Abstract
Objective
To determine the diagnostic yield of CT-guided percutaneous biopsy of densely sclerotic bone lesions.
Materials and methods
We retrospectively analyzed CT-guided percutaneous bone biopsies performed at our institution from September 2008 through August 2011 (329 cases) and from September 2012 through August 2015 (324 cases) after adoption of a battery-powered drill system (OnControl). Bone lesions were included in the analysis if they were >70% sclerotic by visual inspection, had a density > 2 times that of adjacent trabecular bone, and had an attenuation of ≥250 HU. Pathological fractures, diskitis–osteomyelitis, and osteoid osteomas were excluded. Eligible cases were characterized by lesion location, maximum lesion diameter, mean density, biopsy needle type and gauge, reported complications, and histological diagnosis. Clinical and imaging follow-up was used to confirm histological diagnosis. Cases in which a benign histological diagnosis could not be confirmed by imaging over a minimum period of 1 year were excluded.
Results
A total of 37 biopsies of sclerotic bone lesions met the inclusion criteria, 17 of which were performed with a power drill needle and 20 of which were performed with a manually driven needle. The mean lesion density was 604.1 HU. The overall diagnostic yield was 78.4%; overall diagnostic accuracy was 94.6%, and the false-negative rate was 5.4%. Diagnostic yield and accuracy were 82.4% and 100% respectively, with a power drill and 75% and 90% respectively, with a manual device. Diagnostic yield for lesions ≥700 HU was 90% (9 out of 10).
Conclusion
Densely sclerotic bone lesions are amenable to percutaneous needle biopsy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Imaging-guided percutaneous needle biopsy of bone and soft-tissue musculoskeletal lesions has gained wide acceptance as the preferred method in lieu of open biopsies because of its cost-effectiveness, better safety profile, and generally high diagnostic accuracy [1,2,3,4,5]. Some authors have reported high diagnostic accuracy, sensitivity, and specificity with imaging-guided percutaneous needle biopsy of sclerotic bone lesions [6, 7]. However, other investigators have found that percutaneous needle biopsy of sclerotic bone lesions is associated with a relatively low diagnostic yield and significant false-negative rate [8,9,10,11,12], prompting some experts to advocate open biopsy of these lesions instead [13, 14]. In our anecdotal experience, we have had good diagnostic yield with percutaneous needle biopsy of these lesions, especially when using large-gauge power drill biopsy needles.
To further investigate our anecdotal experience and explore whether adoption of a battery-powered drill has affected our diagnostic yield, we conducted a systematic review of CT-guided sclerotic bone lesion biopsies performed at our institution to determine the diagnostic yield, false-negative rate, and diagnostic accuracy of this technique.
Materials and methods
This retrospective study was approved by the Institutional Review Board with a waiver of informed consent in compliance with HIPAA guidelines. The musculoskeletal (MSK) radiology section at our institution performed 329 CT-guided percutaneous bone biopsies from September 2008 through August 2011 and 324 CT-guided bone biopsies from September 2012 through August 2015. These two time periods were chosen to reflect the introduction of OnControl (Vidacare Corporation, Shavano Park, TX, USA), a battery-powered drill needle biopsy system, at our institution in November 2011. CT-guided bone biopsies performed between September 2011 and August 2012 were excluded from the study to prevent skewing of the data by an inflated failure rate related to radiologists learning a new technique.
To best approximate the technical challenges associated with percutaneous biopsy of sclerotic bone lesions, we used very rigorous criteria to select what we considered to be “unequivocal” sclerotic bone lesions. For the purpose of this study, a bone lesion was considered unequivocally sclerotic if at least 70% of its volume appeared sclerotic on visual inspection. Additionally, the lesion had to have an attenuation of at least 250 Hounsfield units (HU) and a density at least twice that of the adjacent normal trabecular bone. The attenuation of each bone lesion was averaged over three consecutive slices whenever possible.
We excluded mixed lytic sclerotic lesions with focal areas of lucency that could be targeted for biopsy, regardless of how much sclerosis involved. Patients who did not have a target sclerotic lesion on imaging (e.g., routine imaging-guided bone marrow biopsy, biopsy of pathological fracture, and suspected diskitis–osteomyelitis) were excluded. Suspected osteoid osteoma cases were also excluded, as the areas of sclerosis in these lesions represent reactive bone change rather than an actual pathological process. Cases in which a final pathological diagnosis could not be confirmed by surgical resection or clinical management and cases without adequate follow-up to demonstrate imaging stability of at least 1 year were also excluded from the study.
