Abstract
Targeted transfer of a functionally active sodium iodide symporter (NIS) into tumour cells may be used for radioiodine therapy of cancer. Therefore, we investigated radioiodine uptake in a hepatoma cell line in vitro and in vivo after transfer of the sodium iodide symporter (hNIS) gene under the control of a tumour-specific regulatory element, the promoter of the glucose transporter 1 gene (GTI-1.3). Employing a self-inactivating bicistronic retroviral vector for the transfer of the hNIS and the hygromycin resistance genes, rat Morris hepatoma (MH3924A) cells were infected with retroviral particles and hNIS-expressing cell lines were generated by hygromycin selection. 125I− uptake and efflux were determined in genetically modified and wild type hepatoma cells. In addition, the iodide distribution in rats bearing wild type and genetically modified hepatomas was monitored. hNIS-expressing MH3924A cell lines accumulated up to 30 times more iodide than wild type hepatoma cells, with a maximal iodide uptake after 30 min incubation time. Competition experiments in the presence of sodium perchlorate revealed a decrease in the iodide uptake (80–84% decrease). Moreover, ouabain led to a loss of accumulated I− (81% decrease) whereas 4,4'-diisothiocyano-2,2'-disulphonic acid stilbene (DIDS) increased the I− uptake into cells (87% increase). However, a rapid efflux of the radioactivity (70%) was observed 20 min after 125I−-containing medium had been replaced by non-radioactive medium. Lithium had no significant effect on iodide efflux. In rats, the hNIS-expressing tumours accumulated 22 times more iodide than the contralateral wild type tumour. In accordance with the in vitro data, we also observed a rapid efflux of the radioactivity out of the tumour in vivo. Dosimetric calculations resulted in an absorbed dose of 85 mGy in the wild type tumour and 830 mGy in the hNIS-expressing tumour after administration of 18.5 MBq 131I. In conclusion, transduction of the hNIS gene under the control of the GLUT1 promoter element induces iodide transport in Morris hepatoma cells in vitro and in vivo. However, for therapeutic application additional conditions need to be defined which inhibit the iodide efflux out of the tumour cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A well-described strategy for gene therapy of cancer is to render tumour cells susceptible to non-toxic chemotherapeutic agents using suicide genes. Besides the combination of gene transfer and chemotherapy, combination of gene transfer and radioactive isotopes is another promising avenue for the treatment of cancer. For each of these potential treatments to achieve clinical utility, the therapeutic gene must be delivered to tumour cells in vivo while avoiding undesired expression in normal tissues [1]. This can be achieved with a specific promoter directing the expression of a heterologous gene to the cells of interest.
It is known that tumour cells have a higher glucose metabolism compared with normal tissue cells [2], and tumour-related changes in the expression of glycolysis-associated genes have been reported by several investigators. Especially the type 1 glucose transporter (GLUT1) gene has been described as one of the early genes which are activated after transformation of cells with oncogenes such as src, ras or fps [3, 4, 5, 6, 7, 8, 9, 10]. An increase in GLUT1 mRNA was found 4–6 h after induction of the p21 c-H-ras oncoprotein, whereas morphologic changes occurred 72–96 h later [8, 11]. The facilitative glucose transporter GLUT1 has also been shown to be upregulated in human tumours [12, 13]. Besides the regulatory elements for vascular endothelial growth factor (VEGF) and most glycolytic enzymes, the GLUT1 promoter is a target of the hypoxia inducible factor 1α (HIF1α). HIF1α increases in hypoxic areas and during stimulation with vasoactive hormones or factors such as angiotensin II, thrombin and platelet-derived growth factor. As a consequence of increased HIF1α levels, VEGF, GLUT 1 and glycolytic enzymes are upregulated, especially in hypoxic areas of the tumors. Therefore, expression of the therapeutic gene in these problematic areas can be obtained with the GLUT1 promoter. These features make the regulatory element of the GLUT1 gene a promising tool for tumour-specific gene therapy. In mice the GLUT1 promoter sequence was mapped at 1.3 kb to +137 bp [14, 15] and two enhancer elements have been characterised.
The characteristic ability of thyroid follicular cells to accumulate iodide enables benign thyroid diseases and differentiated thyroid carcinoma to be successfully treated with radioiodide therapy. The complex process of iodide trapping in the thyroid tissue is initiated by the active transport of iodide and sodium ions into follicular cells, which is mediated by the sodium-iodide symporter (hNIS). The energy-dependent transport, which proceeds against an electrochemical gradient, is coupled to the action of Na+/K+-ATPase and is stimulated by TSH [16, 17, 18, 19, 20].
