format. Depending on our laboratory protocol, either a low dose or high dose of 99mTc-sestamibi was injected. Sestamibi was injected either at peak treadmill exercise, at 4 min after completion of dipyridamole infusion, or at 1.5 min into adenosine infusion. Of 86 patients, 30 underwent treadmill exercise, 49 had adenosine vasodilation, 5 had dobutamine adrenergic stress and 2 had dipyridamole vasodilation. Fifty-six patients had high-dose studies (27.5±2.72 mCi, range 23.5–37 mCi) and 30 had low-dose studies (15.9±0.48 mCi, range 14.9–16.5 mCi).
Image acquisition
SPET imaging was performed using a triple-head gamma camera (Prism 3000XP: Philips, Cleveland, Ohio) equipped with low-energy high-resolution parallel-hole collimators. A 15% energy window was centered over the 140-keV photopeak of 99mTc. Images were obtained by using 360° orbit and a step-and-shoot acquisition (20 stops, 20–40 s per stop). Sixty projections were acquired for each patient. The image matrix size was 64×64 pixels, with a pixel size of 5.25×5.25 mm. ECG gated images were acquired with 16 frames per cardiac cycle with a beat acceptance window of 30% of average R-R interval. Uniformity correction was applied to all acquired images.
Generation of 8-frame images from 16-frame gated raw images
All studies were acquired as 16-frame gated SPET images. To generate a set of 8-frame gated SPET images, two contiguous frames of 16-frame gated raw projection data were summed (1+2, 3+4, 5+6, etc.) prior to filtered back-projection.
Reconstruction
The original 16-frame image data and summed 8-frame image data were reconstructed using conventional filtered back-projection. Each set of images was reconstructed in two ways: (a) all frames were summed and processed as a single ungated SPET study, and (b) the frames were processed individually as separate gated SPET images. A three-dimensional (3-D) Butterworth filter, with an order of 4.0 and a cut-off frequency of 0.23 × Nyquist frequency for ungated SPET images and an order of 2.6 and a cut-off frequency of 0.19 × Nyquist frequency for gated SPET images, was used to smooth the reconstructed images. No photon scatter or attenuation correction was applied. The reconstructed transverse images were reoriented according to the anatomical axes of the left ventricle. Reconstructed slices were displayed in a standard manner as short axis, vertical long axis and horizontal long axis slices.
Generation of circumferential profiles and determination of LV contours
Quantification of ungated SPET images was performed using the Wackers-Liu circumferential quantification method (WL-CQ method, Eclipse Systems, Branford, Conn.). This method has been described and validated previously [20, 21, 22, 23, 24]. In brief, the region of the left ventricle in the ungated SPET slices was determined using the K-means algorithms [25]. Each of the automatically determined short axis slices from the apex to the base of the LV was divided into 128 radial sectors, and the "apex cap" defined by the horizontal long axis slices was divided into 64 sectors. Circumferential count profiles were derived from maximal pixel values in each of the radial sectors. The "mid-wall" of the LV myocardium was determined by a set of concentric circles using a median search algorithm, in which each radius of the circles was calculated as the distance between the center of the corresponding slice and the location of the maximal count detected. A uniform myocardial thickness of 12 mm was assumed. The endocardial contours were determined by the mid-wall of the LV minus one-half of the assumed thickness inward, while the epicardial edges were defined by the mid-wall of the LV plus one-half of the thickness outward.
Calculation of myocardial perfusion defect size
The set of the radii determining the LV contours was linearly extrapolated into 36 sets to standardize the LV myocardial mass for calculation of myocardial perfusion defect size. Each of the derived circumferential count profiles was normalized to the sector with maximal counts in the corresponding profile. The normalized count profiles were also extrapolated in the same way into 36 counts profiles.
A normal database was generated from patients with a low (<3%) likelihood of coronary disease. The lower limits of normal were calculated as mean minus two standard deviations (mean−2SD) of the normal quantitative profiles. The myocardial perfusion defect size was thus defined as the area between the patient circumferential count profile described above and the lower limit of normal circumferential profile, divided by the sum of that area and the area below the patient count profile [20]. The total defect size was calculated as the sum of defects in all 36 linearly extrapolated short axis slices and eight long axis slices containing the apex cap in the 3-D space, weighted for the LV myocardial mass of each slice. The total defect size was expressed as percent of the entire left ventricle (% LV).
