Abstract
Glenohumeral osteoarthritis is a prevalent degenerative disease that can lead to excruciating pain and debility. End-stage osteoarthritis can be treated by both conservative and surgical interventions. Along with a comprehensive history and physical exam, pre-operative imaging with plain radiographs, computerized tomography, and magnetic resonance imaging plays an essential role in the decision-making process guiding whether the patient undergoes a shoulder hemiarthroplasty, anatomic total shoulder arthroplasty, or a reverse total shoulder arthroplasty. The most important pre-operative imaging factors are the integrity of the rotator cuff and presence of significant glenoid erosion. Imaging is also critical postoperatively, as signs of prosthetic loosening, rotator cuff failure (especially involving the subscapularis), periprosthetic fracture, and stress fractures are important entities to recognize. This article will review pertinent imaging findings related to the pre- and post-operative management of patients with glenohumeral osteoarthritis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
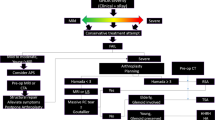
Glenohumeral arthritis can be due to primary osteoarthritis of the shoulder from overuse, posttraumatic arthritis (e.g., secondary to proximal humerus fractures), inflammatory arthritis (e.g., rheumatoid arthritis), reactive arthritis (e.g., Lyme), secondary to proximal humerus AVN or secondary to a rotator cuff tear (i.e., rotator cuff tear arthropathy) [1, 2]. Patients present with pain with use of the affected shoulder and night pain, often accompanied by weakness and limited range of motion. The role of the radiologist is critical, as a variety of imaging modalities are used to make or confirm the diagnosis and play a key role in surgical planning. The surgical options for patients with glenohumeral osteoarthritis consist of arthroscopic debridement procedures and joint replacement surgeries including hemiarthroplasty, total shoulder arthroplasty, and reverse total shoulder arthroplasty. Resurfacing arthroplasty is controversial and will not be discussed in this review. In addition to patient factors such as level of activity and goals of surgery, several imaging characteristics are crucial in deciding which joint replacement surgery is appropriate for each patient. The integrity of the glenoid bone stock, degree of osteoarthritis, and presence of rotator cuff injury are three essential imaging features of high importance to the surgeon in determining the most appropriate surgery to perform.
Routine radiographic imaging is standard following shoulder arthroplasty in order to monitor for any developing complications. The most common complications after shoulder arthroplasty are implant loosening, instability, overstuffing, rotator cuff failure, periprosthetic fracture, and stress fractures. Imaging plays a key role in diagnosing or confirming these complications, and as such it is important for radiologists to be aware of them.
The purpose of this article is to review the common current surgical treatments for glenohumeral osteoarthritis and to outline the role of imaging in the peri-operative management of patients undergoing these procedures. Pre-operatively, imaging is critical in confirming the diagnosis of glenohumeral osteoarthritis and in the decision-making process of which joint replacement option is the most appropriate. Post-operatively, imaging is a routine component of the post-operative evaluation process and is also integral in monitoring for and diagnosing complications.
Surgical treatment of glenohumeral osteoarthritis
Glenohumeral arthritis, whether due to primary osteoarthritis, rotator cuff arthropathy, or inflammatory arthritis, can be a debilitating condition. The initial treatment of glenohumeral arthritis is conservative. This varies patient to patient, but in general consists of non-steroidal anti-inflammatory medications, physical therapy to focus on range of motion and strengthening, and corticosteroid injections. If these treatments fail and patients have refractory pain and loss of shoulder function that negatively affects their life, then surgical treatment may be warranted. Surgical treatment options include hemiarthroplasty, total shoulder arthroplasty, and reverse total shoulder arthroplasty. Normal-appearing radiographs of anatomic total shoulder arthroplasty and reverse total shoulder arthroplasty can be seen in Fig. 1.
Normal radiographs. Normal Grashey and axillary lateral of anatomic right shoulder TSA with lesser tuberosity osteotomy that is well reduced (a, b). Normal appearing AP and axillary lateral after left shoulder reverse TSA where a subscapularis peel was performed (c, d). Normal-appearing AP and axillary lateral after right shoulder hemiarthroplasty where a subscapularis peel was performed (e, f)
Hemiarthroplasty
Dr. Charles Neer was the first to describe the use of a metallic hemiarthroplasty implant in the setting of proximal humerus fractures [3]. By definition, shoulder hemiarthroplasty is replacement of the humeral side of the shoulder joint with a stemmed implant leaving the glenoid intact. Modern implants have customizable stem, neck, and head implants that allow surgeons to recreate the patient’s natural anatomy. Normal positioning consists of having the humeral stem centered within the humeral shaft and having the humeral head centered within the glenoid. Current indications for hemiarthroplasty include severe proximal humerus fracture where the humeral head is at risk for avascular necrosis, primary osteoarthritis without glenoid involvement, arthritis with severe glenoid bone loss such that there is inadequate bone stock to support a glenoid component, cuff tear arthropathy with enough remaining rotator cuff to allow for active elevation on clinical exam, and early stage osteonecrosis without glenoid involvement [1]. Hemiarthroplasty is commonly used in the setting of proximal humerus fractures, as Moeckel et al. have shown that it is effective at controlling pain and improving functional outcomes in this setting [3]. Compared with total shoulder arthroplasty, hemiarthroplasty is associated decreased functional outcomes and higher rates of revision when used in the setting of primary glenohumeral arthritis and sufficient glenoid bone stock [4]. Thus, in the setting of primary glenohumeral osteoarthritis, hemiarthroplasty is most often utilized in the setting of inadequate glenoid bone stock or lack of glenoid involvement.
