Abstract
Objective
The purpose of this study was to determine whether SWE can detect biomechanical changes in the supraspinatus muscle that occur with increasing supraspinatus tendon abnormality prior to morphologic gray-scale changes.
Materials and methods
An IRB approved, HIPAA compliant retrospective study of shoulder ultrasounds from 2013–2018 was performed. The cohort consisted of 88 patients (mean age 55 ± 15 years old) with 110 ultrasounds. Images were acquired in longitudinal orientation to the supraspinatus muscle with shear wave velocity (SWV) point quantification. The tendon and muscle were graded in order of increasing tendinosis/tear (1–4 scale) and increasing fatty infiltration (0–3 scale). Mixed model analysis of variance, analysis of covariance, and Spearman rank correlation were used for statistical analysis.
Results
There was no statistically significant age or sex dependence for supraspinatus muscle SWV (p = 0.314, 0.118, respectively). There was no significant correlation between muscle SWV and muscle or tendon grade (p = 0.317, 0.691, respectively). In patients with morphologically normal muscle on gray-scale ultrasound, there were significant differences in muscle SWV when comparing tendon grade 3 with grades 1, 2, and 4 (p = 0.018, 0.025, 0.014, respectively), even when adjusting for gender and age (p = 0.044, 0.028, 0.018, respectively). Pairwise comparison of tendon grades other than those mentioned did not achieve statistical significance (p > 0.05).
Conclusion
SWE can detect biomechanical differences within the supraspinatus muscle that are not morphologically evident on gray-scale ultrasound. Specifically, supraspinatus tendon partial tears with moderate to severe tendinosis may correspond to biomechanically distinct muscle properties compared to both lower grades of tendon abnormality and full-thickness tears.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well-established that rotator cuff muscle fatty infiltration correlates with poor functional outcomes after rotator cuff repair [1,2,3]. Semiquantitative evaluation of fatty infiltration using Goutallier grading is already for most musculoskeletal radiologists a requisite part of the preoperative interpretation. While this is typically evaluated on magnetic resonance imaging (MRI), prior studies have demonstrated good correlation and comparable diagnostic performance between ultrasound (US) and MRI for the evaluation of fatty infiltration and atrophy of the supraspinatus muscle, in addition to being comparable for assessing rotator cuff integrity [4,5,6,7].
Recent application of ultrasound shear wave elastography (SWE), a quantitative method of assessing tissue biomechanical properties, has shown promise in a variety of musculoskeletal applications. In patients with skeletal myopathy, resulting in fatty infiltration of muscle, SWE has shown decreased muscle shear wave velocity (SWV) when compared to healthy controls [8]. In the setting of rotator cuff disease, SWE has demonstrated tendon softening with increasing rotator cuff tendinopathy and a general trend toward decreased muscle SWV with increasing Goutallier grades, except for Goutallier grade 4 [9, 10].
The purpose of this study was to determine whether SWE can detect biomechanical changes in the supraspinatus muscle that precede morphologic gray-scale changes, as rotator cuff tendon abnormality increases. A secondary aim was to determine whether a correlation exists between SWV and gray-scale indices of tendon and muscle quality. We hypothesized that supraspinatus muscle SWV would decrease with increasing tendon abnormality and muscle fatty infiltration.
Materials and methods
Study design
This study was approved by the NYU School of Medicine Institutional Review Board, and a waiver of consent was obtained. A HIPAA-compliant retrospective study was performed, which included retrospective review of consecutive clinical shoulder ultrasound examinations performed by a single radiologist (R.S.A.) from 2013 to 2018. These examinations were obtained through a Radiology Information System search for all patients who had undergone shoulder ultrasound and who had shear wave elastography performed during the examination, yielding 144 shoulder ultrasound examinations. Thirty-four examinations were excluded from the study for poor image quality based on the elastogram and quality maps, such as poor penetration or artifact leading to image dropout of parametric shear wave data. Six patients were excluded from tendon grading due to prior rotator cuff repair but included for muscle grading. The patient cohort included 88 patients (mean age 55 ± 15 years old) with 110 shoulder examinations, as 22 patients had bilateral examinations (Table 1). For 73% (80/110) of the examinations, the clinical indication was for rotator cuff pathology, either evaluating for rotator cuff tendinosis or rotator cuff tear.
Gray-scale image acquisition
Gray-scale and shear wave images were acquired by a single radiologist with 26 years of musculoskeletal ultrasound experience (R.S.A.) on a Siemens Acuson S3000 equipped with Virtual Touch™ IQ software. Gray-scale imaging of the rotator cuff was performed using a 9 MHz linear transducer on all patients and additionally with a 14 MHz linear transducer if patient body habitus permitted. Supraspinatus and infraspinatus tendons were evaluated in standard Crass or modified Crass positions, and muscle assessment was performed with the arm resting on the lap in cross-adduction.