Employing the rat Morris hepatoma cell line MH3924A as an in vitro/in vivo system, we investigated the effect of retroviral transfer of the hNIS gene under control of the promoter element of the GLUT1 gene on iodide accumulation in transfected cells. This type of study combines the use of a tumour-specific promoter and a gene leading to the accumulation of a beta-emitting isotope in cancer cells. In this case trapping centres in the tumour could create a cross-firing of beta particles, resulting in destruction of both transduced and non-transduced tumour cells. We report here that the transduction of the hNIS coding sequence under control of a tumour-specific regulatory element is sufficient to induce increased iodide uptake which is associated with a considerable efflux and no significant effects of lithium on iodide trapping.
Materials and methods
Construction of a bicistronic retroviral vector for the transfer of the hNIS gene and the hygromycin gene
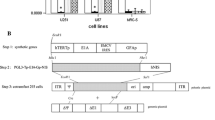
For transfer of the hNIS gene, a bicistronic retroviral vector based on the pSIR self-inactivating retroviral vector (Clontech, USA) was constructed. The hNIS gene and the hygromycin resistance gene were cloned downstream of the glucose transporter 1 promoter (GTI-1.3) taken from the GTI-1.3CAT vector (obtained from Murakami et al. [14]) (Fig. 1). To ensure simultaneous expression of the genes coding for the hNIS and for the hygromycin resistance and stability of the mRNA, a synthetic intron and an internal ribosomal entry site (IRES) from encephalomyocarditis virus (EMCV) were inserted between the genes. Synthetic intron, IRES and hygromycin resistance gene were excised from pIRES1hyg (Clontech, USA).
Structure of the pSIR retroviral vector. pSIR, derived from Moloney murine leukaemia virus, is a self-inactivating retroviral vector which contains a deletion in the 3´LTR resulting in inactivation of the 5´LTR promoter. The vector contains GTI-1.3 as a regulating element for the expression of hNIS and the hygromycin resistance gene. A synthetic intron (IVS) is inserted to stabilise mRNA. To ensure simultaneous expression of the genes, an internal ribosomal entry site (IRES) from encephalomyocarditis virus (EMCV) is inserted
The self-inactivating vector contains a 176-bp deletion in the 3' LTR which removes enhancer sequences [21]. After reverse transcription, the 3' LTR is copied and replaces the 5' LTR, thereby inactivating the 5' LTR promoter and leaving the internal promoter (GTI-1.3) as the only promoter which drives the expression of downstream-located genes.
Cell culture, retroviral infection and generation of recombinant cell lines
The rat Morris hepatoma cell line MH3924A was cultured in RPMI1640 medium with glutamax-I supplemented with 20% fetal calf serum (FCS), and the transient packaging cell line BOSC23 [22], employed for the production of ecotropic retroviral particles, was cultured in RPMI1640 medium with glutamax-I supplemented with 10% FCS. All cells were supplemented with 100 IU/ml penicillin and 100 mg/l streptomycin (Invitrogen, Germany) and were cultured at 37°C, in an atmosphere of 95% air and 5% CO2.
For transient packaging of the retroviral DNA containing the genes of interest and a hygromycin resistance gene (Fig. 1), a lipofection of BOSC23 cells was done. After 2 days the medium containing the retroviral particles was centrifuged to remove detached BOSC23 cells and used for the infection of the MH3924A cells in the presence of polybrene at 8 μg/ml overnight. To select for cells infected with retroviral particles containing the NaI symporter and hygromycin resistance gene under control of the GLUT1 promoter and stable integration of the virus DNA into the host genome, the cells were treated with 425 μg/ml hygromycin for 3 weeks until resistant cell lines were established.
Measurement and modulation of the 125I uptake and efflux
The iodide uptake was performed as described previously by Haberkorn et al. [23]. All experiments were done in triplicates and repeated at least twice. In the presence of 74 kBq Na125I (Amersham, Germany; specific activity 625 MBq/μg; radioactive concentration 3.7 GBq/ml; radiochemical purity 99.3%), wild type MH3924A tumour cells and recombinant cell lines were incubated for 1 h. The cell counts were determined in a Coulter counter (Beckman Coulter, Germany). The cell viability was assessed by trypan blue staining. Cells treated with Na125I were washed twice with ice-cold PBS and lysed with 0.3 M sodium hydroxide on ice. The radioactivity in both cell lysates and medium was measured using an automated NaI(Tl) well counter (Cobra II, Canberra Packard, USA).
To determine the iodide uptake in relationship to the incubation time, the recombinant cell line GTI-1.3hNIS43 and wild type MH3924A cells were cultured with 74 kBq Na125I for 1, 2, 5, 10 or 30 min or 1, 2 or 4 h. Washing and counting was performed as described. For modulation of the iodide uptake, hNIS-expressing and wild type cells were incubated for 1 h in Na125I medium (74 kBq) or Na125I medium supplemented with 10 or 50 μM sodium perchlorate (Sigma), 100 or 300 μM of the anion channel blocker 4,4'-diisothiocyano-2,2'-disulphonic acid stilbene (DIDS; Sigma) or 500 µM ouabain (Sigma), an inhibitor of the Na+/ K+-ATPase. Thereafter, the cells were washed, lysed and counted as described.