Contraindications to hemiarthroplasty are a compromised rotator cuff and/or coracoacromial arch that could lead to anterosuperior humeral escape, extensive subchondral cysts, and/or an osteopenic glenoid making the bearing surface of the glenoid less than ideal as an articulating surface, a non-concentric glenoid that is uncorrectable by reaming, and, lastly, inadequate soft tissues to help balance the non-constrained prosthesis. If there is concern for inadequate bone stock on radiographs, then a CT scan is obtained to evaluate glenoid bone stock as well as glenoid version. This will be discussed further in the preoperative imaging section. The coracoacromial arch is defined by the region between the coracoid, acromion, and the coracoacromial ligament, which aids in preventing humeral head anterosuperior escape [2]. This arch must be maintained to support the hemiarthroplasty. The presence of a compromised rotator cuff and coracoacromial arch is determined both clinically and radiographically. Clinically, patients will not be able to actively elevate their arm and on radiographs they will have a high-riding humeral head. Relative contraindications to hemiarthroplasty include: history of remote infection of the glenohumeral joint, previous shoulder surgery, alcoholism, cigarette smoking, chronic narcotic use, advanced Parkinson’s disease, and neuropathic arthritis [1].
Total shoulder arthroplasty
Dr. Neer also described the first total shoulder arthroplasty in 1974 utilizing a polyethylene glenoid implant and a stemmed humeral component in the setting of primary osteoarthritis [5]. Total shoulder arthroplasty involves replacement of the humeral and glenoid sides of the shoulder joint. Normal positioning includes the humeral head component being centered within the glenoid component and the humeral stem component being centered within the humeral shaft, without radiolucent lines greater than 1.5 mm thick around either component. Total shoulder arthroplasty has been shown to have better outcomes associated with improved range of motion and better pain relief, and is more cost-effective compared to hemiarthroplasty [6]. Indications for total shoulder arthroplasty are primary glenohumeral osteoarthritis causing pain treated with conservative measures in the setting of an intact rotator cuff and good glenoid bone stock, as well as inflammatory arthritis, osteonecrosis of the humeral head with glenoid involvement, and posttraumatic osteoarthritis [1]. Glenoid component loosening is frequently cited as the most common reason for revision arthroplasty, ranging from 1.7 to 4.7% (Fig. 2).
A 72-year-old male with glenoid component loosening. a Malpositioning of the humeral component not concentric on the glenoid component. b Anatomic TSA failure and resultant humeral head elevation. c Axial and d coronal CT arthrogram images showing periprosthetic lucency indicative of loosening of the glenoid component
In the setting of primary osteoarthritis, multiple studies have demonstrated superior outcomes with anatomic total shoulder arthroplasty versus that of shoulder hemiarthroplasty [4, 7, 8]. While failure rates associated with total shoulder arthroplasty have been declining, with 95% 15-year survival with current designs, the polyethylene glenoid continues to be the “weak link” in total shoulder arthroplasty (Fig. 2) [9, 10]. For this reason, some authors prefer hemiarthroplasty, as there is no glenoid component to loosen. However, with hemiarthroplasty, the native glenoid itself becomes the weak link with glenoid erosion by the metallic humeral head, leading to pain. This has led to a conversion rate from hemiarthroplasty to total shoulder arthroplasty of 8.1% [1, 4, 11].
Reverse total shoulder arthroplasty
The reverse total shoulder arthroplasty reverses the ball and socket of the shoulder joint. It consists of modular glenoid components, including a baseplate that is fixed to the glenoid with screws and a glenosphere that is secured to the and acts as the new “ball” of the shoulder joint. The glenosphere and baseplate are often connected through a Morse taper. The humeral component consists of a metal stem inserted into the humeral shaft as well as a polyethylene-lined humeral tray, which acts as the new “socket.” The humeral stem and tray may be modular, which is important to know as they can fail at the junction between these two components. Normal positioning includes having the glenosphere flush with the glenoid as well as having the humeral component centered on the glenosphere proximally and centered within the humeral shaft distally.
Reverse total shoulder arthroplasty allows the deltoid muscle to raise the arm without a functioning rotator cuff. Therefore, knowledge of the rotator cuff integrity is essential in the decision-making process to determine if reverse total shoulder arthroplasty is the ideal implant. In 1987, Dr. Paul Grammont was the first to design a clinically successful reverse total shoulder arthroplasty implant based on the principles of medialization and lowering of the glenohumeral joint center of rotation to minimize the shear forces on the glenoid implant, and to improve the rotational mechanical advantage of the deltoid [12]. In addition, the reverse total shoulder arthroplasty allows for a more constrained articulation—allowing the deeper polyethylene dish of the socket now based on the humerus to articulate with the glenosphere, thus restoring fixed-fulcrum kinetics of the shoulder (Fig. 3). The deltoid can then raise the arm around this fixed center of rotation in the absence of a functional rotator cuff, instead of simply sliding the humeral head superiorly without the centering effect of the rotator cuff. Classic indications for reverse total shoulder arthroplasty are massive, irreparable rotator cuff tear with or without concomitant arthritis, in a low-demand and elderly patient (typically greater than 70 years old). Contraindications include a non-functioning deltoid, infection, a neuropathic joint, and substantial and non-reconstructable glenoid bone loss [13]. Frankle et al. evaluated 60 patients treated with reverse total shoulder arthroplasty for glenohumeral osteoarthritis and rotator cuff deficiency. Their functional American Shoulder and Elbow Surgeons (ASES) score improved by an average of 34 points and their pseudoparalysis was reversed, with average range of motion improving from 55° to 105.1° with abduction [14].