Shear wave acquisition
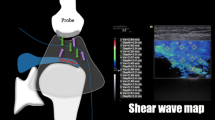
Virtual Touch™ IQ software allows derivation of parametric images from radiofrequency data following an acoustic radiation force impulse (ARFI), as well as subsequent quality factor analysis. Shear wave images were obtained using light compression and a 9 MHz linear transducer in longitudinal orientation to the supraspinatus muscle (Figure 1). Imaging in this plane correlates strongly with muscle biomechanical properties and is less sensitive to the inherent viscoelastic nature of the soft tissue [11, 12]. Likewise, transverse imaging has been shown to result in significantly higher variance in estimated shear wave velocity relative to longitudinal estimates [13]. When scanning over the muscle with the shear wave software turned on, a shear wave parametric image is generated in a preselected rectangular region-of-interest (ROI) (Fig. 1). Shear wave parametric images were evaluated with placement of at least three 3-mm sample volumes in the region of interest (ROI), and at least two separate acquisitions were obtained of the muscle, which were then averaged for analysis. Sample volumes were placed away from the myotendinous junction within each ROI where shear waves were considered of adequate quality, based on homogeneity of the shear wave velocity map and quality map (Fig. 2). Images that did not contain a portion of the ROI meeting these criteria were rejected as being unacceptable. Shear wave velocity was measured in meters per second (m/s). Initial color maps were selected to confirm that the SWV were within the range of detectable velocities, previously determined to be 0.5–15 m/sec. All SWVs fell within this range.
(a) Photograph showing example scanning position and plane for shear wave acquisition. Note the longitudinal orientation of the transducer relative to the supraspinatus muscle. (b) Schematic illustration showing that with the shear wave software on, a shear wave parametric image is generated by the software in the preselected region of interest (blue rectangle within the supraspinatus muscle) over the area of scanning
(a) Example online shear wave acquisition of the supraspinatus muscle in long axis with a region of interest (yellow box), shear wave elastography velocity map (blue-green color box), and several sample volumes taken over the region of interest (yellow circles with hash marks). The shear wave velocity values corresponding to the sample volumes are shown in the lower right hand corner with a symbol key (white text with white arrow). (b) Example shear wave acquisition (off-line analysis) of the supraspinatus muscle in long axis. The 3 images (from left to right) show a grayscale image with a red circle denoting site of point quantification, the shear wave elastography map with a black circle denoting the same point quantification, and a quality map with the same black circle denoting point quantification. Note that the green areas in the quality map are areas with homogeneous shear wave velocity data on the shear wave map (blue areas). The red/yellow areas in the quality map correspond to areas of inhomogeneous shear wave velocity on the shear wave elastography map. Along the bottom left corner of the quality map, the data is considered by the software to be of such low quality as to cause data dropout on the shear wave map
Tendon and muscle grade
Tendon and muscle grading on gray-scale imaging was performed in consensus with two readers (R.S.A., 26 years musculoskeletal ultrasound experience; D.J.L., 1.5 years musculoskeletal ultrasound experience). The two readers each independently reviewed a random subset consisting of 25 examinations for muscle and tendon grading and found that the kappa values for muscle and tendon grading between the two investigators were substantial for both: 0.69 (95% CI: 0.53–0.84) and 0.63 (95% CI: 0.44–0.82), respectively.
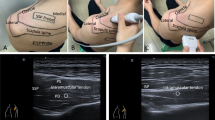
Morphologic tendon grading was classified as follows based on the prior grading system described by Hou et al [9]: grade 1 = normal tendon or mild tendinosis without tear; grade 2 = mild tendinosis with low-grade partial tear or moderate to severe tendinosis without tear; grade 3 = partial tear (< or > 50% tendon thickness) with superimposed moderate to severe tendinosis; and grade 4 = full-thickness tear (Fig. 3). As mentioned above, six patients were excluded from tendon grading due to prior rotator cuff repair.