Quantification of LV volumes and ejection fraction
The LV contour detection algorithms described earlier were also applied to gated SPET images for estimation of LV volumes and ejection fraction. The extrapolation scheme for the radii and the count profiles was not used for the gated SPET, since absolute LV volumes had to be calculated. The LV volumes for the cardiac cycle were derived as follows. First the volume of LV cavity, represented by a stack of short axis slices, was calculated as:
where n represents the number of short axis slices, R i is the radius of slice i, and h denotes the slice thickness. The apex cap was defined by a semi-sphere and the volume of the apex was calculated by:
where R apex represents the radius of the semi-sphere. Consequently, the total LV volume (EDV and ESV) was computed as a summation of V1 and V2, and LVEF was calculated as:
Statistical analysis
Left ventricular parameters derived from 8-frame and 16-frame images were first compared in the entire study group, and then in patients with high- and low-dose studies, and in patients with and without myocardial perfusion defects. Linear regression and Bland-Altman analysis [26] were used to compare LV volumes and ejection fraction. Two-tailed paired Student's t test was used to determine the significance of difference in calculations of EDV, ESV and LVEF from the 8-frame and 16-frame gated SPET. Data are presented as mean ± standard deviation (mean±SD), and P<0.05 in the analysis was considered statistically significant.
Results
Left ventricular volumes
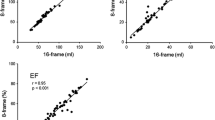
Results of volume measurements of all patient studies are summarized in Table 1. The correlation for EDV calculation by 16-frame and 8-frame method was excellent (r=0.98, SEE=12, P<0.0001, Fig. 1A). However, the mean EDV measured by 16-frame gated SPET (122±72 ml) was larger by 7.0 ml (95% C.I. 4.2–9.7 ml) than that obtained from 8-frame gated SPET studies (115±68 ml). The difference was statistically significant (P<0.0001). Bland-Altman analysis of limits of agreement indicated a larger difference for larger volumes (Fig. 1B).
Similarly, the correlation for ESV calculation by 16-frame and 8-frame method was excellent (r=0.99, SEE=6.7, P<0.0001, Fig. 2A). However, the ESV measured by 16-frame gated SPET (64±58.6 ml) was smaller by 3.6 ml (95% C.I. 5.3 to −2 ml) than that obtained from 8-frame gated SPET studies (67.6±59.5 ml). The difference was statistically significant (P<0.0001). Bland-Altman plots showed excellent agreement over a wide range of volumes (Fig. 2B).
Left ventricular ejection fraction
There was good overall correlation between the two gated framing methods (r=0.94, SEE=5.7, P<0.0001, Fig. 3A) for calculation of LVEF. However, as shown in Table 1, mean ejection fraction measured by 16-frame gated SPET (55.3%±18%) was 6.3% (95% C.I. of 5.1%–7.5%) higher than that obtained from 8-frame gated SPET (49%±17.4%) studies. The difference was statistically significant (P<0.0001). Bland-Altman analysis showed excellent agreement, with no apparent trend over a wide range of ejection fractions (Fig. 3B).
Effect of 99mTc dose on estimation of LV parameters
The effect of image count density on the estimation of LV volumes was evaluated by comparing 16-frame images and 8-frame images in high-dose and low-dose SPET studies. Fifty-six patients had high-dose studies (27.5±2.72 mCi) and 30 patients had low-dose studies (15.9±0.48 mCi). Results of comparisons are shown in Fig. 4. In high-dose studies, 16-frame gated SPET studies resulted in larger EDV (116.5±66.4 ml vs 109.7±63.8 ml, P=0.0002), smaller ESV (60.5±54.3 ml vs 64.3±56.2 ml, P<0.0001), and a higher ejection fraction (56.3%±19% vs 50.1%±18.4%, P<0.0001) as compared to 8-frame gated SPET studies. A similar trend was noted in the low-dose studies—a larger EDV (132.4±81.7 ml vs 125±75.4 ml, P=0.004), a smaller ESV (70.5±66.5 ml vs 74±65.7 ml, P=0.013), and a higher ejection fraction (53.6%±16.3% vs 46.9%±15.5%, P<0.0001). The magnitude of the difference in the estimation of LV parameters was similar in both groups, as shown in Fig. 4.
Effect of defect size
To evaluate the differential effect of myocardial perfusion defects on calculation of LV volumes by 16-frame gating, the patients were divided into two groups based on the quantified perfusion defect size. Group A consisted of 51 SPET studies with no, or very small defects (≤5% LV), whereas group B consisted of 35 SPET studies with large defects (>10% LV). The average size of the defects in patients in group B was 18%±6% LV (range 11%–33% LV).