Preoperative imaging evaluation
Radiography
Radiographs are the first imaging modality used to evaluate the presence and degree of arthritis of the glenohumeral joint. Common projections include an anteroposterior view, a Grashey view, and an axillary view. These views allow for evaluation of the presence, type, and degree of arthritis as well to rule out other pathology including but not limited to fractures, dislocations, and bone lesions. Various radiographic classifications have been established to determine the extent of osteoarthritis of the glenohumeral joint. The most widely adopted classification is the Samilson–Prietro classification (Fig. 4). In this classification, grade 0 is normal; grade 1 is mild with osteophytes less than 3 mm on the humeral head; grade 2 is moderate with osteophytes between 3 and 7 mm on the humeral head or glenoid rim; and grade 3 is severe with osteophytes of more than 7 mm with or without articular incongruity [15].
The status of the rotator cuff can be inferred by radiographic evaluation on the Grashey view. The Grashey view is obtained by a 30° lateral oblique projection, tangential to the glenohumeral joint, in order to obtain the view directly parallel to the glenoid face to reveal any degenerative changes. The radiographic classification utilized for rotator cuff integrity is the Hamada–Fukuda classification, a radiographic morphological description of the natural course of massive rotator cuff tear assessing the height of the acromiohumeral space. There are five distinctions within this classification [16]:
-
Type 1: Normal joint morphology and acromiohumeral distance of more than 6 mm
-
Type 2: Acromiohumeral distance of less than 5 mm
-
Type 3: Type 2 plus acetabularization (i.e., exaggerated undersurface concavity) of the acromion
-
Type 4: Types 2 and 3 plus narrowing of glenohumeral joint space
-
Type 5: Types 2, 3, and 4 plus collapse of the humeral head.
Lastly, an axillary view is essential in preoperative planning as it allows for assessment of posterior glenoid wear and deficiency, which has ramifications on the glenoid preparation (concentric reaming) necessary to center the glenoid-based implant.
Computerized tomography
Computed tomography (CT) provides greater bony detail than radiographs and is often used to evaluate the status of humeral and glenoid bone as well as for preoperative planning. If there is concern for glenoid bone loss, cysts, or retroversion on radiographs, CT should be obtained as this can affect the choice of which type of arthroplasty to perform or the position of the glenoid component. In general, CT should be performed on all patients undergoing an arthroplasty procedure requiring glenoid resurfacing (i.e., total shoulder arthroplasty and reverse total shoulder arthroplasty), as glenoid pathology can affect implant positioning and fixation. Table 1 includes a checklist of items that should be evaluated when a CT is ordered for preoperative planning prior to shoulder arthroplasty.
CT of the affected shoulder aids in the evaluation of glenoid bone loss that may require preoperative planning prior to eccentric reaming, bone graft augmentation, utilization of augmented glenoid components, or consideration of reverse total shoulder arthroplasty [17]. CT evaluation should be performed on all patients undergoing an arthroplasty procedure requiring glenoid resurfacing (i.e., total shoulder arthroplasty and reverse total shoulder arthroplasty), as it allows for quantification of glenoid version and recognition of glenoid cysts and other forms of glenoid bone loss that may impact implant fixation and placement. Glenoid version is defined as the angle between a line drawn from the medial border of the scapula to the center of the glenoid and the line perpendicular to the face of the glenoid on an axial CT scan [18]. Average version is 5° of retroversion with a range from 7° of retroversion to 10° of anteversion being considered normal.
The Walch classification is a commonly used scheme to evaluate glenoid version, the relationship of the humeral head to the glenoid, and the amount of posterior glenoid wear. Glenoid types are divided into categories of A, B, and C (Fig. 5) based upon the relationship of the humeral head to the glenoid. Walch “Type A” has a concentric articulation with either minor or deep erosion of the glenoid (A1 or A2, respectively). Walch “Type B” has a biconcave glenoid with asymmetric wear and posterior humeral subluxation with either simple narrowing of the posterior joint space versus actual posterior wear (B1 versus B2, respectively). Lastly, a Walch “Type C” is a dysplastic glenoid with greater than 25° of glenoid retroversion. Thus, the Walch classification distinguishes true retroversion from posterior wear [19].