To determine the 125I efflux, recombinant and wild type cells were incubated for 1 h with medium containing 74 kBq Na125I. After the cells had been washed twice, three wells were lysed immediately. To the remaining wells was added fresh non-radioactive medium. The cells were again incubated for 2, 4, 6, 8, 10, 12, 16 or 20 min and immediately lysed as described. To investigate the effect of lithium on the 125I efflux, cells were incubated for 24 h with 2, 10 or 50 mM lithium chloride (Merck, Germany). Thereafter, an efflux experiment was performed in the presence of LiCl2 as described above. After incubation for 1 h in the presence of 74 kBq Na125I, the radioactive medium was removed, and the cells were washed twice and incubated in non-radioactive lithium-containing medium for 10 or 20 min and lysed.
Measurement of the 131I uptake in tumour tissue of rats
The experiments were performed in compliance with the current version of the German Law on the Protection of Animals. GTI-1.3hNIS43 tumour cells (8×106) were transplanted subcutaneously into the right thigh or 4×106 wild type MH3924A cells were transplanted into the left thigh of 6-week-old male ACI rats weighting 200–250 g. For imaging studies, which were performed under general gaseous anaesthesia (40% O2/ 60% N2O/1% halothane), only animals bearing tumours with a minimum size of 10 mm in diameter were accepted. After injection of 14.8–18.5 MBq 131I (corresponding to 481–601 MBq/m2) in 200 µl of 0.9% NaCl into the lateral tail vein of the rats, scintigraphic images were taken using a 10-inch scintillation-camera (Searle-Siemens, Germany). The time-dependent relative accumulation of radioactivity in different regions of interest (ROIs), e.g. the heart, the liver, the tumour, the bladder and the whole animal, was monitored in four animals at 1, 2, 4, 6 and 24 h post injection. The absolute amount of radioactivity (% injected dose/g tissue) was determined in 20 animals which were sacrificed 1, 2, 4, 6 or 24 h post injection, by analysing the organs using an automated NaI(Tl) well counter (Cobra II, Canberra Packard, USA).
Results
Generation of hNIS-expressing MH3924A cell lines
After infection of MH3924A cells with recombinant retroviruses and selection with hygromycin, stable cell lines were established. In order to investigate the hNIS activity in the recombinant MH3924A cell lines, iodide uptake experiments were performed and the cell line GTI-1.3hNIS43 with the highest 125NaI uptake was selected for further experiments. For the MH3924A and MHGTI-1.3hJTr43 cells we calculated an in vitro doubling time of 19 h and 18 h respectively. To exclude variations in iodide accumulation due to different cell volumes of wild type cells and transformed cells, the cell size was determined using a Coulter counter, revealing a diameter of 15.2 µm for MH3924A cells and 15.5 µm for MHGTI-1.3hJTr43 cells.
Na125I uptake and efflux in recombinant MH3924A cell lines
After incubation with Na125I for 1 h, up to 30-fold more iodide was transported into the hNIS-expressing hepatoma cell lines as compared with the wild type hepatoma cells (Fig. 2). The GTI-1.3hNIS43 cells presented the highest 125I− uptake and, therefore, were employed for the following experiments. The iodide uptake was dependent on incubation time (Fig. 3), with a maximal 125I− uptake after 30 min exposure. The radioactivity measured after 1 and 2 h 125I− incubation was at a plateau level, implying a steady state.
Iodide uptake in MH wild type (wt) cells and MH cell lines that were stably transfected with hNIS under the control of GTI-1.3. The cells were incubated with 125NaI for 1 h and the intracellular radioactivity was determined. Up to 30-fold higher iodide uptake was seen in MHGTI-1.3hNIS clones compared with MH wt cells. Data are mean values (n=3) ±SD
Time course of iodide uptake by MH wild type (wt) and MHGTI-1.3hNIS43 cells. The cells were incubated with 125NaI for 1, 2, 5, 10, 30, 60, 120 or 240 min and intracellular radioactivity was determined. Iodide uptake by MHGTI-1.3hNIS43 was rapid and became maximal within 30 min, whereas uptake by MH wt remained low. Data are mean values (n=3) ±SD
Figure 4 presents the effect of DIDS, ouabain and sodium perchlorate on Na125I uptake in the GTI-1.3hNIS43 cell line. In the presence of 10 or 50 μM sodium perchlorate we observed an inhibition of iodide accumulation in the GTI-1.3hNIS43 cells by 80% and 84% respectively. Addition of ouabain, which is an inhibitor of the Na+/K+-ATPase led to the loss of 81% of the accumulated I−. The anion channel blocker DIDS had the opposite effect, as it increased the I− uptake into the cells by 87% with no difference at concentrations of 100 and 300 μM.
