Abstract
Calcific tendinopathy typically affects the shoulder rotator cuff tendons. Its management includes shock wave therapy and US-guided percutaneous irrigation, with surgery being less and less used. Extra-shoulder calcific tendinopathy is relatively infrequent and typically affects the hip. While the diagnostic techniques and the treatment options for shoulder calcific tendinopathy have been extensively described, there are only anecdotic reports on the other sites. In this paper, we have reported an unusual case of non-insertional Achilles calcific tendinopathy which occurred many years after Achilles surgical repair. This condition, which presented similar appearance to that of the rotator cuff calcific tendinopathy, is totally different from the well-known and more common insertional calcific Achilles tendinopathy in terms of pathophysiological, imaging, and clinical findings. Further, we have shown that US-guided percutaneous irrigation might be a safe, technically feasible, mini-invasive, and effective treatment also for Achilles calcific tendinopathy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calcific tendinopathy is a common condition caused by the deposition of calcium, mostly hydroxyapatite crystals, within the tendons. The most affected regions are, in order, the shoulder and the hip [1], but it can virtually involve every tendon; the involvement of the ankle, in particular of the Achilles tendon, is only anecdotal in literature. The pathogenesis of the calcific tendinopathy is still not completely clear, but it seems to be related to the fibrocartilaginous metaplasia secondary to hypoxia, which leads to the formation of calcium deposits, typically in healthy tendons [2]. At any rate, the calcific tendinopathy is a dynamic process that progresses through different phases, characterized by specific imaging, pathological, and clinical features. Uhthoff et al. [2] described four stages: pre-calcific, typically asymptomatic, in which fibrocartilaginous transformation occurs within tendon fibers (stage 1); formative-calcific, usually poorly symptomatic, in which calcifications are formed (stage 2); resorptive, often very painful, in which calcium deposits are usually removed by phagocytes thanks to an increased vasculature of the tendon in an inflammatory context (stage 3); and post-calcific, in which fibroblasts restore the normal tendon collagen pattern in a self-healing repair, a process that may be associated with pain and restricted function (stage 4). Calcific tendinopathy can be easily diagnosed with imaging studies as conventional radiography (CR) or ultrasound (US) [3, 4]; MRI, for the low amount of resonating protons contained in calcific deposits, usually leads to poor visibility of the calcification itself [5] .
Calcific tendinopathy is usually a self-limiting condition, with conservative treatment based on anti-inflammatory drugs, painkillers, and infiltrative therapy often representing the first-line approach [1, 6]. If symptoms persist, other therapeutic options are available, essentially referring to the shoulder, but no standard of care has been yet established. Calcific tendinopathy management includes surgery [7], shock wave therapy [8], and image-guided procedures [9,10,11,12]. If compared to other non-surgical procedures, US-guided percutaneous irrigation (US-PICT) has shown to reduce the risk of adverse events [13]. Nevertheless, the clinical value of this treatment on extra-shoulder calcific tendinopathy still needs to be demonstrated, having been scarcely reported for Achilles calcific tendinopathy [14].
In this paper, we report an unusual case of non-insertional Achilles calcific tendinopathy which occurred many years after Achilles surgical repair and was successfully treated by US-PICT.
Case report
A 47-year-old male patient was referred to our Institution, reporting a 5-month history of slightly painful swelling on the posterior side of the right ankle. Pain had led to partial limitations during daily activities, especially limiting jogging with moderate pain also during walking. He had neither history of recent trauma nor metabolic and systemic disorders: the only relevant medical history was that he had undergone a surgical repair of the Achilles tendon for a complete preinsertional rupture 20 years earlier. At physical examination, the patient reported local pain and swelling over the posterior ankle with intact soft tissues and without skin changes. Then, CR and US were performed. Standard ankle CR (Fig. 1) revealed a large soft tissue opacity in the posterior side of the ankle, about 3 cm proximal to the calcaneum, suggesting a calcification within the mid-portion of the Achilles tendon; swelling of the neighboring soft tissues was associated. There were also some linear opacities deeper than the calcification consistent with small linear intratendinous calcifications. The US examination (Fig. 2) confirmed the presence of a calcific deposit within the preinsertional portion of the Achilles tendon. The calcification was soft in appearance, homogeneously hyperechoic, almost isoechoic to the tendon, without posterior acoustic shadow. Pericalcific hypervascularization was observed at Power-Doppler evaluation, and sonoelastography confirmed the soft content of the calcification. The whole tendon was also thickened and hypoechoic due to long-lasting post-surgical changes. Given these clinical and imaging features, the radiologist recommended an orthopedic examination and suggested the US-PICT as a treatment option. The orthopedic surgeon, in turn, proposed the surgical treatment that the patient refused. Therefore, the US-PICT was done by the same musculoskeletal radiologist who performed the US examination. After US-guided injection of local anesthetic (10 ml of 2% lidocaine chlorhydrate, S.A.L.F., Italy) into the skin/subcutaneous tissue around the calcification, one 16-gauge needle was inserted within the calcification under continuous US monitoring with craniocaudal and in-plane approach. The calcification was soft; thereby, most of the calcification content got out of the needle under the pressure of the transducer immediately after needle insertion, with no need of aspiration. The remaining calcium content was irrigated using a 10-mL syringe of saline (NaCl 0.9%) heated to 42 °C (107 °F) with few cycles of pushing and releasing of the syringe plunger (Figs. 3 and 4). After the procedure, an ice pack and a compressive bandage were applied on the Achilles tendon. No complications occurred. For the first 7 days, an elastic wrap was used to reduce the swelling and the patient was advised to avoid running, although weight bearing and walking were allowed. The patient was also advised to take oral painkillers if needed, but it was not necessary.
