Abstract
Objective
To assess the added value of serial 2-deoxy-2-[18F]fluoro-D-glucose (FDG) uptake analysis in predicting clinical response to treatment in infectious spondylodiscitis (IS). We sought to analyze changes in quantitative FDG-PET/CT parameters among patients with clinical response or treatment failure and to compare the sensitivity and specificity of serial FDG-PET/CT and MRI in predicting treatment response in IS.
Materials and methods
This retrospective study consisted of 68 FDG-PET/CT examinations in 34 patients performed before and after at least 2 weeks of antibiotic treatment. Serial MRI scans were available in 32 (94%) patients before and after treatment. FDG-avid lesions were quantified as maximum standardized uptake value (SUVmax), partial-volume corrected lesion metabolic volume (LMV), and partial-volume corrected lesion metabolic activity (LMA).
Results
All FDG-PET/CT parameters significantly decreased in patients with clinical improvement (31/34, 91%, P < 0.001), while patients with disease progression did not show FDG-PET/CT improvement. FDG uptake decrease was similar between patients undergoing early assessment (< 6 weeks) compared with those performing FDG-PET/CT after 6 weeks of treatment. SUVmax, LMV, and LMA decrease over time was 39.0%, 97.4%, and 97.1%, respectively. In predicting clinical responses, SUVmax reduction > 15% and > 25% showed 94% and 89% sensitivity and 67% and 100% specificity compared with 37% and 50% of MRI, respectively. Low degree of agreement with clinical response was shown for MRI compared with FDG-PET/CT parameters using the Cohen kappa coefficient.
Conclusions
FDG-PET/CT monitoring is a valuable tool to predict clinical response to treatment in IS and has greater sensitivity and specificity compared with MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Infectious spondylodiscitis (IS) involves the intervertebral disc and neighboring vertebral bodies, accounting for 2 to 7% of all osteomyelitis [1,2,3]. IS incidence has progressively increased in the last decades due to a higher prevalence of immunocompromised patients and the increased number of spinal surgery performed [4, 5]. Acute clinical presentation of IS is often associated with pyogenic spondylodiscitis (PSD), while tuberculous spondylodiscitis (TBSD) has usually a more indolent course [6]. Both PSD and TBSD, however, can be extremely debilitating and cause potentially incapacitating sequelae [7, 8]. IS diagnosis is often delayed and can take between 2 and 6 months due to nonspecific clinical presentations and confounding concomitant comorbidities [9,10,11]. Increased C-reactive protein (CRP) and presentation with back pain represent nonspecific parameters, bur may achieve high sensitivity (> 90%) in IS diagnosis [9,10,11]. Nevertheless, laboratory tests can show variable results in patients with IS, and treatment responses mainly rely on clinical parameters. Symptoms and signs such as fever or back pain, however, may be resolved slowly due to concomitant comorbidities, resulting in inappropriate protraction of antimicrobial therapy [12,13,14]. Although a 6-week regimen is currently recommended in the majority of IS, treatment duration in some instances can be as long as 12 weeks [14]. Appropriate imaging techniques can expedite IS diagnosis, define the extent of the disease, and assess treatment efficacy thus limiting prolonged and/or inappropriate therapy [5]. Magnetic resonance imaging (MRI) and 2-deoxy-2-[18F]fluoro-D-glucose (FDG) positron emission tomography/computed tomography (FDG-PET/CT), in particular, have demonstrated to be valuable in IS diagnosis and in discriminating between PSD and TBSD [9,10,11, 15]. Compared with FDG-PET/CT, however, MRI has lower sensitivity in early diagnosis and in monitoring responses to treatment [16, 17]. Furthermore, promising applications of FDG-PET/CT in various types of infections have been described [18]. Quantitative FDG-PET/CT analysis, consisting of quantifying FDG uptake using various parameters, has been extensively studied in cancer medicine and in non-infectious inflammatory disorders such as sarcoidosis, showing its superiority over qualitative evaluation [19, 20]. Such approach may represent a valuable option also in monitoring treatment response to infections over time [21]. Nevertheless, studies analyzing the effectiveness of quantitative FDG-PET/CT parameters in documenting the response to antimicrobial treatment in IS over time are limited.