Modulation of iodide uptake by sodium perchlorate, ouabain and DIDS. After 1 h incubation with 125NaI, the uptake of iodide was decreased in the presence of ouabain (−81%) and sodium perchlorate (−84% at 50 µM) and increased with DIDS (+87% at 100 µM) compared with the untreated control. Data are mean values (n=3) ±SD
To determine the isotope efflux, the iodide uptake was allowed to proceed for 1 h, at which time a steady state level of accumulation was achieved. After the medium had been replaced by non-radioactive medium, the amount of 125I− present in the GTI-1.3hNIS43 cell lysates was determined as a function of time. The cellular radioactivity was constantly released into the medium and 67% efflux was observed after 20 min, indicating that the radiotracer was not trapped in the recombinant hepatoma cells (Fig. 5A). The addition of 2 and 10 mM lithium to the culture medium led to an increase in the initial 125I− uptake. However, a 62% efflux during 20 min was observed. The addition of 50 mM lithium led to an inhibition of 50% in the initial iodide uptake compared to cells without lithium, with 70% efflux after 20 min (Fig. 5B).
131I uptake in Morris hepatoma in rats
Consistent with the data obtained from the in vitro studies the hNIS-expressing tumour tissue significantly accumulated 131I−, leading to scintigraphic visualization, whereas only low iodide uptake was observed in the wild type hepatoma (Fig. 6). In the genetically modified tumours the tracer accumulation increased to a maximum level during the first hour after administration, followed by a decrease in the intratumoral radioactivity, resulting in a biological 131I− half-life in hNIS-expressing tumours of 14.1 h within the first 24 h after tracer administration. In contrast, the thyroid showed increasing tracer accumulation during the examination period (Fig. 7). The ex vivo quantitation of the 131I− uptake (% injected dose/gram tissue) of the tumours and of various organs was evaluated 1, 2, 4, 6 and 24 h after tracer administration (Fig. 8). In the hNIS-expressing tumours, up to 23-fold higher iodide accumulation after 1 h was detected when compared with the wild type tumour, corresponding to 4.7% of the injected dose (ID)/g tissue. As a positive control, 23% ID/g could be achieved in the thyroid gland at 1 h after tracer administration. However, 2 h after tracer administration a decrease of radioactivity in the hNIS-expressing tumour occurred (4.2% ID/g) in contrast to the thyroid gland, where iodide is actively organified, resulting in 32% ID/g tissue. At 24 h p.i. only a twofold accumulation (0.4% ID/g) was observed compared with the wild type tumour. Using MIRDOSE 3* [24] we calculated a cumulated activity of 2,879 MBq×s and 2.8×104 MBq×s for the wild type and the hNIS-expressing tumours, respectively. This activity resulted in an absorbed dose of 85 mGy (wild type tumour) and 830 mGy (hNIS-expressing tumour) after administration of 18.5 MBq 131I.
Scintigraphic images of tumour-bearing ACI rats. Wild type (left thigh) or hNIS-expressing (right thigh) Morris hepatoma cells were transplanted subcutaneously. 131I− was injected i.v. and accumulation in the hNIS-expressing tumours was determined after 1 h (A), 2 h (B), 4 h (C) and 6 h (D). Images are scaled from 0% to 100%
131I− uptake in hNIS-expressing and wild type Morris hepatomas at 1, 2, 4, 6 and 24 h after radiotracer administration in rats [impulses (imp)/pixel]. A decrease in radioactivity occurred in the hNIS-expressing tumours within 24 h after administration, whereas an increase in tracer accumulation could be observed in the thyroid gland. Data are mean values (n=4) ±SD
Discussion
Thyroid cancer can be very effectively treated by radioiodine therapy. This is due to the expression of thyroidal genes such as thyroid peroxidase (TPO) and NIS, which lead to trapping of radioactive iodine in these tumours. Functioning thyroid cancer metastases can be detected and treated by administration of radioiodine, while avoiding adverse effects of ionising radiation to other organs, which do not concentrate radioiodine.
The cloning of the gene coding for the human sodium iodide symporter offered the possibility of hNIS gene transfer into non-thyroidal tumour cells, thereby inducing radioiodine uptake which may be used for imaging and treatment of non-thyroidal tumours with radioiodine [25]. Quantitation of iodide transport into genetically modified tissues may be done by dynamic positron emission tomography (PET) measurements of 121I or 124I uptake and pharmacokinetic models calculating transport rates. For scintigraphy with 99mTcO4 or 123I, semiquantitative approaches using ratios of transfected to non-transfected tissues may be useful. Since radioiodine therapy of differentiated thyroid cancer has been proven to be very effective with no severe side-effects in the gastrointestinal tract or other tissues, transfer of the hNIS gene into non-thyroid tumours may be an attractive new therapeutic approach. However, to avoid adverse effects on the normal thyroid, this procedure makes sense only after thyroidectomy. Mandell et al. [26] demonstrated in vitro and in vivo iodide accumulation in several cancer cell lines, including melanoma, liver, colon carcinoma and ovarian carcinoma cell lines, after transfection with the rat NIS gene. Stable cell lines [23, 27] and gene therapeutic approaches with tissue specific promoters [28, 29, 30, 31] were performed to direct the expression of the NIS gene to cells of interest.