Comparisons of LV volumes for groups A and B are shown in Tables 2 and 3. For both groups, the mean EDV obtained from 16-frame gated SPET studies was significantly larger than that obtained from 8-frame gated SPET studies. However, the mean ESV measured by 16-frame gated SPET studies was significantly smaller than that derived from 8-frame gated SPET studies. The correlation between 16-frame gating and 8-frame gating for estimation of LV volumes was excellent in both groups (Fig. 5). Also, Bland-Altman analysis showed excellent agreement over a wide range of volumes in both groups (Fig. 6) and a trend towards larger difference for larger EDVs (Fig. 6A, C).
In comparisons of LVEF for both groups (Tables 2 and 3), the ejection fractions obtained from 16-frame gated SPET studies were again significantly higher than those obtained from 8-frame gated SPET studies (63.4%±15.7% vs 56%±15.3% for group A, P<0.0001; 43.5%±14.4% vs 38.8%±15.3% for group B, P<0.0001), regardless of whether the defect was small (group A) or large (group B). This was consistent with the pattern of LVEF for all patients, as demonstrated in Fig. 3.
In Fig. 7, the difference between the 16-frame gated SPET data and 8-frame gated SPET data for LV volumes and LVEF is plotted against the defect size. As can be seen, the magnitude of perfusion defects did not affect the difference in estimation of LV parameters (EDV, ESV, LVEF) by the two gating methods (8-frame and 16-frame). There is no apparent trend in the magnitude of the difference over a wide range of defect sizes.
Reproducibility of 16-frame studies
To assess whether the accuracy of processing was significantly affected by the dose of radiotracer administered, 20 randomly selected high-dose studies and 20 low-dose studies were processed twice by two experienced nuclear medicine technologists. The correlation coefficients for EDV, ESV, and LVEF were excellent for both low-dose and high-dose studies (low dose: r=0.98, r=0.99, and r=0.97, respectively; high dose: r=0.95, r=0.96, and r=0.91, respectively).
Discussion
The present study shows that the LV volumes calculated from 16-frame imaging data differ significantly from those calculated from 8-frame imaging data. Sixteen-frame studies result in larger EDVs and smaller ESVs as compared to 8-frame studies. These findings are consistent with those of other studies [19].
The differences in LV volumes mentioned can be explained by the superior temporal resolution of 16-frame studies as compared with 8-frame studies. The end-systolic frame in an 8-frame study contains residual volume from systole/diastole on either side and hence is larger than that in a 16-frame study. Higher temporal resolution of SPET should result in more accurate determination of end-systolic and end-diastolic frames and hence more accurate assessment of ventricular volumes.
The results were consistent even in patients receiving lower doses of 99mTc. There appear to be no grounds for concern that lower count density may result in inferior image quality and impair estimation of LV parameters. Thus the implementation of a 16-frame protocol will not necessitate the use of higher doses of radiotracer to achieve "higher count densities."
We used stress studies for our analysis since only stress studies are routinely obtained by the 16-frame method in our laboratory. However, these results can be easily extrapolated to rest studies. Typically rest studies use lower doses of radiotracer (in the range of 10–15 mCi). We have shown in the present study that the results are independent of the dose of the radiotracer and that the differences in LV volumes are seen even with the lower doses.
The presence of myocardial perfusion defects may present a particular problem for 16-frame gating due to low or absent counts. There have been a number of reports regarding the effect of perfusion defect on the estimation of LV function. Vallejo et al. reported overestimation of volumes due to perfusion defects [27], while Everaert et al. [28] and Chua et al. [29] reported no effect of perfusion defects on estimation of volumes. Manrique et al. reported that both thallium-201 and 99mTc-MIBI gated SPET underestimated LVEF in patients with significant perfusion defects [30]. Using the same raw patient data, our study shows that the effect of 16-frame gated imaging on estimation of LV volumes, as compared to 8-frame gated imaging, was consistent and similar whether significant perfusion defects were present or not.
The large amount of computer storage space and longer acquisition and processing times required for 16-frame gated SPET studies may result in an increase in costs as compared to 8-frame gated SPET. The cost of storage media was a major concern a few years ago. Now this is no longer an issue owing to their decreasing cost. The use of newer data compression technologies may further reduce the storage requirements. The availability of faster and more sophisticated computers equipped with multiple processors and multi-tasking capability allows for fast and simultaneous acquisition and processing of multiple programs. This compensates for the longer acquisition and processing times necessary for 16-frame gated SPET.
Study limitations
A limitation of the present study is that LV volumes and ejection fractions, derived from 16-frame and 8-frame gated SPET, were not compared with an independent gold standard. Hence there is no direct proof that the larger EDV, higher LVEF and lower ESV from 16-frame gated SPET are indeed more accurate. However, prior studies with 8-frame gating have shown that calculated LV EDV and LVEF are lower as compared to a "gold standard" such as angiography or cine MRI [12, 16, 18]. Thus the change in LV volumes and ejection fraction with 16-frame gating is in the direction that makes them more comparable with established standards.