A total of 15 cases met the criteria for unequivocal sclerotic bone lesions from September 2008 through August 2011 (Fig. 1); all 15 of these biopsies were performed using manually driven biopsy needles. The types of manually driven biopsy needles used included Bonopty 14G Coaxial Bone Biopsy System (Apriomed, Londonbery, NH, USA); 11-gauge and 13-gauge Osteo-Site Bone Biopsy Needle Set (Cook Medical, Bloomington, IN, USA); and 14-gauge, 16-gauge, and 17-gauge Ostycut Disposable Bone Biopsy Needle (Bard, Tempe, AZ, USA). A total of 22 cases met the criteria from September 2012 through August 2015 (Fig. 1); 17 of these biopsies were performed with the 11-gauge OnControl Bone Marrow Biopsy Needle Set and Powered Bone Access Driver, and 5 were performed using manually driven biopsy needles. In total, 20 biopsies were performed using manually driven biopsy needles, and 17 were performed using power drill needles (Fig. 1). Details of the biopsies, including lesion characteristics and biopsy technique, are listed in Tables 1 and 2.
Biopsies were performed on one of two CT scanners in the department: Somatom Definition AS or Somatom Definition AS+ (Siemens Healthcare, Forchheim, Germany). For all cases, CT fluoroscopy (i-Sequence, Siemens) was performed using the following parameters: 120 kV (kilovolt), 35 mAs (milliampere-second), 2.4- or 4.8-mm slice thickness, and medium-smooth reconstruction kernel (B30f or B31f). All biopsies were performed by MSK fellows under direct supervision or by one of the seven fellowship-trained MSK staff radiologists with 4–31 years of experience. Review of these biopsy cases was performed by one MSK fellow under guidance of a fellowship-trained MSK staff radiologist with 10 years of experience. Equivocal cases were resolved by consensus.
Each case that met the inclusion criteria was characterized by:
-
1.
Patient age and sex
-
2.
Lesion location, size, and attenuation
-
3.
Biopsy needle size and type
-
4.
Complications
-
5.
Histological diagnosis
-
6.
Final diagnosis
Surgical resection histology, clinical management, and imaging follow-up were used to determine diagnostic accuracy. It is important to differentiate diagnostic yield from diagnostic accuracy. A positive diagnostic yield was obtained when a definitive histological diagnosis was made from the biopsy specimens submitted. A nondiagnostic (or indeterminate) yield was defined as a biopsy in which a definitive histological diagnosis was not made. Diagnostic accuracy was defined as the concordant rate of the needle biopsy result and final diagnosis made from resection or follow-up; this was the product of true-positive and true-negative biopsies.
Statistical analysis was performed by a statistician not involved with collection of the data; R version 3.2.2 (http://www.R-project.org/) was used for analysis. Fisher’s exact test was used to compare the diagnostic yield and accuracy between the power drill and the manual devices and between the 11-gauge and the ≤11-gauge biopsy needles. A P value of 0.05 or less was used to indicate statistical significance.
Results
Overall, the mean patient age was 59 years (48.6% women), the mean lesion size was 3.4 cm (range, 0.8–14 cm), the mean lesion attenuation was 604.1 HU (range, 264–1058 HU), and the mean number of core specimens was 1.7. For the manually driven needle group (mean patient age, 61.7 years; 50% women; mean lesion size, 3.8 cm; mean lesion attenuation, 567.5 HU; mean number of core specimens, 1.7), the median needle size used was 12-gauge (range, 11–17; Table 1). For the power drill needle group (mean patient age, 55.9 years; 47% women; mean lesion size, 2.8 cm; mean lesion attenuation, 647.2 HU; mean number of core specimens, 1.8), every biopsy was performed using an 11-gauge needle (Table 2). The overall diagnostic yield for all sclerotic lesions in this study was 78.4%, and overall diagnostic accuracy was 94.6% (Table 3). A total of 75.7% of the lesions were malignant, with metastasis being the most common diagnosis (67.6%; 25 out of 37 cases). There was one benign case of sarcoid (2.7%). In 8 cases, no malignant cells were visible, and a definite pathological diagnosis could not be established from the needle biopsy; these cases were considered nondiagnostic. Six of these cases remained stable on imaging follow-up, confirming their benignity, and were considered true-negatives. In the other 2 cases (5.4%), the patients were later diagnosed with metastatic cancers, and follow-up imaging showed progression of soft-tissue and bone lesions (Figs. 2, 3); these were considered false-negatives.