The efficacy of radioiodine therapy of hepatoma after transfer of the hNIS gene could be enhanced using a tumour-specific promoter for the regulation of gene expression. The gene for the glucose transporter GLUT1 is known to be upregulated in most tumours [12, 13] and oncogene transformed cells [3, 4, 5, 6, 7, 8, 9, 10]. Therefore, the GLUT1 promoter should provide an efficient regulatory unit for tumour-specific gene expression. We could show in a series of experiments that under regulation of the GLUT1 promoter, reporter gene and HSVtk suicide gene expression was restricted to tumour cells and oncogene transformed cells [Sieger S et al., unpublished work]. In this study we investigated whether the transduction of the hNIS gene under the control of the GLUT1 promoter element is sufficient to induce iodide uptake in Morris hepatoma cells and tumours in rats. Employing a bicistronic self-inactivating retroviral vector for the hNIS and hygromycin resistance expression, we established MH3924A cell lines expressing the hNIS gene under the transcriptional control of the promoter region of the GLUT1 gene (GTI-1.3). With respect to the wild type hepatoma cells, increased iodide uptake was observed in all recombinant cell lines. As the expression of viral DNA after its integration in the host genome can be affected by the chromatin structure of contiguous cellular sequences, the level of 125I− accumulation varied significantly among individual cell lines. Additionally the balance of positive and negative acting host transcription factors determines the level of transcriptional activity of integrated viral DNA [32]. We observed an up to 30-fold higher radiotracer uptake when compared with the wild type cells, with a maximal accumulation after 30 min incubation (Fig. 3). In like manner, Cos 7 cells transiently infected with the hNIS expression vector pcDNA3 accumulated tenfold more Na125I than controls [25]. Mandell et al. [26] modified human melanoma, mouse colon carcinoma and human ovarian adenocarcinoma cells with a retroviral vector bearing the rat NIS gene and also observed an up to 35-fold increase in iodide uptake.
To evaluate whether the iodide accumulation was specifically induced by the hNIS activity, iodide uptake was determined in the GTI-1.3hNIS43 cells in the presence of sodium perchlorate, DIDS or the Na+/K+-ATPase inhibitor ouabain. Iodide uptake was decreased in the presence of ouabain as well as of sodium perchlorate, indicating that a functional hNIS is expressed in the genetically modified cell lines (Fig. 4). In contrast, the potent erythrocytes anion channel blocker DIDS, which has been described to stimulate the initial influx of iodide in the rat thyroid cell line FRTL5 [17] and in porcine thyroid cells [20], caused an 87% increase in uptake in the GTI-1.3hNIS43 cell line.
Non-thyroid, hNIS-expressing cells do not organify iodide, with the consequence that the concentration of intracellular iodide will drop proportionally to the external iodide concentration (Fig. 5A). Lithium was found to inhibit the release of iodine from the thyroid. Therefore, lithium was used to enhance the efficacy of radioiodine treatment of differentiated thyroid cancer [33, 34, 35]. In FRTL-5 rat thyroid cells and in primary cultures of porcine thyroid follicles, 2 mM lithium suppressed TSH-induced iodide uptake, iodide uptake stimulated by 8-bromo-cAMP, iodine organification and de novo thyroid hormone formation [35]. Koong et al. [36] described a lithium-induced prolongation (by up to 50%) of the biological and effective half-lives and increased accumulation of 131I in metastases and thyroid remnants. A variety of actions have been described for lithium. These include inhibitory effects on the adenosine triphosphatase (ATPase) activity and on the inositol phospholipid metabolism with an impact on signal transduction, as well as alterations of cyclic adenosine monophosphate (cAMP), intracellular enzymes and in vitro responses of cultured cells to thyrotropin-releasing hormone. We performed efflux experiments with hNIS-expressing hepatoma cells in the presence of different concentrations of lithium. Lithium-treated cells revealed a higher initial iodide uptake compared with untreated cells when lithium concentrations of 2 and 10 mM were used, but still a 62% efflux was observed after 20 min. When lithium was used at a concentration of 50 mM, the initial iodide uptake was decreased by 50% in comparison to untreated cells, with a 70% efflux after 20 min (Fig. 5B). Our data showed only a marginal effect of lithium in hepatoma cells and cannot confirm the data obtained with thyroid cells. Therefore, it remains questionable whether the mechanisms described can be transferred to non-thyroid cells transfected with the NIS gene.