Another limitation of our study was the exclusion of the portion of the ventricular cavity bordered by the membranous septum in the calculation of LV volumes. This limitation is inherent to the technique of gated SPET, as the membranous septum does not accumulate radiotracer. This could potentially lead to underestimation of absolute LV volumes by gated SPET.
Clinical implications and conclusions
Measurement of LV volumes may have an important impact on the management of patients with variety of cardiac disorders, such as coronary artery disease and valvular heart disease. ESV has been shown to be a powerful predictor of mortality in patients after myocardial infarction [31] and coronary artery bypass surgery [32]. Sharir et al. showed that ESV derived by gated SPET had an incremental prognostic value in patients with coronary artery disease [33]. Our study shows that 16-frame gated SPET provides volume measurements that are significantly different than those provided by 8-frame gated SPET. Sixteen-frame gating provides better temporal resolution than 8-frame gating in the estimation of LV volumes and ejection fraction. Thus the acquisition of 16 frames per cycle may be the preferred mode for gated SPET studies.
References
Germano G, Kiat H, Kavanagh PB, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med 1995; 36:2138–2147.
DePuey EG, Nichols K, Dobrinsky C. Left ventricular ejection fraction assessed from gated technetium-99m sestamibi SPECT. J Nucl Med 1993; 34:1871–1876.
Williams KA, Taillon LA. Left ventricular function in patients with coronary artery disease assessed by gated tomographic myocardial perfusion images: comparison with assessment by contrast ventriculography and first-pass radionuclide angiography. J Am Coll Cardiol 1996; 27:173–181.
Mannting F, Morgan-Mannting MG. Gated SPECT with technetium-99m-sestamibi for assessment of myocardial perfusion abnormalities. J Nucl Med 1993; 34:601–608.
Smith WH, Kastner RJ, Calnon DA, Segalla D, Beller GA, Watson DD. Quantitative gated single photon emission computed tomography imaging: a count based method for display and measurement of regional and global ventricular systolic function. J Nucl Cardiol 1997; 4:451–463.
Calnon DA, Kastner RJ, Smith WH, Segalla D, Beller GA, Watson DD. Validation of a new count based gated single photon emission computed tomography method for quantifying left ventricular systolic function: comparison with equilibrium radionuclide angiography. J Nucl Cardiol 1997; 4:464–471.
Faber TL, Cooke CD, Folks RD, et al. Left ventricular function and perfusion from gated SPECT perfusion images: an integrated method. J Nucl Med 1999; 40:650–659.
Ziffer J, Cooke CD, Folks RD, LaPidus A, Alzaraki N, Garcia EV. Quantitative myocardial thickening assessed with sestamibi: clinical evaluation of a count based method [abstract]. J Nucl Med 1991; 32 (Suppl):1006.
Cooke CD, Garcia EV, Cullom SJ, et al. Determining the accuracy of calculating systolic wall thickening using a fast Fourier transform approximation: a simulation study based on canine and patient data. J Nucl Med 1994; 35:1185–1192.
DePuey EG, Rozanski A. Using gated technetium 99m sestamibi SPECT to characterize fixed myocardial defects as infarct or artifact. J Nucl Med 1995; 36:952–955.
Mochizuki T, Murase K, Tanaka H, Kondoh T, Hamamoto K, Tauxe WN. Assessment of left ventricular volume using ECG-gated SPECT with technetium-99m-MIBI and technetium-99m-tetrofosmin. J Nucl Med 1997; 38:53–57.
Vaduganathan P, He ZX, Vick GW III, Mahmarian JJ, Verani MS. Evaluation of left ventricular wall motion, volumes and ejection fraction by gated myocardial tomography with technetium 99m-labelled tetrofosmin: a comparison with cine magnetic resonance imaging. J Nucl Cardiol 1999; 6:3–10.
Atsma DE, Kayser HWM, Croon C, et al. Good correlation between left ventricular ejection fraction, end systolic and end diastolic volume measured by gated SPECT as compared to magnetic resonance imaging [abstract]. J Am Coll Cardiol 1999; 33 (Suppl):436A.
Iskandrian AE, Germano G, VanDecker W, et al. Validation of left ventricular volume measurements by gated SPECT99mTc-labeled sestamibi imaging. J Nucl Med 1998; 5:574–578.