We performed a retrospective study to assess the added value of serial FDG uptake analysis in predicting clinical response to antimicrobial treatment in patients with IS. Aims of the study were (1) to analyze changes in quantitative FDG-PET/CT parameters before and after IS treatment among patients with clinical response or failure to treatment and (2) to compare the sensitivity and specificity of serial FDG-PET/CT and MRI in predicting treatment response in patients with IS.
Material and methods
Study population
This was a retrospective observational study. The study population was selected from 70 consecutive patients with diagnosis of IS referred to our institution between January 2010 and December 2017. Inclusion criteria were (1) diagnosis of infectious spondylodiscitis; (2) availability of MRI and FDG-PET/CT at baseline (within 2 weeks of the initiation of antibiotic therapy); (3) at least one serial FDG-PET scan performed to assess response to therapy; and (4) adult age (18 years or older).
A total of 34 patients with diagnosis of IS based on suggestive clinical and laboratory data were included in the study. Blood cultures were obtained from all patients, and CT-guided or ultrasound-guided aspiration biopsy was performed in 19 patients. Biopsies were performed if a microbiologic diagnosis for a known associated organism (e.g., Staphylococcus aureus, streptococci) was not established by blood cultures [22], with the exception of patients who refused to undergo the procedure or those with contraindications (e.g., bleeding diathesis, sites with limited anatomical access) [23]. During biopsy, pathological bone tissue with aspiration of paravertebral collections was performed to obtain histopathological and microbiological samples. All biopsies were performed after the first FDG-PET/CT to avoid the detection of an increased FDG uptake due to inflammation associated with the procedure. Diagnosis of spondylodiscitis was based on microbiological, clinical, and histopathological features consistent with spinal infection as previously described [15]. IS was considered proven when microorganisms (including acid-fast bacilli and/or granuloma/caseous necrosis for TBSD) were present in specimens obtained from the affected site and/or blood cultures. IS was considered probable if microorganisms were absent but histologic aspects suggestive of infection were still present in combination with clinical or radiological manifestations (X-ray, CT scan, MRI) consistent with spinal infection [22]. Metastatic disease was excluded by clinical data (e.g., no risk factors or history of cancer), radiological follow-up, and, when available, histopathological investigation confirming the negativity for cancer cells in the collected samples [22].
Data collected included patients’ clinical characteristics, MRI and FDG-PET/CT monitoring, and outcome.
FDG-PET/CT
FDG-PET/CT was obtained from all patients at baseline and repeated after at least 2 weeks of antimicrobial therapy according to the treating team’s clinical judgment. Median time interval between two serial FDG-PET/CT scans was 55 days (IQR 34.5; 77.0). FDG-PET/CT scans performed within 6 weeks of treatment were considered early assessment (EA). Among patients with PSD, 13/28 (46%) received FDG-PET/CT EA. Nine (32%) patients underwent FDG-PET/CT between 6 and 10 weeks, and 6 (22%) after 10 weeks of treatment. Compared with PSD, patients with TBSD received longer treatments and underwent FDG-PET/CT at different time points between month 2 and 12 of treatment.
FDG-PET/CT was performed using a hybrid PET/CT scanner (Biograph, Siemens Medical Solutions, Erlangen, Germany) following at least 6-h fasting after an unrestricted diet [15]. Fasting plasma glucose levels were measured prior to FDG injection and required to be less than 140 mg/dl. Whole-body images were acquired approximately 60 min after intravenous injection of 185 MBq (5.034 mCi) of FDG, as previously described [15, 24]. No intravenous contrast agent was administered for the CT part of the PET-CT scan. CT scan of the area between the proximal femora and base of the skull was acquired for attenuation correction of PET data and anatomic correlation. CT acquisition was performed with a spiral dual-slice CT scanner with 130 kV, 60 mAs, 5-mm slice thickness, and a pitch of 1. Images were acquired using a matrix of 512 Å~512 pixels and a pixel size of 1 mm. Approximately 6 to 7 bed positions were used in the three-dimensional acquisition mode for scanning the entire patient. The acquisition time was tailored to patient weight ranging from 2 min per bed position for patients under 60 Kg to 4 min per bed position for patients over 90 Kg. PET data were acquired using a matrix of 128 Å~128 pixels, and PET images were reconstructed using the iterative ordered subset expectation maximization method (four iterations and eight subsets) with a slice thickness of 5 mm. After completion of PET acquisition, the reconstructed attenuation-corrected PET images, CT images, and fused matched PET and CT images were available for review in the axial, coronal, and sagittal planes, as well as maximum intensity projections (MIP), and in 3D cine mode.