Another approach to achieve prolonged iodine retention in non-thyroidal tumours is simultaneous transfer of genes responsible for the iodide organification. Boland et al. [37] observed low levels of iodide organification in cells co-infected with both the NIS and the TPO gene in the presence of exogenous hydrogen peroxide. In a former study with rat hepatoma cells we could achieve high amounts of hTPO protein after retroviral transfer of the human TPO gene, but we were not able to measure TPO enzyme activity or enhanced accumulation of iodide [38]. Future approaches in our laboratory are directed towards stabilization of TPO by co-transfer of calreticulin and also the induction of thyroid-specific gene expression by transfer of thyroid specific transcription factors.
In vivo experiments with stable transduced MHGTI-1.3hNIS cells revealed maximal iodide uptake into the genetically modified hepatoma 1 h after the rats had been injected with 131I. A decrease in iodide accumulation occurs as early as 2 h after tracer administration, whereas the thyroid gland accumulates 131I until 24 h p.i. Although the hNIS protein favours iodide transport into the cells rather than efflux, the radioactivity continuously disappeared from the hNIS-expressing tumours and from different organs of the body except the thyroid (Figs. 6, 7 and 8). In a recent study Haberkorn et al. [23] transferred the hNIS gene with the EF1α regulatory element in rat Morris hepatoma cells and achieved an up to 235-fold higher iodide accumulation in vitro and a 17-fold higher iodide uptake 1 h after tracer administration in vivo. Using the GLUT1 promoter as a regulating element for the hNIS gene, we observed relatively less iodide accumulation with the GLUT1 promoter in vitro, but we achieved a higher (23-fold) iodide accumulation after 1 h in vivo. EF1α, the adult type elongation factor 1α, is one of the most abundant proteins in eukaryotic cells and therefore its promoter serves as a strong constitutive regulating element. The higher iodide accumulation achieved in vivo by using the GLUT1 regulatory element is evidence for the high activation of the GLUT1 promoter in the hepatoma model used in this study. However, different integration sites for exogenous genes in the cell lines may be responsible for differences in iodide uptake. An influence may also be expected from the different vector systems used: a retroviral vector with an intact long terminal repeat (LTR) and an internal promoter (EF1α) versus a self-inactivating retroviral vector with the GLUT1 promoter as the only active regulatory element. Furthermore, the copy number of integrated retroviral sequences may account for differences in activity. However, as we used the same stable cell line in vitro and in vivo with the same copy number in both types of studies, the observed higher uptake in vitro for the EF1α promoter but higher uptake in vivo for the GLUT1 promoter does not seem to be influenced by the copy number or the integration site. One possible interpretation may be the strong induction of the GLUT1 promoter in vivo by the transcription factor HIF1α. HIF1α is upregulated especially in hypoxic areas, as well as in response to factors such as angiotensin II, thrombin and platelet-derived growth factor. Since tumours are highly heterogeneous with widely distributed hypoxic areas, we may expect induction of the GLUT1 regulatory elements by increased levels of HIF1α. Furthermore, the use of a self-inactivating retrovirus where the 5'LTR promoter is lost during reverse transcription leaves the GLUT1 promoter as the only active promoter in the construct, and no interference occurs between the 5'LTR and the internal promoter.
We determined a biological 131I− half-life in hNIS-expressing tumours of 14.1 h within the first 24 h after tracer administration. Compared with the amount of radioactivity in the blood, where we observed a biological 131I− half-life of 4 h, we achieved a prolonged accumulation of 131I in the hNIS-expressing tumour. But still we expect the exposure time of genetically modified tumour cells to 131I− radiation to be too short for therapeutic relevance unless iodide is organified. From the accumulated activity of 2,879 MBq×s and 2.8×104 MBq×s for the wild type and the hNIS-expressing tumours respectively, we calculated an absorbed dose of 85 mGy (wild type tumour) and 830 mGy (hNIS-expressing tumour) after administration of 18.5 MBq 131I. Shimura et al. [39] also reported an achieved dose of 4 Gy after administration of 37 MBq 131I, which was not sufficient to inhibit the growth of rat FRTL-Tc cells.