Germano G, VanDecker W, Mintz R, et al. Validation of left ventricular volumes automatically measured with gated myocardial perfusion SPECT [abstract]. J Am Coll Cardiol 1998; 31 (Suppl):43A.
Yoshiko J, Hasegawa S, Yamaguchi H, et al. Left ventricular volumes and ejection fraction calculated from quantitative electrocardiographic-gated99mTc-Tetrofosmin myocardial SPECT. J Nucl Med 1999; 40:1693–1698.
Mathew D, Zabrodina Y, Mannting F. Volumetric and functional analysis of left ventricle by gated SPECT: a comparison with echocardiographic measurements [abstract]. J Am Coll Cardiol 1998; 31 (Suppl):44A.
Nichols K, Tamis J, DePeuy EG, Meires J, Malhotra S, Rozanski A. Relationship of gated SPECT ventricular function parameters to angiographic measurements. J Nucl Cardiol 1998; 5:295–303.
Kumita S, Cho K, Nakajo H, et al. Assessment of left ventricular diastolic function with electrocardiography-gated myocardial perfusion SPECT: comparison with multigated equilibrium radionuclide angiography. J Nucl Cardiol 2001; 8:568–574.
Liu YH, Sinusas AJ, DeMan P, Zaret BL, Wackers FJT. Quantification of SPECT myocardial perfusion images: methodology and validation of the Yale-CQ method. J Nucl Cardiol 1999; 6:190–204.
Liu YH. A new approach of quantification of left ventricular volume for gated SPECT imaging: preliminary validation using a Phantom [abstract]. J Nucl Cardiol 2001; 8:S61.
Liu YH, Harrell CR, Tsatkin V, et al. Evaluation of a new quantification method for gated SPECT imaging: comparison with the quantitative gated SPECT (QGS) method in patients [abstract]. J Nucl Cardiol 2001; 8:S65.
Lam PT, Wackers FJT, Liu YH. Validation of a new method for quantification of left ventricular function from ECG-gated SPECT [abstract]. J Nucl Med 2001; 42:93P.
Hwang WS, Fernando GP, Natale D,et al. Comparison and validation of 3 gated SPECT programs for volume determination using ventricular casts of excised canine hearts [abstract]. J Nucl Med 2001; 42:46P.
Tou JT, Gonzalez RC. Pattern recognition principles. Reading, Mass.: Addison-Wesley, 1974.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; i:307–310.
Vallejo E, Dione DP, Bruni WL, et al. Reproducibility and accuracy of gated SPECT for determination of left ventricular volumes and ejection fraction: experimental validation using MRI. J Nucl Med 2000; 41:874–882.
Everaert H, Bossuyt A, Franken PR. Left ventricular ejection fraction and volumes from gated single photon emission tomographic myocardial perfusion images: comparison between two algorithms working in three-dimensional space. J Nucl Cardiol 1997; 4:472–476.
Chua T, Yin LC, Thiang TH, Choo TB, Ping DZ, Leng LY. Accuracy of the automated assessment of left ventricular function with gated perfusion SPECT in the presence of perfusion defects and left ventricular dysfunction: correlation with equilibrium radionuclide ventriculography and echocardiography. J Nucl Cardiol 2000; 7:301–311.
Manrique A, Faraggi A, Vera P, Vilain D, Lebtahi R, Cribier A, Le Guludec D.201Tl and 99mTc-MIBI gated SPECT in patients with large perfusion defects and left ventricular dysfunction: comparison with equilibrium radionuclide angiography. J Nucl Med 1999; 40:805–809.
White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 1987; 76:44–51.
Hamer AW, Takayama M, Abraham KA, et al. End-systolic volume and long term survival after coronary artery bypass graft surgery in patients with impaired left ventricular function. Circulation 1994; 90:2899–2904.
Sharir T, Germano G, Kavanagh PB, et al. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation 1999; 100:1035–1042.
Acknowledgements
We thank Anne-Marie Quirk M.D., for her assistance in data collection and Wendy Bruni, Jeff Pestallozi, Maryjo Zito, Maung Khin and Vera Tastkin for their technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: Frans J.T. Wackers and Yi-Hwa Liu, through an arrangement with Yale University School of Medicine (New Haven, Conn.), receive royalties from the sale of Wackers-Liu CQ software.
Rights and permissions
About this article
Cite this article
Navare, S.M., Wackers, F.J.T. & Liu, YH. Comparison of 16-frame and 8-frame gated SPET imaging for determination of left ventricular volumes and ejection fraction. Eur J Nucl Med Mol Imaging 30, 1330–1337 (2003). https://doi.org/10.1007/s00259-003-1231-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1231-0