FDG-PET/CT analysis
Qualitative analysis was performed by two independent nuclear medicine experts blinded to clinical and outcome data, while quantitative analysis was performed by an expert investigator blinded to clinical and outcome data. For qualitative analysis axial, coronal and sagittal FDG-PET/CT fusion image reconstructions and maximum intensity projection (MIP) images were reviewed, and an FDG activity higher than that from adjacent vertebras as well as presence of soft tissue uptake was deemed positive for infection [25].
To compare the results of repeated FDG-PET/CT scans, improvement was defined as resolution or reduction of FDG uptake in involved spinal segments. Unchanged or increased metabolic gradient were considered worsening.
In all patients, maximum standardized uptake value (SUVmax) of the FDG-avid lesion was determined by placing a region of interest (ROI) over the area surrounding the lesion suspected of infection and using the following equation: (activity/unit volume)/(injected dose/total body weight). FDG-PET/CT scans performed between 2015 and 2017 (n = 38) were also quantitatively analyzed using ROVER software (ABX advanced biochemical compounds GmbH, Radeberg, Germany) to determine partial-volume corrected lesion metabolic volume (LMV) and partial-volume corrected lesion metabolic activity (LMA) [19, 20]. For quantitative analysis, a ROI was placed on the fused axial image encompassing the entire vertebral lesion (coronal and sagittal images were reviewed to ensure the entire lesion and no adjacent FDG-avid structures were included, manual editing was performed if necessary). LMV (defined as the volume of FDG-avid voxels inside the ROI) was automatically determined using a lower threshold setting of 40% SUVmax with a minimum volume of 1 cc as previously described [19, 20]. Partial-volume corrected LMA (defined as the product of LMV and SUVmean) was then automatically calculated [19, 20]. Due to lack of storage of DICOM (Digital Imaging and Communications in Medicine) files, ROVER analysis was not possible for FDG-PET/CT scans acquired prior to 2015. Quantitative changes in FDG uptake were evaluated by calculating ΔSUVmax, ΔSUVmean, ΔLMV, and ΔLMA as the percentage change.
MRI acquisition protocol and data analysis
In 32 (94%) patients, a repeat MRI was available during treatment follow-up. Interval between repeat FDG-PET/CT and MRI at end of treatment (EOT) varied from 3 to 5 weeks.
Magnetic resonance images (MRI) of the spine were obtained with a 1.5 T scanner (Avanto, Siemens, Erlangen, Germany). The MRI protocol included T1 and T2 weighted sagittal and axial images with and without intravenous injection of gadolinium contrast enhancement. Images were evaluated by two expert neuroradiologists blinded to clinical and outcome data. Criteria used to identify IS included abnormalities such as disc enhancement with associated surrounding end plate irregularity, increased T2 signal with enhancement of the adjacent marrow space, reduced cortical definition, persistence of epidural abscesses, and inflammation of paraspinal soft tissues. To compare longitudinally the results of repeated MRI scans, a negative examination (resolution) or a reduction of features such as paraspinal inflammation, bone marrow edema, epidural, and disc space enhancement was defined as improvement, whereas no change or deterioration of MRI findings were considered worsening.
Patient follow-up and outcomes
At baseline, before initiation of antimicrobial treatment (T0), all patients underwent a diagnostic workup comprehensive of clinical evaluation, laboratory tests, FDG-PET/CT, and MRI. Duration of PSD treatment varied from 6 to 12 weeks according to the physician’s judgment. TBSD was treated with antitubercular drugs for 9 to 12 months. Clinical and laboratory data at EOT and after 6 to 12 months following treatment termination, according to the persistence of symptoms and/or signs suggestive of IS (e.g., back pain, fever, and neurological symptoms such as weakness, paresthesias, and bowel or bladder incontinence), were also collected.