This study focussed on characterisation of cells, transfected with the Na+/I− symporter under control of a tumour-specific regulatory element in vitro and in vivo. The transduction of the hNIS gene induces high accumulation of iodide in non-I−-concentrating hepatoma cells, indicating a strong GLUT1 promoter-induced transcription of the hNIS gene in tumour cells. Furthermore, the GLUT1 promoter seems to be attractive because of the heterogeneous pattern of tumours, including the presence of hypoxic areas. In these areas GLUT1 may be strongly induced by the transcription factor HIF1α. However, since the iodide is not organified in the genetically modified cells, the high influx is followed by a rapid efflux in vitro and in vivo. Nevertheless, the approach of targeted radioiodide therapy with the help of a tumour-specific regulatory element can lead to accumulation of iodide in cancer tissue in vivo. However, several problems remain to be overcome: for a gene therapeutic application of the NIS gene under control of a tumour-specific promoter, the delivery mechanisms (viral vectors as gene shuttles) for efficient tumour cell transduction with the recombinant DNA for the NIS gene need to be improved, and 131I must be retained in the target tissue sufficiently long to obtain therapeutically relevant radiation doses in the tumours. A further option to improve therapy outcome is the use of biologically more effective isotopes such as the high LET-emitter astatine-211 [40]. In addition to its therapeutic application, tumour-specific NIS gene expression could provide a diagnostic tool for imaging gene transfer in non-thyroidal tumours and their metastases using 99Tc, 123I or 121I [41].
References
Dachs GU, Dougherty GJ, Startford IJ, Chaplin DJ. Targeting gene therapy to cancer: a review. Oncol Res 1997; 9:313–323.
Warburg O, Wind F, Negelein E. On the metabolism of tumors in the body. In: Warburg O, ed. The metabolism of tumors. London: Constable; 1939:254–270.
Flier JS, Mueckler MM, Usher P, Lodish HF. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science 1987; 235:1492–1495.
Hiraki Y, deHerreros AG, Birnbaum MJ. Transformation stimulates glucose transporter gene expression in the absence of protein kinase C. Proc Natl Acad Sci U S A 1989; 86:8252–8256.
Godwin AK, Lieberman MW. Elevation of glucose transporter, c-myc, and transin RNA levels by Ha-rasT24 is independent of its effect on the cell cycle. Mol Carcinogenesis 1991; 4:275–285.
White MK, Weber MJ. Transformation by the src oncogene alters glucose transport into rat and chicken cells by different mechanisms. Mol Cell Biol 1988; 8:138–144.
White MK, Weber MJ. The src oncogene can regulate a human glucose transporter expressed in chicken embryo fibroblasts. Mol Cell Biol 1990; 10:301–306.
Sistonen L, Hölttä E, Mäkelä TP, Keski-Oja J, Alitalo K. The cellular response to induction of the p21c-Ha-ras oncoprotein includes stimulation of jun gene expression. EMBO J 1989; 9:815–822.
Birnbaum MJ, Haspel HC, Rosen OM. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science 1987; 20; 235:1495–1498.
Shawver LK, Olson SA, White MK, Weber MJ. Degradation and biosynthesis of the glucose transporter protein in chicken embryo fibroblasts transformed by the src oncogene. Mol Cell Biol 1987; 7:2112–2118.
Godwin AK, Lieberman MW. Early and late responses to induction of ras T24 expression in Rat-1 cells. Oncogene 1990; 5:1231–1241.
Nishioka T, Oda Y, Seino Y, Yamamoto T, Inagaki N, Yano H, Imura H, Shigemoto R, Kikuchi H. Distribution of the glucose transporters in human brain tumours. Cancer Res 1992; 52:3972–3979.
Yamamoto T, Seino Y, Fukumoto H, Koh G, Yano H, Inagaki N, Yamada Y, Inoue K, Manabe T, Imura H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun 1990; 170:223–230.
Murakami T, Nishiyama T, Shirotani T, Shinohara Y, Kan M, Ishii K, Kanai F, Nakazuru S, Ebina Y. Identification of two enhancer elements in the gene encoding the type 1 glucose transporter from the mouse which are responsive to serum, growth factor, and oncogenes. J Biol Chem 1992; 267:9300–9306.
Todaka M, Nishiyama T, Murakami T, Saito S, Ito K, Kanai F, Kan M, Ishii K, Hayashi H, Shichiri M. The role of insulin in activation of two enhancers in the mouse GLUT1 gene. J Biol Chem 1994; 269:29265–29270.
Marcocci C, Cohen JL, Grollman EF. Effect of actinomycin D on iodide transport in FRTL-5 thyroid cells. Endocrinology 1984; 115:2123–2132.
Weiss SJ, Philp NJ, Grollman EF. Iodide transport in a continuous line of cultured cells from rat thyroid. Endocrinology 1984; 114:1090–1098.
Paire A, Bernier-Valentin F, Selmi-Ruby S, Rousset B. Characterization of the rat thyroid iodide transporter using anti-peptide antibodies. J Biol Chem 1997; 272:18245–18249.
Nakamura Y, Ohtaki S, Yamazaki I. Molecular mechanism of iodide transport by thyroid plasmalemmal vesicles: cooperative sodium activation and asymmetrical affinities for the ions on the outside and inside of the vesicles. J Biochem 1988; 104:544–549.
Nakamura Y, Kotani T, Ohtaki S. Transcellular iodide transport and iodination on the apical plasma membrane by monolayer porcine thyroid cells cultured on collagen-coated fibers. J Endocrinol 1990; 126:275–281.