Lateral ankle CR showing a large calcification (arrows) along the Achilles tendon, about 3 cm proximal to the calcaneum. Swelling of the neighboring soft tissues was associated. Note also some linear opacities deeper than the calcification consistent with small intratendinous calcifications (headarrows)
Long-axis US confirmed the presence of a soft calcification of 20 mm (a, b, c, d; arrows). Note also the small intratendinous calcifications (headarrows). Power-Doppler image (c) showed the pericalcific hypervascularization. In the longitudinal strain sonoelastography (d), the calcification (arrows) appeared red to green (asterisk) indicating soft tissue, while the deeper small calcification fragment (headarrow) appeared blue, which represents stiff tissue
US-PICT procedure. Long-axis US images (a, b) used as a guidance with a 16-gauge needle (headarrows) approaching (a) and then inserted (b) within the calcification (asterisk) with craniocaudal approach. Most of the calcification content got out of the needle under the pressure of the transducer without the need to be aspirated (c; curved arrow). Note also the swelling over the posterior ankle due to the calcification leading to a skin bulging (d; arrows)
The patient reported a progressive swelling and pain relief, until complete resolution about two months later, when he completely got back to his normal daily activities. Follow-up imaging examinations at two months, including both CR (Fig. 5) and US (Fig. 6), showed size reduction and fragmentation of the calcification, with thin residual calcific thin walls, complete resorption of the calcific content, remarkable reduction of soft tissue swelling close to the tendon, and disappearance of pericalcific hypervascularization at Power-Doppler evaluation. The US examination showed the integrity of the tendon.
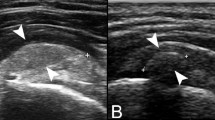
Long-axis US two months after treatment showed reduction of the calcification presenting thin residual calcific walls and complete resorption of the calcific core (a, b; arrows), with the small linear intratendinous calcifications being still present (b; headarrows). Power-Doppler image (c) showed the disappearance of pericalcific hypervascularization
Discussion
Extra-shoulder calcific tendinopathy is relatively infrequent and typically affects the hip. While the diagnostic techniques and the treatment options for shoulder calcific tendinopathy have been extensively described, there are only anecdotic reports on the other sites. Draghi et al. recently published a systematic review of the literature to highlight the localizations of calcific tendinopathy beyond the rotator cuff, its ultrasound characteristics, and the therapeutic options [15].
In particular, non-insertional calcific tendinopathy of the Achilles tendon is very rare and represents a different clinical and pathological entity from the more common insertional calcific tendinopathy. Kurtoğlu et al. reported it as an early manifestation of primary hyperparathyroidism due to a parathyroid adenoma in a young patient [16]; however, it was not a real calcific tendinopathy but only calcium deposition within Achilles tendon related to hypercalcemia, which spontaneously resolved after parathyroidectomy. Indeed, the association of calcific tendinopathy with hyperparathyroidism has not been demonstrated yet, since the pathophysiology and imaging features of calcium deposits within the tendons of patients with hyperparathyroidism is quite different. In 2014, Nazi et al. [14] evaluated 10 patients with calcific tendinopathy treated with US-PICT, two of whom with calcific tendinopathy of the Achilles tendon, with excellent clinical and imaging results. However, the authors did not precisely describe the imaging features of Achilles calcific tendinopathy. Insertional calcific tendinopathy is characterized by the development of calcific spurs—mostly traction enthesophytes at the calcaneal insertion of the tendon, typically in active people [17]. The histopathologic process, that is the ossification of the enthesial fibrocartilage [18], could represent an adaptive structural change to a repeated tensional stress [19]. Unlike non-insertional tendinopathy, which typically occurs in healthy tendons as in the rotator cuff, the Achilles tendon may in this case show tears, especially at the bone-tendon junction [18].