Clinical outcome was classified as clinical response (resolution or improvement of fever, back pain, neurological symptoms) or as progressive disease, in case of worsening of symptoms. Laboratory data in support of IS diagnosis included C-reactive protein (CRP) levels and white blood cell (WBC) count.
Statistical analysis
Continuous and categorical data were reported as median (25th and 75th percentile) and frequency distributions, respectively. Repeated measures of FDG-PET/CT parameters were compared using paired-sample parametric (paired Student t) or nonparametric (paired Wilcoxon signed rank) tests. One-way analysis of variance (ANOVA) test was used to determine the significance of differences between groups of patients. Categorical variables were evaluated using chi-square or, when appropriate, the two-tailed Fisher exact test. For variables that were not normally distributed, Mann-Whitney test was used.
The sensitivity, specificity, positive predictive value, and negative predictive value of FDG-PET/CT and MRI for detection of clinical response to treatment were estimated using standard statistical formulas. The area under the receiver operating characteristic (ROC) curve and Youden’s index were calculated to establish cutoff values for ΔSUVmax with the highest sensitivity and specificity.
The degree of agreement between different modalities to define response to therapy was assessed using Cohen’s kappa coefficient. Analyses were performed using SPSS v. 20.0 (IBM, SPSS, Chicago, Illinois). All tests were two-tailed, and a P value less than 0.05 was determined to represent statistical significance.
Results
A total of 68 FDG-PET/CT scans were analyzed in 34 patients with IS receiving repeat FDG-PET/CT. Patients’ characteristics are summarized in Table 1. Median duration of symptoms prior to antimicrobial treatment was 84 days (IQR 45–117). Overall, 27 (79%) patients presented with back pain at diagnosis and 18 (53%) required pain medications. Among patients undergoing spinal biopsy, 38% tested positive. Microbiological diagnosis was available in 24 (71%) cases from biopsy or blood cultures. The majority of patients showed involvement of the lumbar spinal tract. In 14 (41%) cases, the presence of abscesses (defined as spinal or paraspinal fluid collections) was detected, and two patients required surgical drainage. Overall treatment duration for PSD was 53 days (range 40–86 days). The most common antimicrobial regimen was the association of levofloxacin and rifampin (11/34, 33%). TBSD was microbiologically confirmed in 4 (12%) and probable in 2 patients. Patients with TBSD were younger and had a higher number of vertebrae involved compared with patients with PSD (Supplementary Table 1).
Clinical outcome
Clinical resolution or improvement of symptoms was reported in 4 (67%) patients with TBSD and 25 (89%) patients with PSD at T1. Two patients undergoing FDG-PET/CT scan at 2 and 3.6 months after antitubercular treatment still reported significant back pain at T1. Three patients with PSD had progressive disease and required treatment change. Among these, two patients who were empirically treated for PSD with levofloxacin and levofloxacin plus rifampicin, respectively, were switched to a different therapy. One patient received antitubercular treatment based on a repeat biopsy showing granulomatous necrosis, while the other was empirically switched to anti-MRSA therapy. The third patient had an infection caused by multidrug-resistant MRSA and required combination treatment with teicoplanin, rifampin, and fusidic acid. In these patients, non-infectious reasons for progressive disease (e.g., metastatic disease) were excluded by clinical evaluation and negativity of histological specimens for malignant cells. Clinical improvement was reported in all patients following treatment change. At EOT, overall clinical response was 91% (6/6 and 25/28 patients with TBSD and PSD, respectively). Leukocytosis (WBC > 11,000/mmc) was detected in 9 (26%) cases and normalized during treatment in 7 (78%) patients. CRP levels were increased (> 5 mg/L) at presentation in 19 (56%) patients and decreased at EOT in 10 (53%) patients (median values 9.4 mg/L, IQR 2.2–27.6). During the 12-month follow-up, one patient died from cardiac complications (not related to IS) 8 weeks after treatment, and one patient with previous history of acute leukemia and stem cell transplantation was diagnosed with a new episode of IS caused by a different pathogen.
FDG-PET/CT monitoring
Qualitative FDG-PET/CT monitoring showed overall improvement in 82% of cases, with resolution of abnormal FDG uptake in 12% of patients. Lesions appeared unchanged in 4 (12%) patients and worsened in 2 (6%) patients. Qualitative FDG-PET/CT analysis at EOT showed a sensitivity of 87% (Table 2).