Assessing and understanding glenoid morphology prior to the operation can prevent common causes of implant failure. If the glenoid is not placed in its anatomic location, the articulation and movement between the resurfaced glenoid and humeral head will be abnormal, causing glenoid implant loosening and early arthroplasty failure. For example, in the setting of a non-concentric glenoid, edge loading can occur that leads to a “rocking-horse effect” in either the frontal or horizontal plane, which causes glenoid implant liftoff, and thus failure [9]. Three-dimensional, volume-rendered images of the scapula obtained from either a CT or magnetic resonance (MR) imaging dataset provide extremely accurate, detailed images of the glenoid, particularly as the scapula may be tilted due to thoracic kyphosis making the glenoid morphology and version more difficult to assess on standard two-dimensional datasets. Budge et al. assessed the glenoid version of 34 cadaveric shoulders with both standard axial two-dimensional images, and modified two-dimensional reconstructed images along the transverse scapular plane as defined on a reconstructed surface-rendered three-dimensional model of the scapula, and found a 5–15° difference in glenoid version between the two methods, concluding that making the measurement on a plane defined by the scapular body allowed for more accurate and reliable assessment [20].
MR imaging
MRI of the shoulder is indicated in patients where there is concern for rotator cuff deficiency on clinical exam. As mentioned previously, an intact rotator cuff is required for hemiarthroplasty and total shoulder arthroplasty and thus the integrity of the rotator cuff is the key factor in determining whether a patient is a candidate for an anatomic total shoulder arthroplasty or hemiarthroplasty versus a reverse total shoulder arthroplasty. MRI provides great soft tissue detail and can provide information on the presence of rotator cuff tears as well as the presence and degree of muscle atrophy.
The Goutallier grading scale of fatty infiltration of the rotator cuff muscles was originally described using CT to assess the degree of fatty infiltration of the individual muscles of the rotator cuff [21]. This method was then modified by Fuchs et al. utilizing MR imaging, which demonstrated acceptable interobserver and intraobserver reliability [22]. The Goutallier classification consists of: grade 0 being normal muscle, grade 1 being some fatty streaks, grade 2 being less than 50% fatty muscle atrophy, grade 3 being 50% fatty muscle atrophy, and grade 4 being greater than 50% fatty muscle atrophy [21]. The importance of this grading scale is its implication on reparability of the rotator cuff: a Goutallier or Fuchs fatty infiltration grade of 3 or higher (i.e., fatty infiltration equal to or greater than 50% of muscle bulk) has a re-tear rate of 50–70% [21, 23, 24]. As indicated earlier, a total shoulder arthroplasty requires a stable joint. A glenohumeral joint with a massive rotator cuff tear and fatty degeneration is predisposed to dynamic and static elevation of the humeral head as the deltoid force vector is no longer adequately opposed by the centering effect of the rotator cuff. This creates unequal forces on the glenoid component as the head now articulates with the superior edge of the glenoid—the so-called “rocking-horse” phenomenon that leads to early glenoid loosening in total shoulder arthroplasty patients with a large or massive rotator cuff tear [9].
It is important to note that the majority of patients with primary osteoarthritis of the glenohumeral joint have an intact rotator cuff. Thus, MRI is only obtained if there is concern for rotator cuff deficiency on clinical exam. In Neer’s original description, only one patient in 27 with primary osteoarthritis had a full-thickness rotator cuff tear [5]. In another study, Walch and Boileau found that 7.4% of patients with primary glenohumeral osteoarthritis had a partial-thickness supraspinatus tear and 7.6% had a full-thickness tear [25]. Total shoulder arthroplasty in the setting of a rotator cuff tear, specifically of the infraspinatus and subscapularis tendons, had poor clinical results with decreased Constant scores (objective and subjective data points which include pain, activities of daily living (ADLs), range of motion and strength), decreased active external rotation, decreased forward flexion and decreased subjective patient outcomes [25]. Small tears isolated to the supraspinatus have little to no effect on the outcome of arthroplasty for primary glenohumeral osteoarthritis; however, fatty degeneration of the infraspinatus has been found to be a negative prognostic indicator in arthroplasty longevity [25].
3D specific imaging in shoulder arthroplasty
The glenoid component accounts for a large proportion (24%) of the complications associated with total shoulder arthroplasty [26]. Furthermore, there is an emphasis on accurate placement of the glenoid in primary arthroplasty as revision total shoulder arthroplasty outcomes are inferior to those of primary total shoulder arthroplasty [26, 27]. Bone loss and severe retroversion lead to difficulties of accurate glenoid placement in both anatomic and reverse total shoulder arthroplasty, and if not accounted for, can lead to early glenoid-based implant failure [28]. In order to mitigate these possible complications, 3D reformatted images have been utilized in preoperative planning and have been shown to help in the accurate placement of the glenoid component.
A variety of implant companies have designed software to help preoperative planning for both anatomic total shoulder arthroplasty and reverse total shoulder arthroplasty (i.e., Athrex VIP System, Tornier BLUEPRINT, etc.). With the utilization of 3D preoperative planning software, inter-rater reliability of the assessment of intraoperative bone loss, assessment of glenoid fit, and improvement in glenoid preparation has been demonstrated [29]. As a result, the positioning of the glenoid implant has greatly improved with the ability to place the implant within 5° of desired inclination and 10° of planned version compared to standard techniques [30]. Furthermore, in the setting of reverse total shoulder arthroplasty, utilization of 3D modeling has shown to improve both accuracy of baseplate placement as well as screw placement within the scapula [31]. Due to these technical improvements, surgeons will continue to rely on preoperative planning tools as they can aid in intraoperative surgical decision-making and surgical site preparation to reduce risks of future complications.