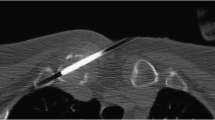
a A 69-year-old man with a sclerotic lesion in the left ischium. b The lesion was biopsied using a manually driven 17-gauge Ostycut needle. The biopsy was nondiagnostic, with no malignant cells visible. c The patient was subsequently diagnosed with metastatic prostate cancer, with a follow-up CT study performed 13 months later, demonstrating progression of the lesion. This was considered a false-negative biopsy
a A 59-year-old man with a sclerotic lesion in the right humerus. b The lesion was biopsied using a manually driven 16-gauge Ostycut needle. The biopsy was nondiagnostic with no malignant cells visible. c The patient was subsequently diagnosed with metastatic nasopharyngeal carcinoma, with a follow-up positron emission tomography (PET)-CT study performed 1 month later demonstrating progression of hypermetabolic bone, including lesions in both proximal humeri (arrows); these results were compatible with metastatic disease. This was considered a false-negative biopsy
The power drill group demonstrated higher diagnostic yield (82.4%; P = 0.701) and diagnostic accuracy (100%; P = 0.490) than the manually driven device group (75% diagnostic yield and 90% accuracy; Table 3). Both of the false-negative cases occurred when the manually driven biopsy needles were used.
The 11-gauge needle group showed higher diagnostic yield (84.6%; P = 0.204) and diagnostic accuracy (100%; P = 0.083) than the <11-gauge needle group (63.6% diagnostic yield and 81.8% accuracy) (Table 4). Both of the false negative cases occurred in the <11-gauge needle group. Specifically, 16-gauge and 17-gauge Ostycut needles were used in these two instances.
When assessing only densely sclerotic lesions with a density of at least 700 HU, we observed a diagnostic yield of 90% and diagnostic accuracy of 100% (Table 5). There was 1 case of an extremely dense sclerotic lesion (density > 1,000 HU). Percutaneous core biopsy of this lesion demonstrated crushed marrow and no malignant cells. Although this biopsy did not yield a specific pathological diagnosis, stability on imaging follow-up confirmed the nonmalignant biopsy result.
In terms of complications and technical issues, there was 1 case of pneumothorax (2.7%) and no reported cases of excess blood loss. There were 2 specimens with substantial crush artifact (5.4%) for which a definitive diagnosis could not be made; however, no malignant cells were seen. Benign etiology in both of these cases was confirmed with the stable appearance of the lesions on a 2-year follow-up MR study of the lumbar spine (sacral lesion) and CT of the abdomen and pelvis (L1 lesion). One of these biopsies (L1 lesion) was obtained using a power drill device (11-gauge needle), and the other (sacral lesion) was obtained using a manually driven needle (11-gauge needle). There was 1 case in which the core specimen became irretrievably trapped inside an 11-gauge OnControl needle, requiring the placement of a 13-gauge OnControl needle inside the 11-gauge needle to obtain the specimen. Despite this complication, the retrieved specimen was diagnostic for metastatic disease in a patient with known pancreatic adenocarcinoma.
Discussion
Imaging-guided percutaneous needle biopsy of bone and soft-tissue lesions is now routinely performed in preference to open biopsy because of the widely recognized cost-effectiveness, lower complication rate, and high diagnostic accuracy of percutaneous needle biopsy [1,2,3,4,5]. Although some authors have reported high diagnostic accuracy, sensitivity, and specificity with imaging-guided percutaneous needle biopsy of sclerotic bone lesions [6, 7], others have reported a relatively low diagnostic yield (48.5–66%) [8,9,10,11] and significant false-negative rate (24%) [12]. Berning et al. [13] asserted that biopsy of sclerotic bone lesions is susceptible to crush artifacts and that sclerotic bone lesions have inherent low cellularity, providing pathologists with little to no chance of making a histological diagnosis. Similarly, McCarthy stated that needle biopsy of sclerotic lesions has a low probability of positive diagnostic yield and that open biopsy of these lesions is “almost always preferable” [14]. However, the MSK radiologists at our institution routinely perform CT-guided percutaneous biopsy of sclerotic bone lesions, and our results appear more favorable than those of earlier studies.