In this paper, we have reported an unusual case of non-insertional calcific tendinopathy of the Achilles tendon occurred many years after surgical repair of the tendon itself. Nevertheless, we do not know how this factor may have influenced its development. Indeed, several cases of mineralization developed as complication of Achilles tendon surgical repair have been described [20,21,22]: in all cases, however, it was more about ossification than calcification, as typically happening in the post-surgical context. As described by O’Brien E.J et al. [23], in fact, heterotopic mineralization within the tendons may be due to calcification or ossification: ossification is characterized by the formation of bone, more frequently through an enchondral process, while the calcium deposition in the calcification is secondary to the over-described cell-mediated response [2]. This difference has a diagnostic counterpart too, in particular in US imaging: ossification appears as a marked hyperechogenicity with lack of through-transmission [24], whereas calcifications are typically amorphous, without a strong posterior acoustic shadowing. Notably, in our patient, the calcific tendinopathy, presenting as a soft calcification, co-existed with small linear intratendinous calcifications. This could mean that the large superficial calcification might result from a migration of the adjacent tendon calcification, also explaining the link between the two types of calcification facing each other. Such migrations are quite frequently encountered in clinical practice, particularly at the shoulder where, during migration, calcifications are extruded from the tendon into the subacromial bursa more frequently [25]. This generally occurs in the resorptive phase and is associated with pain and fragmentation of the calcification itself.
Diagnostic techniques for calcific tendinopathy in the ankle are not different from those in other locations, since US and CR are sufficient and satisfactory to make the diagnosis [26]. As mentioned earlier, for the treatment of calcific tendinopathy in general, there is no codified standard of care. However, US-PICT is currently accepted as the first-line therapy among interventional treatments in rotator cuff calcific tendinopathy, due to its high efficacy and low complication rate [27,28,29,30,31].
In conclusion, we have reported an unusual case of non-insertional Achilles calcific tendinopathy. This clinical entity, that is quite common in the shoulder rotator cuff tendons, has been scarcely described in the Achilles tendon. Indeed, this condition is totally different from the well-known insertional calcific Achilles tendinopathy in terms of pathophysiological, imaging, and clinical findings. Further, we have shown that US-guided percutaneous irrigation might be a safe, technically feasible, mini-invasive, and effective treatment for Achilles calcific tendinopathy.
References
Hayes CW, Conway WF. Calcium hydroxyapatite deposition disease. Radiographics. 1990;10:1031–48.
Uhthoff HK, Sarkar K. Calcifying tendinitis. Baillieres Clin Rheumatol. 1989;3:567–81.
Chianca V, Albano D, Messina C, et al. Rotator cuff calcific tendinopathy: from diagnosis to treatment. Acta Biomed. 2018;89:186–96.
Sconfienza LM, Albano D, Allen G, et al. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur Radiol. 2018;28:5338–51.
Filippou G, Adinolfi A, Cimmino MA, et al. Diagnostic accuracy of ultrasound, conventional radiography and synovial fluid analysis in the diagnosis of calcium pyrophosphate dihydrate crystal deposition disease. Clin Exp Rheumatol. 2016;34:254–60.
Greis AC, Derrington SM, McAuliffe M. Evaluation and nonsurgical management of rotator cuff calcific tendinopathy. Orthop Clin North Am. 2015;46:293–302.
Yoo JC, Park WH, Koh KH, Kim SM. Arthroscopic treatment of chronic calcific tendinitis with complete removal and rotator cuff tendon repair. Knee Surg Sports Traumatol Arthrosc. 2010;18:1694–9.
Cacchio A, Paoloni M, Barile A, et al. Effectiveness of radial shock-wave therapy for calcific tendinitis of the shoulder: single-blind, randomized clinical study. Phys Ther. 2006;86:672–82.
Serafini G, Sconfienza LM, Lacelli F, Silvestri E, Aliprandi A, Sardanelli F. Rotator cuff calcific tendonitis: short-term and 10-year outcomes after two-needle us-guided percutaneous treatment--nonrandomized controlled trial. Radiology. 2009;252:157–64.
Farin PU, Jaroma H, Soimakallio S. Rotator cuff calcifications: treatment with US-guided technique. Radiology. 1995;195:841–3.
Sconfienza LM, Chianca V, Messina C, Albano D, Pozzi G, Bazzocchi A. Upper limb interventions. Radiol Clin N Am. 2019;57:1073–82.