Complications of shoulder arthroplasty
Although shoulder arthroplasty can reliably relieve shoulder pain and restore motion, there are notable complications that can adversely affect patient outcomes. Overstuffing of the implant, rotator cuff failure, periprosthetic fractures and stress fractures, implant loosening, instability, and notching may occur and can be detected on postoperative imaging studies. Some complications such as periprosthetic humerus fractures are common to all types of arthroplasty whereas other complications are more specific to a surgical technique, such as notching with reverse total shoulder arthroplasty. Table 2 shows a list of common complications for each arthroplasty procedure.
Overstuffing
Hemiarthroplasty is an anatomic arthroplasty and, as such, the goal is to match the patient’s anatomy. There are multiple implant systems with various types of modularity that attempt to restore the native anatomy. It is paramount for the surgeon to know both the individual patient’s anatomy and the specifics of the implant being used in order to attempt to restore offset, retroversion, head size, and the inclination of the proximal humerus [32]. Pearl et al. demonstrated that 95% of the patient’s native anatomy can be restored with a modern hemiarthroplasty implant if proper technique is used [33]. Care must be taken to not oversize the implant as it can overstuff the glenohumeral joint limiting the ability to repair any remaining cuff, and especially places a repaired subscapularis tendon at risk. Overstuffing can lead to increased joint reactive force with resultant pain and glenoid erosion [34]. Williams et al. showed that even if the humeral head is the proper size, malpositioning with overhang of 4 mm or more can lead to rotator cuff tear [35]. The acromiohumeral interval is a means to evaluate for overstuffing and can be assessed and measured as early as the first standing post-operative radiograph with a Grashey view of the shoulder. The normal acromiohumeral interval measures at least 7 mm; an acromiohumeral interval less than 2 mm suggests either overstuffing of the glenohumeral joint or a new rotator cuff tear. Clinical exam can be utilized to distinguish between overstuffing and a new rotator cuff tear. The normal head height distance—the vertical distance between the highest point of the implant humeral head and the supraspinatus (i.e., superior) facet of the greater tuberosity—should be between 2 and 5 mm. A distance greater than 5 mm can possibly indicate overstuffing, while sequential radiographs with decreasing head height distance are indicative of subsidence of the humeral component [36] [37]. Lastly, Alolabi et al. described a reproducible radiographic assessment for overstuffing, which necessitates obtaining a near-perfect post-operative Grashey view of the extremity [38]. This method uses three bony landmarks (medial edge of the greater tuberosity, lateral edge of the greater tuberosity, and medial calcar) to create a best-fit circle to define the anatomic center of rotation. A second best-fit circle is drawn to fit the curvature of the prosthetic humeral head to define the implant center of rotation. A deviation of greater than 3 mm between the anatomic and implant centers of rotation results in overstuffing of the reconstructed joint (Fig. 6).
Best-fit method to evaluate for overstuffing. One best-fit sphere representing the native humerus (solid line) is made from a circle around the lateral edge of the greater tuberosity, and the medial calcar. The other best-fit sphere representing the prosthetic humeral head is drawn to fit the curvature of the prosthetic humeral head. The centers of rotation of each best-fit sphere are annotated by the star for the prosthesis and the circle for the native humeral head, with difference between the two > 3 mm significant for concern for overstuffing
Rotator cuff tear
Subscapularis tendon failure is the second most common complication following shoulder arthroplasty, reported in as high as 51% of patients in some studies [39, 40]. To provide adequate exposure and access to the glenohumeral joint for all three arthroplasty options (hemiarthroplasty, anatomic total shoulder arthroplasty, and reverse total shoulder arthroplasty), the subscapularis typically is detached either through a lesser tuberosity osteotomy, a subscapularis tenotomy, or by peeling the subscapularis tendon off of the humerus. An example of normal post-operative radiographs following a lesser tuberosity osteotomy can be seen in Figs. 1 and 7.
In the setting of shoulder arthroplasty, complications can occur with lesser tuberosity exposure. Shi et al. followed patients for greater than 2 years and found lesser tuberosity failure at an average of 9 weeks from the date of surgery; the lesser tuberosity failures occurred with and without trauma [41]. When a lesser tuberosity osteotomy was performed intraoperatively, the subscapularis tendon failure is likely to be a fracture through the osteotomy and thus radiographs or CT can be used to identify the displacement of the lesser tuberosity. In cases where a subscapularis tenotomy or peel was performed intraoperatively, the subscapularis tendon is likely to avulse off of the bone. In these cases, there will be no displaced bony fragments. The only radiographic clue may be a subtle anterior translation of the humeral component with respect to the glenoid component on radiographs or CT. As the subscapularis provides important function to the shoulder, it is essential to report osteotomy failures and anterior subluxation on post-operative radiographs.