Supraspinatus tendon grading examples. (a) Grade 1 tendons were normal tendons or those with mild tendinosis without tear. Specifically, these tendons had little to no inhomogeneity or thickening and no fluid clefts to suggest presence of a tear. (b) Grade 2 tendons were those with mild tendinosis with low-grade partial tear or moderate to severe tendinosis without tear. Specifically, these were tendons with mild tendon inhomogeneity with a partial fluid cleft involving less than 50% tendon thickness or tendons with moderate or severe inhomogeneity and tendon thickening without a fluid cleft to suggest presence of a tear. (c) Grade 3 tendons were those with a partial tear with superimposed moderate to severe tendinosis. Specifically, these were tendons that had at least moderate inhomogeneity and thickening with a partial-thickness ( < or > 50% tendon thickness) fluid cleft. (d) Grade 4 tendons were those with full-thickness tears
Morphologic muscle grading was classified as follows based on the prior grading system described by Meng et al [14]: grade 0 = muscle hypoechoic to perimyseal fat (normal); grade 1 = areas of mild increased echogenicity relative to normal background muscle; grade 2 = nearly as echogenic as perimyseal fat; and grade 3 = isoechoic to perimyseal fat (Fig. 4). Of the 69 shoulder examinations with morphologically normal muscle on ultrasound, 16 of these had available corresponding MRI examinations within 6 months. These MRI examinations were reviewed in consensus by two musculoskeletal radiologists, and each muscle was confirmed as Goutallier grades 0–1 on MRI.
Supraspinatus muscle grading examples. (a) grade 0 = muscle hypoechoic to perimyseal fat (normal) with thin white arrow denoting the supraspinatus muscle; (b) grade 1 = areas of mild increased echogenicity (thick short white arrows) relative to normal background muscle; (c) grade 2 = nearly as echogenic as perimyseal fat (thick short white arrows denoting more confluent areas of increased echogenicity); (d) grade 3 = isoechoic to perimyseal fat (thin white arrows denoting the outer margin of the supraspinatus muscle, which is diffusely echogenic with volume loss). For all image parts, “S” denotes the scapula
The tendon/muscle grading distribution is shown in Tables 2, 3, 4.
Statistical analysis
Data from 110 examinations provides 80% power at the two-sided 5% significance level to detect correlations of magnitude 0.26, 90% power to detect correlations of magnitude 0.30, and 95% power to detect correlations of magnitude 0.33. This implies that the study had high ( > 95%) power to detect even relatively small correlations so that failure to detect correlations as significant is probably not a mere consequence of inadequate statistical power.
Mixed model analysis of variance (ANOVA) and analysis of covariance (ANCOVA) were used to compare tendon grades and compare muscle grades with respect to shear wave velocity without and with adjustment for the potential confounding effects of age and gender, respectively.
As part of each mixed model analysis, the grades were pairwise compared, and p values for the comparisons are provided with and without a Tukey-Kramer multiple comparison correction. An unequal variance t-test was used to compare males and females in terms of the shear wave velocity measurements represented for each subject as an average over replicate measurements.
Due to the ordinal nature of tendon and muscle grade, the Spearman rank correlation was used to assess the association of the subject-level average velocity with muscle grade, tendon grade, and age. All statistical analyses were conducted using SAS 9.3 software (SAS Institute, Cary, NC) with statistical significance defined as p < 0.05.
Results
There was no statistically significant age or sex dependence (age, r = −0.097, p = 0.314; sex, p = 0.118) for supraspinatus muscle SWV for all patients as well as when controlling for muscle and tendon status. There was no statistically significant correlation between muscle SWV and muscle grade (r = −0.096, p = 0.317) or muscle SWV and tendon grade (r = −0.039, p = 0.691, Fig. 5).
In patients with morphologically normal muscle on gray-scale ultrasound, there was a statistically significant difference in muscle SWV between tendon subgroups. Specifically, there were statistically significant differences when comparing tendon grade 3 with grades 1, 2, and 4 (p = 0.018, 0.025, 0.014, respectively; Fig. 6), even when adjusting for gender and age (p = 0.044, 0.028, 0.018, respectively). Pairwise comparison of tendon grades other than those mentioned did not achieve statistical significance (p > 0.05); however, there was trend behavior toward a positive correlation across tendon grades 1–3 (Table 5).
Discussion
To our knowledge, this is the first study in the literature to examine whether SWE can detect biomechanical changes in the supraspinatus muscle that precede morphologic gray-scale changes as supraspinatus tendon abnormality increases. We have demonstrated a statistically significant difference in muscle SWV between tendon subgroups and specifically that there are statistically significant differences in muscle SWV between tendon grade 3, which is defined as partial tears with superimposed moderate to severe tendinosis, and other tendon subgroups.
Several recent biochemical and biomechanical studies from the literature may provide a potential explanation for why patients with a partial tear and moderate to severe tendinosis may exhibit increased SWV compared to less severe degrees of tendon abnormality and compared to full-thickness tears. Supraspinatus muscle passive tension and fatty degeneration of the musculature are correlated with tear size [15, 16]. Furthermore, Silldorff et al. found a correlation between the increases in supraspinatus muscle passive tension and increases in collagen content in the extracellular matrix.