Various statistical instruments have been used in previous studies, including sensitivity, diagnostic accuracy, and diagnostic yield. This lack of consistency in the reporting of biopsy outcomes in the literature makes data interpretation and comparison across studies difficult. Sensitivity refers to the ability of a test to correctly identify patients with the disease of interest. It measures the true-positive rate according to the formula [sensitivity = TP/(TP + FN)]. Sensitivity is used to answer simple binary questions, such as “cancer” or “no cancer.” This instrument is insufficient for the assessment of the biopsy success rate, as the formula does not account for “indeterminate” or “nondiagnostic” biopsy results. Diagnostic accuracy refers to the reliability of biopsy results when a histological diagnosis is made. In statistics, accuracy equals the total sum of true-positives and true-negatives divided by the total number of data points, as stated by the formula [accuracy = (TP + TN)/(TP + TN + FP + FN)]. However, accuracy also ignores the significance of indeterminate/nondiagnostic results, thereby overestimating the ability of percutaneous biopsy to provide a definitive answer. Diagnostic yield refers to the likelihood that a test or procedure will provide the information needed to establish a diagnosis. In pathology, positive diagnostic yield refers to cases in which a specific histological diagnosis can be made from a biopsy. A nondiagnostic or intermediate result refers to cases in which a specific histological diagnosis cannot be made from the biopsy specimen. Diagnostic yield accounts for nondiagnostic results according to the formula [diagnostic yield = diagnostic results/(diagnostic results + nondiagnostic results)]. Therefore, diagnostic yield is the most direct and accurate representation of biopsy success rate.
At the inception of this study, it became clear that a standardized definition for sclerotic bone lesions must be established, as previous studies provided varying definitions or no definition at all [6,7,8,9,10,11,12,13,14]. Not every bone lesion with a sclerotic component should be considered a sclerotic lesion, even if the sclerotic component accounts for 50% or more of its volume (e.g., a mixed density lytic–sclerotic lesion in which the lytic component is the primary pathological process). On the other hand, sclerotic components in many bone lesions do not represent true osteoblastic pathology, but rather reactive change (e.g., pathological fractures, osteoid osteoma, and diskitis–osteomyelitis). Previous studies have demonstrated that increased trabecular bone density is associated with increased bone tissue stiffness, making these lesions more difficult to biopsy [15, 16]. Chang et al. [17] reported cases of bent needle tips and difficulty retrieving specimens during biopsy of densely sclerotic lesions of more than 700 HU. Therefore, to best approximate the technical challenges associated with percutaneous biopsy of sclerotic lesions, we chose unequivocally sclerotic lesions that were at least 70% sclerotic by volume, had an attenuation of at least 250 HU, and had a density at least twice that of the adjacent normal trabecular bone.
Despite the high density of the sclerotic lesions chosen in this study, the overall diagnostic yield (78.4%) in our analysis was still higher than those reported in past studies. Our diagnostic yield of sclerotic lesions biopsied with a power drill device was 82.4%, slightly higher than the 75% yield obtained with manually driven needles. This observation is concordant with a recent study that also reported a higher diagnostic yield with a power drill device (73%) than with a manual device (55.9%) [11]. We suspect that the lower diagnostic yield in this previous study may be in part a result of a higher proportion of biopsies performed with a manual device and smaller gauge needles. Compared with a manual device, the power drill device tends to produce specimens of greater length and diameter [18,19,20] with less force needed to advance into the lesion because of the rapid rotation of the needle [21].
In our study, the diagnostic yield of 11-gauge biopsy needles (84.6%) was higher than that obtained with smaller caliber needles (63.6%). Two false-negative results occurred when smaller caliber biopsy needles were used, whereas none occurred among the biopsies performed using 11-gauge needles. This difference may be explained by discrepancies in the volume of core specimens retrieved with different needles. Assuming equal specimen lengths of 10 mm, an 11-gauge needle yields a core specimen volume of 44.8 mm2, which is 303% higher than the volume of 14.78 mm2 produced with a 15-gauge needle. Wu et al. [9] demonstrated that diagnostic yield increases as the length of the specimen and number of cores submitted increase and therefore recommended that at least three cores should be submitted to optimize diagnostic yield. The mean number of core specimens submitted in our study was only 1.7. We believe that our frequent use of 11-gauge biopsy needles and tendency to obtain longer specimens of 15–20 mm likely contributed to the high diagnostic yield in this study, despite the lower mean number of cores submitted.