Accurate imaging of implants with MRI requires sequences limiting the dephasing and artifacts due to metal. Metal suppression protocols are institution-dependent and can offer valuable information in regard to the integrity of the rotator cuff, soft tissue hematoma formation, and occult fractures. Patients with absolute contraindications to MRI (e.g., cochlear implants, many cardiac pacemakers, and some other implanted devices) require evaluation utilizing different imaging modalities. Ultrasound is a fast and reliable way to diagnose rotator cuff tears after arthroplasty as the prosthesis does not limit the diagnosis of rotator cuff compromise [39]. Sofka et al. recommends an extended field-of-view imaging and tissue harmonic imaging, and power Doppler imaging when utilizing ultrasound to evaluate the periprosthetic soft tissues, including the rotator cuff [42]. CT arthrography can also provide valuable information, identifying the precise location of tears and demonstrating presence and degree of retraction. CT arthrography may also show intratendinous cleavages found in full- and partial-thickness tendon tears, as well as detecting a potentially loose component as contrast may insinuate around the perimeter of the component [43].
Periprosthetic fracture
Periprosthetic fractures are a complication of all types of shoulder arthroplasty with an incidence of 1.5–3%; approximately half of these injuries occur intraoperatively and half occur on a delayed basis as the result of falls or other blunt trauma [44, 45]. Timely recognition is essential in adequately treating this complication. Identification of fracture location and adequacy of humeral stem fixation should be noted as they will dictate operative planning ranging from non-operative treatment, open reduction and internal fixation, and revision arthroplasty [45]. In the setting of total shoulder arthroplasty or reverse total shoulder arthroplasty, periprosthetic scapular neck or glenoid fractures can lead to instability and component loosening. Imaging obtained includes radiographs and CT where quantification of remaining glenoid bone stock remaining should be noted as surgery may require the use of bone graft, bone wedge reinforcements, and/or revision glenoid implants [46].
Implant loosening
Humeral component loosening
Humeral component loosening has an overall prevalence of 1% and accounts for 7% of the total complications after arthroplasty. Loosening of the humeral component is even higher in patients with non-cemented press-fit stems, although radiolucent lines do not necessarily correlate with symptomatic implant loosening requiring revision arthroplasty [47]. Humeral periprosthetic lucency is more common when there is a polyethylene glenoid component than with a hemiarthroplasty, possibly due to effects of wear-related particles [48]. Sperling et al. defined “at risk” humeral components as those with radiographic evidence of subsidence, tilt, or a complete radiolucent line measuring greater than 2 mm around the entire implant [49].
Glenoid component loosening
The majority of symptomatic loosening is associated with the glenoid component (83% of cases with loosening) [46]. Despite high numbers of radiographic lucent lines (80% of cases) and even radiographic signs of loosening (34% of cases demonstrated migration, tilt, or a shift of the component or a complete radiolucent line of greater than 1.5 mm in thickness), only 7% of cases at 13.4 years required revision surgery for glenoid-based loosening [46]. Implant design has demonstrated variability when looking at radiolucent lines: keeled implants, as opposed to pegged implants, have shown statistically higher rates of radiolucent lines and clinically significant loosening [46, 50]. Keeled implants are designed to have one central keel implanted into the glenoid while pegged implants have multiple smaller, peripheral pegs, which are implanted into the glenoid. Examples of these implant types can be seen in Fig. 8. Metal-backed implants have historically also had a higher rate of failure [10, 46]. It is important to note that the majority of glenoid implants on the market now are all-polyethylene, radiolucent implants. Typically, a marker wire, beads, or other embedded radiopaque material will be present for radiographic surveillance. Figure 2 demonstrates an example of glenoid component loosening.
Instability
Anterior and superior instability account for the greater than 80% of cases of post-arthroplasty glenohumeral instability; most of these cases are closely associated with the integrity of the subscapularis tendon [46, 51]. Grashey, axillary, and scapular-Y radiographs should be obtained to provide direction of dislocation and visualization of possible associated fractures. Anterior subluxation of the humeral head by more than 5 mm suggests anterior instability [52]. This typically occurs due to the combination of implant malpositioning, inadequate soft tissue tensioning, and poor soft tissue integrity. Warren et al. noted anterior capsular abnormalities, excessive anteversion of the glenoid component, an oversized humeral head component, and anterior placement of the humeral component also contributed to anterior instability. Similarly, superior instability is noted by an acromiohumeral distance less than 5 mm on the Grashey view. Posterior instability can be due to positioning (glenoid retroversion greater than 20° and/or humeral component retroversion greater than 45°), infraspinatus insufficiency, and/or dorsal glenoid deficiency. Lastly, inferior instability is due to excessive shortening of the humeral implant or deltoid insufficiency, possibly due to iatrogenic axillary nerve damage [52].
Early dislocation of a reverse total shoulder arthroplasty (within 3 months of placement) is an uncommon complication (2.9%) [53]. In this population, the combination of using a deltopectoral approach and subscapularis insufficiency leads to a statistically significant increase in the likelihood of instability. The use of advanced imaging (MRI with metal suppression, CT arthrography, or ultrasound) to determine the integrity of the rotator cuff is recommended [54, 55].
Complications specific to reverse total shoulder arthroplasty
Notching
Notching of the scapular neck is the phenomenon encountered in reverse total shoulder arthroplasty where the cup-like humeral component impinges upon the inferior aspect of the scapular neck, leading to bone resorption (Fig. 9). The loss of bone due to the notching can be seen on the inferior neck of the glenoid on radiographs. Originally described by Boileau et al., scapular notching was seen in 68% of patients in their study; notching extending superior to the inferior screw was seen in 28% of their patients [56].