In patients showing clinical response to treatment, SUVmax significantly decreased over time (Fig. 1), with mean ΔSUVmax of 39.0% (Table 3). Sensitivity and specificity of FDG-PET/CT parameters in predicting clinical responses are summarized in Table 2. ΔSUVmax > 25% had the highest sensitivity and specificity in predicting clinical response as shown by the areas under the receiver operating characteristic (ROC) curve (AUC = 0.93, Supplementary Table 2 and Supplementary Fig. 1).
Agreement between FDG-PET/CT parameters and clinical response is reported in Supplementary Table 3. Of note, we observed a substantial agreement between qualitative FDG-PET/CT and ΔSUVmax > 25% (κ = 0.62). Among patients with disease progression at EOT, 2 out of 3 had increased SUVmax at T1 (Fig. 1c). Patients with improvement at qualitative FDG-PET/CT analysis showed ΔSUVmax of 40.3% compared with 6.7% of patients without improvement (P = 0.001).
FDG-PET/CT parameters were compared in different patients’ groups. SUVmax at T0 was higher in TBSD compared with PSD (P = 0.04). Significant SUVmax reduction over time was reported for both PSD (6.5 vs. 4.5, P < 0.01) and TBSD (9.9 vs. 4.4, P = 0.04). ΔSUVmax did not differ between patients undergoing FDG-PET/CT EA (within 6 weeks of treatment) or after 6 weeks of treatment and among those with or without spinal abscesses (Supplementary Table 1 and 4).
Quantitative analysis with ROVER software was performed in 19 patients (Supplementary Table 5). A sharp reduction of LMV and LMA (97.3% and 97.1%, respectively) on repeat FDG-PET/CT as compared with baseline scans was recorded among patients with clinical response. In two patients, LMV and LMA reduction was limited, showing variation of 18% and 40%, respectively (Fig. 2). In the first case, the patient showed disease progression at EOT despite complete qualitative FDG-PET/CT response and ΔSUVmax > 15%. The other patient was receiving treatment for TBSD and did not report initial clinical improvement at T1 (3.6 months). Among different groups, ROVER parameters were similar (Supplementary Table 3).
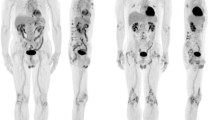
Figure 3 reports a typical FDG-PET/CT scan documenting IS. Figure 4 shows the changes of FDG uptake before and after treatment along with quantitative analysis showing size and metabolic activities of the lesions.
Baseline FDG-PET/CT scan of a patient presenting with thoracic IS. The upper row shows FDG-PET/CT scans in three projections: coronal (a), sagittal (b), and transverse (c), while the lower row shows the quantitation of lesion metabolic volume (LMV) and lesion metabolic activity (LMA) using ROVER software (d–f)
FDG-PET/CT monitoring at T0 (a, b) and T1 (b, c) in a patient with thoracic IS showing significant reduction of FDG uptake after treatment. Coronal, sagittal, and transverse FDG-PET/CT projections as well as a transversal section of the corresponding CT scan and fused FDG-PET/CT scan are shown. Lower rows show the quantitation of lesion metabolic volume (LMV) and lesion metabolic activity (LMA) made by ROVER software
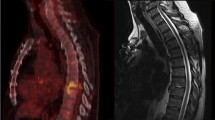
MRI monitoring
Sixty percent of patients receiving MRI monitoring showed no change (46%) or worsened MRI appearance (21%) after treatment. In 40% of patients, an improvement in MRI following treatment was shown. None of the MRI performed at EOT showed complete resolution of the radiological signs of the infection. Overall MRI sensitivity was 37%. We observed poor agreement between MRI responses and both clinical outcome and FDG-PET/CT responses (ĸ = − 0.16, Supplementary Table 3).
A total of 16 (47%) patients performed an MRI also during the 12-month follow-up period following treatment. Five out of 16 (31%) showed complete resolution of IS (at 4, 7, 9, 10, and 11 months after treatment, respectively) while 11/16 (69%) had persistence of radiological signs of infection.
Discussion
Our study demonstrated that FDG-PET/CT is a useful tool to monitor treatment response among patients with IS. Serial FDG uptake analysis showed greater sensitivity (> 85%) in predicting clinical response to antimicrobial treatment compared with MRI (37%).