Our study demonstrated that densely sclerotic bone lesions of 700 HU or more can be biopsied with a high degree of confidence. The diagnostic yield for sclerotic lesions of at least 700 HU was 90% using either the power drill or manual device. In our experience, biopsy of densely sclerotic lesions of at least 700 HU is significantly more difficult than biopsy of less radiodense lesions. Greater care and forward pressure are generally required to secure the needle into the cortex and to advance the needle to the desired trajectory and depth. The biopsy needle can sometimes be advanced no more than 5 to 10 mm, even with a power drill device, because of very high tissue stiffness, and the biopsy needle must be removed prematurely for specimen retrieval. In our study, new needles were infrequently needed for subsequent passes because of the needle becoming dull or bent. In one instance, the core specimen became irretrievably trapped inside an 11-gauge OnControl needle, and a 13-gauge OnControl needle had to be placed inside the 11-gauge needle to perform a biopsy of the trapped specimen. Chang et al. [17] recommended taking short cores of no more than 5 mm from the periphery when biopsying densely sclerotic lesion of at least 700 HU, advice that we found helpful.
Eight of the biopsies in our study were considered nondiagnostic because they did not yield a definite pathological diagnosis, but the biopsy result still provided useful information in many of these cases. In 6 (75%) of these cases, imaging follow-up confirmed the benign diagnosis. This finding is similar to previous studies that found 53–60% of nondiagnostic biopsies to be clinically useful [22, 23]. These studies also demonstrated that diagnostic yield is significantly lower in biopsies of benign lesions than in biopsies of malignant lesions [17, 22, 23], which would explain the greater number of nondiagnostic benign cases in our study. If a patient has no known malignancy and the prebiopsy radiological features suggest a benign process, a nondiagnostic biopsy can provide reassurance and support the decision for imaging follow-up, thereby preventing escalation to open biopsy. Conversely, it is important to follow a nondiagnostic lesion until it is determined to be benign or malignant. Two of our 8 (25%) nondiagnostic biopsies were later believed to be secondary to metastatic disease. Other studies have reported false-negative rates of 24–39% [12, 24]. At our institution, if a biopsy of an indeterminate lesion with a high degree of suspicion for malignancy prebiopsy yields a nondiagnostic result, a repeat percutaneous needle biopsy is offered to the clinical team. If this repeat biopsy remains nondiagnostic, either imaging follow-up or open biopsy is performed after multidisciplinary oncological conference.
This study had several limitations. Because of the small sample size, the unequal number of 11-gauge and <11-gauge needles used, and the small number of biopsies in which a correct diagnosis was not obtained, we could not use a statistical model to estimate the effect of needle type and lesion characteristics on diagnostic yield. A large discrepancy between the number of malignant (75.7%) and benign (2.7%) lesions may have inflated the diagnostic yield in this study, given the reported higher diagnostic yield of malignant lesions [22, 23]. Because of the retrospective nature of the study, the patients were not randomized by needle size or type; thus, inherent bias in needle selection by the operator could not be excluded.
In conclusion, sclerotic bone lesions, including densely sclerotic lesions, are amenable to percutaneous CT-guided needle biopsy with a high diagnostic yield, high diagnostic accuracy, and low false-negative rate. Use of a larger gauge needle may help to avoid false-negative biopsies.
References
Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1996;78:644–9.
Fraser-Hill MA, Renfrew DL. Percutaneous needle biopsy of musculoskeletal lesions. 1. Effective accuracy and diagnostic utility. AJR Am J Roentgenol. 1992;158:809–12.
Fraser-Hill MA, Renfrew DL, Hilsenrath PE. Percutaneous needle biopsy of musculoskeletal lesions. 2. Cost-effectiveness. AJR Am J Roentgenol. 1992;158:813–8.
Pohlig F, Kirchhoff C, Lenze U, et al. Percutaneous core needle biopsy versus open biopsy in diagnostics of bone and soft tissue sarcoma: a retrospective study. Eur J Med Res. 2012;17:29.
Yao L, Nelson SD, Seeger LL, Eckardt JJ, Eilber FR. Primary musculoskeletal neoplasms: effectiveness of core-needle biopsy. Radiology. 1999;212:682–6.