Chianca V, Orlandi D, Messina C, et al. Interventional therapeutic procedures to treat degenerative and inflammatory musculoskeletal conditions: state of the art. Radiol Med. 2019;124:1112–20.
Arirachakaran A, Boonard M, Yamaphai S, Prommahachai A, Kesprayura S, Kongtharvonskul J. Extracorporeal shock wave therapy, ultrasound-guided percutaneous lavage, corticosteroid injection and combined treatment for the treatment of rotator cuff calcific tendinopathy: a network meta-analysis of RCTs. Eur J Orthop Surg Traumatol. 2017;27:381–90.
Niazi G, Hetta W. The role of ultrasound guided percutaneous needle aspiration and lavage (barbotage) in the treatment of calcific tendinitis. Egypt J Radiol Nucl Med. 2015;46:63–70.
Draghi F, Cocco G, Lomoro P, Bortolotto C, Schiavone C. Non-rotator cuff calcific tendinopathy: ultrasonographic diagnosis and treatment. J Ultrasound. 2019. https://doi.org/10.1007/s40477-019-00393-2.
Kurtoğlu S, Akın L, Kendirci M, Çağlı S, Özgöçmen S. An unusual presentation of parathyroid adenoma in an adolescent: calcific Achilles tendinitis. J Clin Res Pediatr Endocrinol. 2015;7:333–5.
Irwin TA. Current concepts review: insertional Achilles tendinopathy. Foot Ankle Int. 2010;31:933–9.
van Dijk CN, van Sterkenburg MN, Wiegerinck JI, Karlsson J, Maffulli N. Terminology for Achilles tendon related disorders. Knee Surg Sports Traumatol Arthrosc. 2011;19:835–41.
Benjamin M, Rufai A, Ralphs JR. The mechanism of formation of bony spurs (enthesophytes) in the Achilles tendon. Arthritis Rheum. 2000;43:576–83.
Ateschrang A, Gratzer C, Weise K. Incidence and effect of calcifications after open-augmented Achilles tendon repair. Arch Orthop Trauma Surg. 2008;128:1087–92.
Kraus R, Stahl JP, Meyer C, et al. Frequency and effects of intratendinous and peritendinous calcifications after open Achilles tendon repair. Foot Ankle Int. 2004;25:827–32.
Leppilahti J, Forsman K, Puranen J, Orava S. Outcome and prognostic factors of Achilles rupture repair using a new scoring method. Clin Orthop Relat Res. 1998;346:152–61.
O'Brien EJ, Frank CB, Shrive NG, Hallgrimsson B, Hart DA. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int J Exp Pathol. 2012;93:319–31.
Chun KA, Cho KH. Postoperative ultrasonography of the musculoskeletal system. Ultrasonography. 2015;34:195–205.
Della Valle V, Basi EM, Calliada F. Migration of calcium deposits into subacromial–subdeltoid bursa and into humeral head as a rare complication of calcifying tendinitis: sonography and imaging. J Ultrasound. 2015;18:259–63.
Harries L, Kempson S, Watura R. Calcific tendonitis of the tibialis posterior tendon at the navicular attachment. J Radiol Case Rep. 2011;5:25–30.
de Witte PB, Selten JW, Navas A, et al. Calcific tendinitis of the rotator cuff: a randomized controlled trial of ultrasound-guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41:1665–73.
Pasquotti G, Faccinetto A, Marchioro U, et al. US-guided percutaneous treatment and physical therapy in rotator cuff calcific tendinopathy of the shoulder: outcome at 3 and 12 months. Eur Radiol. 2016;26:2819–27.
Sconfienza LM, Randelli F, Sdao S, Sardanelli F, Randelli P. Septic bursitis after ultrasound-guided percutaneous treatment of rotator cuff calcific tendinopathy. Pm r. 2014;6:746–8.
Sconfienza LM, Adriaensen M, Albano D, et al. Clinical indications for image-guided interventional procedures in the musculoskeletal system: a Delphi-based consensus paper from the European Society of Musculoskeletal Radiology (ESSR)—part I, shoulder. Eur Radiol. 2020;30:903–13.
Silvestri E, Barile A, Albano D, et al. Interventional therapeutic procedures in the musculoskeletal system: an Italian survey by the Italian College of Musculoskeletal Radiology. Radiol Med. 2018;123:314–21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by our Institutional Review Board. The patient provided written informed consent. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Albano, D., Vicentin, I., Messina, C. et al. Post-surgical Achilles calcific tendinopathy treated with ultrasound-guided percutaneous irrigation. Skeletal Radiol 49, 1475–1480 (2020). https://doi.org/10.1007/s00256-020-03453-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03453-5