IS is characterized by delayed recognition and low microbiological yields (19–41% from aspiration biopsies), and usually requires prolonged empirical treatment that may lead to toxicity and antimicrobial resistance [9,10,11, 22]. Russo A et al. [26] reported the results of the UDIPROVE clinical protocol aiming at optimizing the microbiological yields in the cohort of patients with vertebral osteomyelitis at our Center between January 2009 and January 2017. Overall, the etiology of infection obtained from combined blood cultures and vertebral biopsy or drainage was 74%. In this cohort, quantitative (e.g. LMA and LMV) analysis as well as MRI monitoring was not performed; however, a significant reduction of SUV over time (p < 0.001) was confirmed among patients undergoing PET/CT during the study period (Russo A et al. Int J Infect Dis [26]). FDG-PET/CT appears promising to evaluate the response to antimicrobial therapy in IS [13, 27,28,29,30]. Since an effective treatment limits WBC migration to the site of infection, reduced FDG uptake may become evident as early as 48 h following therapy. Timely evaluation of treatment response using FDG-PET/CT can therefore support clinicians’ decision to prolong or change treatment.
FDG-PET/CT monitoring can also be useful for the management of TBSD, in which the persistence of symptoms usually lasts for several weeks and may represent a confounding factor to assess clinical responses. In our study, we observed limited LMV and LMA reduction in a patient with biopsy-confirmed TBSD after 3.6 months of treatment who complained of back pain, consistent with the prolonged course of TBSD compared with PSD. These data suggest that performance of FDG-PET/CT in TBSD within 4 months of treatment could be premature; furthermore, in this indication LMA and LMV were more accurate than SUVmax, which appeared significantly reduced at T1 in all TBSD patients. FDG-PET/CT may also be useful in discriminating between TBSD and PSD. As previously shown, we confirmed higher SUVmax values at baseline in patients with TBSD compared with PSD [15].
Although qualitative FDG-PET/CT assessment showed high sensitivity and a substantial agreement with ΔSUVmax in predicting clinical response to treatment in IS, quantitative assessment may offer further advantages. A ΔSUVmax cutoff that can be predictive of treatment responses, for example, could be a useful tool for the management of patients with IS. In our report, ΔSUVmax > 25% had the highest Youden index compared with other ΔSUVmax and may represent an acceptable cutoff value to assess FDG-PET/CT responses, if validated in further studies. Furthermore, LMA and LMV appeared promising in infectious spondylodiscitis and may be more precise than SUVmax in documenting positive and negative responses to treatment. A patient in our study failed initial treatment for PSD and was subsequently switched to TBSD treatment based on histological results. In this case, FDG-PET/CT showed reduction of SUVmax of 17% and qualitative improvement, while ΔLMA and ΔLMV were only marginally reduced. LMA and LMV results were consistent with a partial response to treatment, since levofloxacin—that was empirically employed to treat PSD—is also active against Mycobacterium tuberculosis. Furthermore, the most dramatic FDG changes over time (> 95%) were indeed noted for LMA and LMV compared with SUVmax. Although these results should be interpreted with caution due to the limited number of patients enrolled in the study, the use of LMA and LMV has previously proven beneficial in predicting treatment response in other diseases [31,32,33]. Also, SUVmax compared with LMA and LMV may be altered by the size of the lesion and does not take into account global disease activity.
We also explored the efficacy of MRI compared with FDG-PET/CT in monitoring treatment responses in IS. Low (< 50%) MRI sensitivity has been previously reported, suggesting that its use in this indication should be avoided [12, 34, 35]. Our results support the lack of indication for MRI in monitoring early responses to antimicrobial treatment in IS and the superiority of FDG-PET/CT. Improvement or normalization of MRI scans was observed in a minority of patients and only after several months following antimicrobial treatment. Conversely, improvement of FDG-PET/CT was evident also in early assessments (within 6 weeks of treatment), suggesting that that FDG-PET/CT could be useful in IS to predict early responses to antimicrobial treatment.