Jelinek JS, Murphey MD, Welker JA, et al. Diagnosis of primary bone tumors with image-guided percutaneous biopsy: experience with 110 tumors. Radiology. 2002;223:731–7.
Leffler SG, Chew FS. CT-guided percutaneous biopsy of sclerotic bone lesions: diagnostic yield and accuracy. AJR Am J Roentgenol. 1999;172:1389–92.
Vieillard MH, Boutry N, Chastanet P, Duquesnoy B, Cotten A, Cortet B. Contribution of percutaneous biopsy to the definite diagnosis in patients with suspected bone tumor. Joint Bone Spine. 2005;72:53–60.
Wu JS, Goldsmith JD, Horwich PJ, Shetty SK, Hochman MG. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy? Radiology. 2008;248:962–70.
Li Y, Du Y, Luo TY, et al. Factors influencing diagnostic yield of CT-guided percutaneous core needle biopsy for bone lesions. Clin Radiol. 2014;69:e43–7.
Cohen MG, McMahon CJ, Kung JW, Wu JS. Comparison of battery-powered and manual bone biopsy systems for core needle biopsy of sclerotic bone lesions. AJR Am J Roentgenol. 2016;206:W83–6.
Lis E, Bilsky MH, Pisinski L, et al. Percutaneous CT-guided biopsy of osseous lesion of the spine in patients with known or suspected malignancy. AJNR Am J Neuroradiol. 2004;25:1583–8.
Berning W, Freyschmidt J, Ostertag H. Percutaneous bone biopsy, techniques and indications. Eur Radiol. 1996;6:875–81.
McCarthy EF. CT-guided needle biopsies of bone and soft tissue tumors: a pathologist’s perspective. Skeletal Radiol. 2007;36:181–2.
Willems NM, Mulder L, den Toonder JM, Zentner A, Langenbach GE. The correlation between mineralization degree and bone tissue stiffness in the porcine mandibular condyle. J Bone Miner Metab. 2014;32:29–37.
Mulder L, Koolstra JH, den Toonder JM, van Eijden TM. Intratrabecular distribution of tissue stiffness and mineralization in developing trabecular bone. Bone. 2007;41:256–65.
Chang CY, Simeone FJ, Huang AJ. Battery-powered bone drill: caution needed in densely blastic lesions. Skeletal Radiol. 2015;44:1845–8.
Lynch DW, Stauffer SL, Rosenthal NS. Adequacy of powered vs manual bone marrow biopsy specimens: a retrospective review of sequential marrow aspirates and biopsies in 68 patients. Am J Clin Pathol. 2015;143:535–9.
Berenson JR, Yellin O, Blumenstein B, et al. Using a powered bone marrow biopsy system results in shorter procedures, causes less residual pain to adult patients, and yields larger specimens. Diagn Pathol. 2011;6:23.
Reed LJ, Raghupathy R, Strakhan M, et al. The OnControl bone marrow biopsy technique is superior to the standard manual technique for hematologists-in-training: a prospective, randomized comparison. Hematol Rep. 2011;3:e21.
Lee RK, Ng AW, Griffith JF. CT-guided bone biopsy with a battery-powered drill system: preliminary results. AJR Am J Roentgenol. 2013;201:1093–5.
Omura MC, Motamedi K, UyBico S, Nelson SD, Seeger LL. Revisiting CT-guided percutaneous core needle biopsy of musculoskeletal lesions: contributors to biopsy success. AJR Am J Roentgenol. 2011;197:457–61.
Didolkar MM, Anderson ME, Hochman MG, et al. Image guided core needle biopsy of musculoskeletal lesions: are nondiagnostic results clinically useful? Clin Orthop Relat Res. 2013;471:3601–9.
Hwang S, Lefkowitz RA, Landa J, et al. Percutaneous CT-guided bone biopsy: diagnosis of malignancy in lesions with initially indeterminate biopsy results and CT features associated with diagnostic or indeterminate results. AJR Am J Roentgenol. 2011;197:1417–25.
Acknowledgements
We would like to thank Megan Griffiths for her help with editing the manuscript and Jennifer Bullen for her help with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards for the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Chang, IY.J., Ilaslan, H., Sundaram, M. et al. CT-guided percutaneous biopsy of sclerotic bone lesions: diagnostic outcomes. Skeletal Radiol 47, 661–669 (2018). https://doi.org/10.1007/s00256-017-2828-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-017-2828-x