A more recent study looking at the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after massive rotator cuff tears in a mouse model found that different types of progenitor cells give rise to fibroblast and adipocyte production to cause fibrosis and fatty infiltration, respectively [17]. This study did not evaluate the relative timing of these two distinct though concomitant processes, however, although it is currently postulated that it is the fibrosis component that results in higher muscle tension rendering rotator cuff repairs more challenging. Fibrosis is also correlated with decreased force production and myofibril architectural distortion [18,19,20].
It is possible that the significantly increased muscle SWV in patients with partial tears and moderate to severe tendinosis as compared to tendon grades above and below grade 3 reflects disproportionate fibrosis relative to the degree of fatty infiltration. Prior animal and human studies have shown increased muscle fibrosis in the setting of rotator cuff tears [21,22,23]. Currently, MRI primarily evaluates the fat fraction rather than fibrosis component of rotator cuff muscle degeneration, while ultrasound may evaluate the contribution of both processes to backscatter. SWE may be able to detect biomechanical changes in the muscle due to fibrosis not readily assessed visually on morphologic imaging.
The lack of statistically significant correlation between muscle SWV and tendon subgroups is consistent with a prior study by Rosskopf et al. that examined not only patients with morphologically normal muscle but also included patients with various grades of fatty infiltration on MRI. There were several other differences between the prior study by Rosskopf et al. and the current study, including differences in probe orientation, the use of ultrasound versus MRI as the modality for grading muscle fatty infiltration, and differing distributions of patients with normal versus abnormal muscle with far fewer patients with severe degrees of fatty infiltration in the current study, as the primary aim was to examine biomechanical differences in morphologically normal muscle.
With respect to probe orientation, there is currently no consensus on whether muscles should be evaluated in transverse or longitudinal orientation. However, studies have shown that shear waves do not propagate well in perpendicular orientation to skeletal muscle and that elasticity strongly increases along the muscle fibers rather than in perpendicular orientation [11, 12]. Secondly, our preliminary work looking at both orientations indicates greater SWV dispersion in the transverse plane, as well as generally higher velocity magnitudes longitudinally, thereby providing a larger dynamic range for velocity assessment [13]. For these reasons, we have elected to scan in longitudinal orientation to the muscle.
There were several limitations to this study. The sample size for abnormal muscle was smaller, which may have affected our ability to detect significant correlations with increasing tendon abnormality. The grading systems for both muscle and tendon quality were subjective, rather than quantitative. In terms of data acquisition, there was also a significant learning curve in optimizing acquisition of SWV data. We learned through the course of the study that fascial boundaries and abrupt tissue interfaces give rise to large changes in SWV likely secondary to abrupt changes in acoustic impedance, which results in significant artifact (Fig. 7). There may in addition be degradation of the ARFI pulse below the tissue boundary. We now acquire data from portions of the muscle that are away from the myotendinous junction when possible in order to obtain more consistent results.
In conclusion, SWE can detect biomechanical differences within the supraspinatus muscle that are not morphologically evident on gray-scale ultrasound. Specifically, supraspinatus partial tears with moderate to severe tendinosis may correspond to biomechanically distinct muscle properties compared to both lower grades of tendon abnormality and full-thickness tears. SWE may be a novel tool to quantitatively evaluate muscle quality in the setting of rotator cuff disease. Further research is needed to determine whether this may aid prognosis for repair outcomes.
Abbreviations
- US:
-
Ultrasound
- MRI:
-
Magnetic resonance imaging
- SWE:
-
Shear wave elastography
- SWV:
-
Shear wave velocity
- ARFI:
-
Acoustic radiation force impulse
- ROI:
-
Region of interest
References
Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–28.
Goutallier D, Postel JM, Lavau L, Bernageau J. Influence of muscular degeneration of the supra- and infraspinatus on the prognosis of surgical repair of the rotator cuff. Acta Orthop Belg. 1998;64(Suppl 2):42–5.
Mellado JM, Calmet J, Olona M, Esteve C, Camins A, Perez Del Palomar L, et al. Surgically repaired massive rotator cuff tears: MRI of tendon integrity, muscle fatty degeneration, and muscle atrophy correlated with intraoperative and clinical findings. AJR Am J Roentgenol. 2005;184(5):1456–63.