Numerous reports analyzing quantitative FDG-PET/CT advantages have recently become available [19, 36]. To our knowledge, however, our study is the first to assess quantitative parameters, including LMV and LMA, to monitor treatment response in IS. Due to the fact that monitoring of clinical symptoms may be slow and subjective and MRI results appear unreliable, the use of FDG-PET/CT in IS represents an excellent instrument to consider, especially if supported by quantitative assessments. Although PET-CT is recognized by the IDSA guidelines as a highly sensitive tool for detecting IS (since a negative PET scan excludes the diagnosis of osteomyelitis) [22], PET scanning is currently not approved in the USA for IS diagnosis or for monitoring IS treatment response. Further studies corroborating its efficacy in spondylodiscitis are necessary to support the use of FDG-PET/CT for this indication.
Our study has several limitations. Firstly, assessment of FDG-PET/CT monitoring was limited by the retrospective nature of the study and the small sample size. Secondly, repeat MRI was not always performed at the same time as FDG-PET/CT but usually within a few weeks. Nevertheless, we do not believe that this could have significantly affected the comparison between FDG-PET/CT and MRI performance, since changes in MRI appearance over time appear very slow, requiring several months. Finally, microbiological confirmation of IS was not obtained in all patients, representing a potential confounder in assessing response to antimicrobial treatment.
In conclusion, we showed significant reduction of FDG uptake among patients with IS who had favorable response to antimicrobial therapy. This significant decrease was noted for all FDG-PET/CT parameters, confirming the utility of quantitative FDG-PET/CT to assess treatment response in IS. Larger prospective studies are warranted in order to confirm these findings.
References
Cottle L, Riordan T. Infectious spondylodiscitis. J Inf Secur. 2008;56:401–12.
Stäbler A, Reiser MF. Imaging of spinal infection. Radiol Clin N Am. 2001;39:115–535.
Sapico F, Montomerie J. Vertebral osteomyelitis. Infect Dis Clin N Am. 1990;77:539–50.
Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery—the case for restraint. N Engl J Med. 2004;350:722–6.
Tyrrell PN, Cassar-Pullicino VN, McCall IW. Spinal infection. Eur Radiol. 1999;9:1066–77.
Tins BJ, Cassar-Pullicino VN. MR imaging of spinal infection. Semin Musculoskelet Radiol. 2004;8:215–29.
Skaf GS, Domloj NT, Fehlings MG, Bouclaous CH, et al. Pyogenic spondylodiscitis: an overview. J Infect Public Health. 2010;3:5–16.
Liebergall M, Chaimsky G, Lowe J, Robin GC, Floman Y. Pyogenic vertebral osteomyelitis with paralysis. Prognosis and treatment. Clin Orthop Relat Res. 1991;269:142–50.
Mahboubi S, Morris MC. Imaging of spinal infections in children. Radiol Clin N Am. 2001;39:215–22.
Galhotra RD, Jain T, Sandhu P, Galhotra V. Utility of magnetic resonance imaging in the differential diagnosis of tubercular and pyogenic spondylodiscitis. J Nat Sci Biol Med. 2015;6:388–93.
Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. Am J Roentgenol. 2004;182:1405–10.
Giacomuzzi F, Rensi M, Geatti O. FDG PET/CT (PET) for early evaluation of response to therapy in spondylodiscitis: experience in 80 patients. J Nucl Med. 2014;55:378.
Nanni C, Boriani L, Salvadori C, et al. FDG PET/CT is useful for the interim evaluation of response to therapy in patients affected by haematogenous spondylodiscitis. Eur J Nucl Med Mol Imaging. 2012;39:1538–44.
Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385:875–82.
Bassetti M, Merelli M, Di Gregorio F, et al. Higher fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) uptake in tuberculous compared to bacterial spondylodiscitis. Skelet Radiol. 2017;46:777–83.
Smids C, Kouijzer IJE, Vos FJ, et al. A comparison of the diagnostic value of MRI and (18)F-FDG-PET/CT in suspected spondylodiscitis. Infection. 2017;45:41–9.
Baxi S, Malani PN, Gomez-Hassan D, Cinti SK. Association between follow-up magnetic resonance imaging and clinical status among patients with spinal infections. Infect Dis Clin Pract (Baltim Md). 2012;20:326–9.