Nakajima K, Ikenaka K, Nakahira K, Morita N, Mikoshiba K. An improved retroviral vector for assaying promoter activity; analysis of promoter interference in pIP211 vector. FEBS Lett 1993; 315:129–133.
Pear WS, Nolan GP, Scott ML, Baltimorre D. Production of high titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A 1993; 90:8392–8396.
Haberkorn U, Henze M, Altmann A, Jiang S, Morr I, Mahmut M, Peschke P, Kubler W, Debus J, Eisenhut M. Transfer of the human NaI symporter gene enhances iodide uptake in hepatoma cells. J Nucl Med 2001; 42:317–325.
Stabin M. MIRDOSE: personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 1996; 37:538–546.
Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL, Jhiang SM. Cloning of the human sodium iodide symporter. Biochem Biophys Res Commun 1996; 226:339–345.
Mandell RB, Mandell LZ, Link CJ. Radioisotope concentrator gene therapy using the sodium/iodide symporter gene. Cancer Res 1999; 59:661–668.
Nakamoto Y, Saga T, Misaki T, Kobayashi H, Sato N, Ishimori T, Kosugi S, Sakahara H, Konishi J. Establishment and characterization of a breast cancer cell line expressing Na+/I− symporters for radioiodide concentrator gene therapy. J Nucl Med 2000; 41:1898–1904.
Spitzweg C, O'Connor MK, Bergert ER, Tindall DJ, Young CY, Morris JC. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res 2000; 60:6526–6530.
Spitzweg C, Dietz AB, O'Connor MK, Bergert ER, Tindall DJ, Young CY, Morris JC. In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Ther 2001; 8:1524–1531.
Spitzweg C, Morris JC. Approaches to gene therapy with sodium/iodide symporter [review]. Exp Clin Endocrinol Diabetes 2001;109:56–59.
Boland A, Ricard M, Opolon P, Bidart JM, Yeh P, Filetti S, Schlumberger M, Perricaudet M. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumours for a targeted radiotherapy. Cancer Res. 2000; 60:3484–3492.
Goff SP. Genetics of retroviral integration. Ann Rev Genet 1992; 26:527–544.
Temple R, Berman M, Robbins J, Wolff J. The use of lithium in the treatment of thyrotoxicosis. J Clin Invest 1972; 51:2746–2756.
Gershengorn MC, Izumi M, Robbins J. Use of lithium as an adjuvant to radioiodine therapy of thyroid carcinoma. J Clin Endocrinol Metab 1976; 42:105–111.
Urabe M, Hershman JM, Pang XP, Murakami S, Sugawara M. Effects of lithium on function and growth of thyroid cells in vitro. Endocrinology 1991; 129:807–814.
Koong SS, Reynolds JC, Movius EG, Keenan AM, Ain KB, Lakshmanan MC, Robbins J. Lithium as a potential adjuvant to131I therapy of metastatic, well differentiated thyroid carcinoma. J Clin Endocrinol Metab 1999; 83:912–916.
Boland A, Magnon C, Filetti S, Bidart JM, Schlumberger M, Yeh P, Perricaudet M. Transposition of the thyroid iodide uptake and organification system in nonthyroid tumor cells by adenoviral vector-mediated gene transfers. Thyroid 2002; 12:19–26.
Haberkorn U, Altmann A, Jiang S, Morr I, Mahmut M, Eisenhut M. Iodide uptake in human anaplastic thyroid carcinoma cells after transfer of the human thyroid peroxidase gene. Eur J Nucl Med 2001; 28:633–638.
Shimura H, Haraguchi K, Miyazaki A, Endo T, Onaya T. Iodide uptake and experimental131I therapy in transplanted undifferentiated thyroid cancer cells expressing the Na+/I− symporter gene. Endocrinology 1997; 138:4493–4496.
Petrich T, Helmeke HJ, Meyer GJ, Knapp WH, Pötter E. Establishment of radioactive astatine and iodine uptake in cancer cell lines expressing the human sodium iodide symporter. Eur J Nucl Med 2002; 29:842–854.
Haberkorn U, Altmann A, Eisenhut M. Functional genomics and proteomics—the role of nuclear medicine. Eur J Nucl Med 2002; 29:115–132.
Acknowledgements
The authors wish to thank M. Mahmut, I. Morr and I. Preugschat-Gumprecht for their technical help. We also thank R. Kühnlein for his help in performing the animal experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sieger, S., Jiang, S., Schönsiegel, F. et al. Tumour-specific activation of the sodium/iodide symporter gene under control of the glucose transporter gene 1 promoter (GTI-1.3). Eur J Nucl Med Mol Imaging 30, 748–756 (2003). https://doi.org/10.1007/s00259-002-1099-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-002-1099-4