The grading scheme for scapular neck notching is the Nerot–Sirveaux classification: Grade 0, no notch; grade 1, small notch stopping short of the inferior screw; grade 2, medium notch reaching the inferior screw; grade 3, large notch extending beyond/superior to the inferior screw [57]. Despite being radiographically impressive, notching has not been shown to consistently affect the Constant score, the adjusted Constant score, nor the ASES score. The surgeon can reduce the rate of notching by placing the glenoid implant as low as possible on the glenoid face [56]. In the presence of excessive glenoid notching, glenoid loosening can occur, which can be observed on plain radiographs [52].
Acromial stress fracture
Acromion stress fractures in reverse total shoulder arthroplasty are believed to be due to increased deltoid tension or acute trauma. These fractures often occur due to increased forces on the acromion by the deltoid leading to an insufficiency fracture in oeteopenic or osteoporotic patients. Patients present with a change in their pain scores after a period of being asymptomatic. They often complain of pain with active abduction and tenderness directly over the acromion (Fig. 10). Typically, patients tolerate these fractures well and conservative treatment is pursued. In rare cases, the fracture extends into the scapular spine, operative fixation may be necessary. These fractures most often occur just posterior to the AC joint and can be difficult to detect on radiographs, particularly in the presence of a metal glenosphere that can obscure their presence on the axillary view [52, 58]. Thus, CT scan is often required to diagnose acromial stress fractures.
Levy et al. described a classification of acromial fractures following reverse total shoulder arthroplasty that was related to the origin of the deltoid. Type I includes fractures through the midpart of the acromion involving a portion of the anterior and middle deltoid origins. Type II fractures involve the entire middle deltoid origin with a portion of the posterior deltoid origin. Type III fractures involve the entire middle and posterior deltoid origins [59].
Conclusions
Current imaging modalities allow for extensive preoperative and postoperative assessment of patients undergoing shoulder arthroplasty for the treatment of painful glenohumeral osteoarthritis. An understanding of both the indications for surgery and then typical post-operative complications is essential for the radiologist to communicate pertinent findings to the orthopedic surgeon. In general, the most important pre-operative factors for the orthopedic surgeon to gain from imaging are the degree of osteoarthritis, the integrity of the rotator cuff, and the presence of any significant glenoid erosion. Post-operatively, the presence of implant loosening, rotator cuff failure (especially the subscapularis), periprosthetic fractures, stress fractures, and instability are the critical findings indicating potential complications. As the number of shoulder arthroplasties performed continues to increase, both the orthopedic surgeon and the radiologist must work together to identify risk factors preoperatively and complications post-operatively.
References
Wiater JM, Fabing MH. Shoulder arthroplasty: prosthetic options and indications. J Am Acad Orthop Surg. 2009;17(7):415–25.
R. Drake WV, A. Mitchell. Gray’s anatomy for students. 1st ed. Philadelphia: Elsevier/Churchill Livingstone; 2005.
Moeckel BH, Dines DM, Warren RF, Altchek DW. Modular hemiarthroplasty for fractures of the proximal part of the humerus. J Bone Joint Surg Am. 1992;74(6):884–9.
Radnay CS, Setter KJ, Chambers L, Levine WN, Bigliani LU, Ahmad CS. Total shoulder replacement compared with humeral head replacement for the treatment of primary glenohumeral osteoarthritis: a systematic review. J Shoulder Elb Surg. 2007;16(4):396–402.
Neer CS 2nd. Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 1974;56(1):1–13.
Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947–56.
Edwards TB, Kadakia NR, Boulahia A, et al. A comparison of hemiarthroplasty and total shoulder arthroplasty in the treatment of primary glenohumeral osteoarthritis: results of a multicenter study. J Shoulder Elbow Surg / Am Shoulder Elbow Surgeons [et al]. 2003;12(3):207–13.
Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality-of-life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am. 2005;87(10):2178–85.
Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplast. 1988;3(1):39–46.
Fox TJ, Foruria AM, Klika BJ, Sperling JW, Schleck CD, Cofield RH. Radiographic survival in total shoulder arthroplasty. J Shoulder Elbow Surg / Am Shoulder Elbow Surgeons [et al]. 2013;22(9):1221–7.
Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg / Am Shoulder Elbow Surgeons [et al]. 2005;14(5):471–9.
Grammont P TP, Lafay JP, Deries X. Concept study and realization of a new total shoulder prosthesis. Rhumatologie. 1987;39.
Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthopaed Surg. 2009;17(5):284–95.
Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697–705.
Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65(4):456–60.
Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92–6.
Stephens SP, Paisley KC, Jeng J, Dutta AK, Wirth MA. Shoulder arthroplasty in the presence of posterior glenoid bone loss. J Bone Joint Surg Am. 2015;97(3):251–9.
Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032–7.
Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplast. 1999;14(6):756–60.
Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg / Am Shoulder Elbow Surg [et al]. 2011;20(4):577–83.
Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83.
Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg/ Am Shoulder Elbow Surg [et al]. 1999;8(6):599–605.
Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–28.
Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg/ Am Shoulder Elbow Surg [et al]. 2003;12(6):550–4.
Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84-a(12):2240–8.
Gonzalez JF, Alami GB, Baque F, Walch G, Boileau P. Complications of unconstrained shoulder prostheses. J Shoulder Elb Surg. 2011;20(4):666–82.
Deutsch A, Abboud JA, Kelly J, et al. Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elb Surg. 2007;16(6):706–16.
Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439–49.
Scalise JJ, Codsi MJ, Bryan J, Brems JJ, Iannotti JP. The influence of three-dimensional computed tomography images of the shoulder in preoperative planning for total shoulder arthroplasty. J Bone Joint Surg Am. 2008;90(11):2438–45.
Iannotti JP, Weiner S, Rodriguez E, et al. Three-dimensional imaging and templating improve glenoid implant positioning. J Bone Joint Surg Am. 2015;97(8):651–8.
Venne G, Rasquinha BJ, Pichora D, Ellis RE, Bicknell R. Comparing conventional and computer-assisted surgery baseplate and screw placement in reverse shoulder arthroplasty. J Shoulder Elb Surg. 2015;24(7):1112–9.
Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons [et al]. 2005;14(1 Suppl S):99s–104s.
Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: how much prosthetic geometry is necessary? J Shoulder Elbow Surg/ Am Shoulder Elbow Surg [et al]. 2009;18(3):366–70.
Sanchez-Sotelo J, Cofield RH, Rowland CM. Shoulder hemiarthroplasty for glenohumeral arthritis associated with severe rotator cuff deficiency. J Bone Joint Surg. 2001;83(12):1814–22.
Iannotti JP, Spencer EE Jr, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. Journal of Shoulder and Elbow Surgery. 2005;14(1, Supplement):S111–21.
Merolla G, Di Pietto F, Romano S, Paladini P, Campi F, Porcellini G. Radiographic analysis of shoulder anatomical arthroplasty. Eur J Radiol. 2008;68(1):159–69.
Sanchez-Sotelo J, Wright TW, O’Driscoll SW, Cofield RH, Rowland CM. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J Arthroplast. 2001;16(2):180–7.
Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg / Am Shoulder Elbow Surg [et al]. 2014;23(11):1740–6.
Ives EP, Nazarian LN, Parker L, Garrigues GE, Williams GR. Subscapularis tendon tears: a common sonographic finding in symptomatic postarthroplasty shoulders. J Clin Ultrasound : JCU. 2013;41(3):129–33.
Cofield RH, Edgerton BC. Total shoulder arthroplasty: complications and revision surgery. Instr Course Lect. 1990;39:449–62.
Shi LL, Jiang JJ, Ek ET, Higgins LD. Failure of the lesser tuberosity osteotomy after total shoulder arthroplasty. J Shoulder Elbow Surg/ Am Shoulder Elbow Surg [et al]. 2015;24(2):203–9.
Sofka CM, Adler RS. Original report. Sonographic evaluation of shoulder arthroplasty. AJR Am J Roentgenol. 2003;180(4):1117–20.
Walch G, Boileau, P. Shoulder Arthroplasty. 2 ed: Springer; 1999.
Athwal GS, Sperling JW, Rispoli DM, Cofield RH. Periprosthetic humeral fractures during shoulder arthroplasty. J Bone Joint Surg Am. 2009;91(3):594–603.
Steinmann SP, Cheung EV. Treatment of periprosthetic humerus fractures associated with shoulder arthroplasty. J Am Acad Orthop Surg. 2008;16(4):199–207.
Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279–92.
Maynou C, Petroff E, Mestdagh H, Dubois HH, Lerue O. Clinical and radiologic outcome of humeral implants in shoulder arthroplasty. Acta Orthop Belg. 1999;65(1):57–64.
Sanchez-Sotelo J, O’Driscoll SW, Torchia ME, Cofield RH, Rowland CM. Radiographic assessment of cemented humeral components in shoulder arthroplasty. J Shoulder Elbow Surg/ Am Shoulder Elbow Surg [et al]. 2001;10(6):526–31.
Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg/ Am Shoulder Elbow Surg [et al]. 2000;9(6):507–13.
Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg/ Am Shoulder Elbow Surg [et al]. 2005;14(3):252–7.
Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492–7.
Buck FM, Jost B, Hodler J. Shoulder arthroplasty. Eur Radiol. 2008;18(12):2937–48.
Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg/ Am Shoulder Elbow Surg [et al]. 2014;23(5):737–44.
Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elb Surg. 2009;18(6):892–6.
Gallo RA, Gamradt SC, Mattern CJ, et al. Instability after reverse total shoulder replacement. J Shoulder Elb Surg. 2011;20(4):584–90.
Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg/ Am Shoulder Elbow Surg [et al]. 2006;15(5):527–40.
Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Brit Vol. 2004;86(3):388–95.
Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res. 2011;469(9):2544–9.
Levy JC, Anderson C, Samson A. Classification of postoperative acromial fractures following reverse shoulder arthroplasty. J Bone Joint Surg Am. 2013;95(15):e104.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dekker, T.J., Steele, J.R., Vinson, E.V. et al. Current peri-operative imaging concepts surrounding shoulder arthroplasty. Skeletal Radiol 48, 1485–1497 (2019). https://doi.org/10.1007/s00256-019-03183-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-03183-3