Strobel K, Hodler J, Meyer DC, Pfirrmann CW, Pirkl C, Zanetti M. Fatty atrophy of supraspinatus and infraspinatus muscles: accuracy of US. Radiology. 2005;237(2):584–9.
Khoury V, Cardinal E, Brassard P. Atrophy and fatty infiltration of the supraspinatus muscle: sonography versus MRI. AJR Am J Roentgenol. 2008;190(4):1105–11.
Wall LB, Teefey SA, Middleton WD, Dahiya N, Steger-May K, Kim HM, et al. Diagnostic performance and reliability of ultrasonography for fatty degeneration of the rotator cuff muscles. J Bone Joint Surg Am. 2012;94(12):e83.
de Jesus JO, Parker L, Frangos AJ, Nazarian LN. Accuracy of MRI, MR arthrography, and ultrasound in the diagnosis of rotator cuff tears: a meta-analysis. AJR Am J Roentgenol. 2009;192(6):1701–7.
Carpenter EL, Lau HA, Kolodny EH, Adler RS. Skeletal muscle in healthy subjects versus those with GNE-related myopathy: evaluation with shear-wave US--a pilot study. Radiology. 2015;277(2):546–54.
Hou SW, Merkle AN, Babb JS, McCabe R, Gyftopoulos S, Adler RS. Shear wave ultrasound elastographic evaluation of the rotator cuff tendon. J Ultrasound Med. 2017;36(1):95–106.
Rosskopf AB, Ehrmann C, Buck FM, Gerber C, Fluck M, Pfirrmann CW. Quantitative shear-wave US elastography of the supraspinatus muscle: reliability of the method and relation to tendon integrity and muscle quality. Radiology. 2016;278(2):465–74.
Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An KN. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013;46(14):2381–7.
Gennisson JL, Deffieux T, Mace E, Montaldo G, Fink M, Tanter M. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med Biol. 2010;36(5):789–801.
Merkle A AB, Lin D, Babb J, Adler R. Shear wave ultrasound evaluation of the supraspinatus muscle: anisotropy and age considerations. Radiological Society of North America 2017 Scientific Assembly and Annual Meeting, November 26 - December 1, 2017. Chicago IL.
Meng C, Adler R, Peterson M, Kagen L. Combined use of power Doppler and gray-scale sonography: a new technique for the assessment of inflammatory myopathy. J Rheumatol. 2001;28(6):1271–82.
Silldorff MD, Choo AD, Choi AJ, Lin E, Carr JA, Lieber RL, et al. Effect of supraspinatus tendon injury on supraspinatus and infraspinatus muscle passive tension and associated biochemistry. J Bone Joint Surg Am. 2014;96(20):e175.
Kim HM, Dahiya N, Teefey SA, Keener JD, Galatz LM, Yamaguchi K. Relationship of tear size and location to fatty degeneration of the rotator cuff. J Bone Joint Surg Am. 2010;92(4):829–39.
Liu X, Ning AY, Chang NC, Kim H, Nissenson R, Wang L, et al. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J. 2016;6(1):6–15.
Davis ME, Korn MA, Gumucio JP, Harning JA, Saripalli AL, Bedi A, et al. Simvastatin reduces fibrosis and protects against muscle weakness after massive rotator cuff tear. J Shoulder Elb Surg. 2015;24(2):280–7.
Choo A, McCarthy M, Pichika R, Sato EJ, Lieber RL, Schenk S, et al. Muscle gene expression patterns in human rotator cuff pathology. J Bone Joint Surg Am. 2014;96(18):1558–65.
Sato EJ, Killian ML, Choi AJ, Lin E, Esparza MC, Galatz LM, et al. Skeletal muscle fibrosis and stiffness increase after rotator cuff tendon injury and neuromuscular compromise in a rat model. J Orthop Res. 2014;32(9):1111–6.
Laron D, Samagh SP, Liu X, Kim HT, Feeley BT. Muscle degeneration in rotator cuff tears. J Shoulder Elb Surg. 2012;21(2):164–74.
Barton ER, Gimbel JA, Williams GR, Soslowsky LJ. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res. 2005;23(2):259–65.
Gigliotti D, Xu MC, Davidson MJ, Macdonald PB, Leiter JRS, Anderson JE. Fibrosis, low vascularity, and fewer slow fibers after rotator-cuff injury. Muscle Nerve. 2017;55(5):715–26.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, D.J., Burke, C.J., Abiri, B. et al. Supraspinatus muscle shear wave elastography (SWE): detection of biomechanical differences with varying tendon quality prior to gray-scale morphologic changes. Skeletal Radiol 49, 731–738 (2020). https://doi.org/10.1007/s00256-019-03334-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-03334-6