Bassetti M, Carnelutti A, Muser D, et al. 18F-Fluorodeoxyglucose positron emission tomography and infectious diseases: current applications and future perspectives. Curr Opin Infect Dis. 2017;30:192–200.
Muser D, Santangeli P, Castro SA, Liang JJ, Enriquez A, Werner TJ, et al. Prognostic role of serial quantitative evaluation of (18)F-fluorodeoxyglucose uptake by PET/CT in patients with cardiac sarcoidosis presenting with ventricular tachycardia. Eur J Nucl Med Mol Imaging. 2018;45(8):1394–404.
Lee PI, Cheng G, Alavi A. The role of serial FDG PET for assessing therapeutic response in patients with cardiac sarcoidosis. J Nucl Cardiol. 2017;24(1):19–28.
Houshmand S, Salavati A, Hess S, Werner TJ, Alavi A, Zaidi H. An update on novel quantitative techniques in the context of evolving whole-body PET imaging. PET Clin. 2015;10:45–58.
Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, et al. Executive summary: 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;15(61):859–63.
Garg RK, Somvanshi DS. Spinal tuberculosis: a review. J Spinal Cord Med. 2011;34:440–54.
Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute trials. J Nucl Med. 2006;47:1059–66.
Fuster D, Tomás X, Mayoral M, et al. Prospective comparison of whole-body 18F-FDG PET/CT and MRI of the spine in the diagnosis of haematogenous spondylodiscitis. Eur J Nucl Med Mol Imaging. 2015;42:264–71.
Russo A, Graziano E, Carnelutti A et al. Management of vertebral osteomyelitis over an eight-year period: The UDIPROVE (UDIne PROtocol on Vertebral osteomyelitis). Int J Infect Dis 2019;89:116–121.
Prodromou ML, Ziakas PD, Poulou LS, et al. FDG PET is a robust tool for the diagnosis of spondylodiscitis: a meta-analysis of diagnostic data. Clin Nucl Med. 2014;39:330–5.
Gunes BY, Onsel C, Sonmezoglu K, et al. Diagnostic value of F-18 FDG PET/ CT in patients with spondylodiscitis: is dual time point imaging time worthy? Diagn Microbiol Infect Dis. 2016;85:381–5.
Riccio SA, Chu AKM, Rabin HR, Kloiber R. Fluorodeoxyglucose positron emission tomography/computed tomography interpretation criteria for assessment of antibiotic treatment response in pyogenic spine infection. Can Assoc Radiol J. 2015;66:145–52.
Skanjeti A, Penna D, Douroukas A, et al. PET in the clinical work-up of patients with spondylodiscitis: a new tool for the clinician? J Nucl Med Mol Imaging. 2012;56:569–76.
Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–36.
Dibble EH, Alvarez ACL, Truong M-T, Mercier G, Cook EF, Subramaniam RM. 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med. 2012;53:709–15.
Basu S, Saboury B, Werner T, et al. Clinical utility of FDG-PET and PET/CT in non-malignant thoracic disorders. Mol Imaging Biol. 2011;13:1051–60.
Niccoli Asabella A, Iuele F, Simone F, Fanelli M, et al. Role of (18)F-FDG PET/CT in the evaluation of response to antibiotic therapy in patients affected by infectious spondylodiscitis. Hell J Nucl Med. 2015;18(Suppl 1):17–22.
Kowalski TJ, Layton KF, Berbari EF, et al. Follow-up MR imaging in patients with pyogenic spine infections: lack of correlation with clinical features. Am J Neuroradiol. 2007;28:693–9.
Berkowitz A, Basu S, Srinivas S, et al. Determination of whole-body metabolic burden as a quantitative measure of disease activity in lymphoma: a novel approach with fluorodeoxyglucose-PET. Nucl Med Commun. 2008;29:521–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The local institutional review board approved the study. Informed consent was waived due to the retrospective nature of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elda Righi and Daniele Muser equally contributed to this manuscript.
Electronic supplementary material
ESM 1
(DOCX 53 kb)
Rights and permissions
About this article
Cite this article
Righi, E., Carnelutti, A., Muser, D. et al. Incremental value of FDG-PET/CT to monitor treatment response in infectious spondylodiscitis. Skeletal Radiol 49, 903–912 (2020). https://doi.org/10.1007/s00256-019-03328-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-03328-4