Abstract
Neuropathy of the posterior femoral cutaneous nerve may manifest as pain and paresthesia in the skin over the inferior buttocks, posterior thigh, and popliteal region. Current treatment options include physical and oral pain therapy, perineural injections, and surgical neurectomy. Perineural steroid injections may provide short-term pain relief; however, to our knowledge, there is currently no minimally invasive denervation procedure for sustained pain relief that could serve as an alternative to surgical neurectomy. Percutaneous cryoablation of nerves is a minimally invasive technique that induces a sustained nerve conduction block through temporary freezing of the neural layers. It can result in long-lasting pain relief, but has not been described for the treatment of neuropathy-mediated PFCN pain. We report a technique of MR-guided cryoablation of the posterior femoral cutaneous nerve resulting in successful treatment of PFCN-mediated sitting pain. Cryoablation of the posterior femoral cutaneous nerve seems a promising, minimally invasive treatment option that deserves further investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
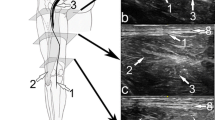
The posterior femoral cutaneous nerve (PFCN) is a purely sensory nerve of the sacral plexus, which travels in close proximity with the sciatic nerve in the subgluteal space [1]. More inferiorly, the thin cutaneous branch (CB) of the PFCN diverges and courses between the gluteus maximus and the hamstring origin to pierce the posterior crural fascia and descend to the popliteal area (Fig. 1). Neuropathy of the CBPFCN typically manifests as pain and paresthesia of the skin over the inferior buttocks, posterior thigh, and popliteal region. It may be diagnosed through the physical findings of pain and altered sensations in the innervated skin territories and relief of symptoms through a selective block with local anesthesia [2].
Current treatment options include physical therapy, oral pain therapy, perineural injections [2], and surgical neurectomy [3]. Perineural steroid injections may provide short-term pain relief; however, there is to our knowledge currently no minimally-invasive denervation procedure for sustained pain relief that could serve as an alternative to surgical neurectomy. Cryoablation is a minimally invasive procedure that consists of the temporary freezing of tissues through the creation of an ice ball at the tip of a probe. Cryoablation of nerves results in the destruction of various layers of neural tissues and sustained conduction blockage of pain signals. Cryoablation of various nerves in the body has been successfully applied for sustained pain relief [4], but has not been described for the treatment of neuropathy-mediated CBPFCN pain.
Successful cryoablation requires accurate visualization and targeting of the nerve in addition to monitoring of the ice ball. Interventional magnetic resonance (MR) imaging appears well suited for this technique because of its ability to visualize the less than 1-mm CBPFCN and monitor the cryoablation ice ball [5–7].
We report a case of MR-guided cryoablation of the PFCN resulting in the successful treatment of chronic neuropathy-mediated sitting pain.
Case report
A 66-year-old active woman presented to our service with intractable pain in the skin over the lower buttocks and posterior thigh regions. Her symptoms occurred after right pudendal nerve surgery, which had provided pain relief in the perineum. She was otherwise healthy, exercised regularly, and lived a healthy life-style. Her pain was continuous on a daily basis, but with varying severity ranging between 3 and 7 on a 10-point visual analog scale. The pain was aggravated by sitting and exercise and alleviated by rest and lying flat on the stomach. She had already undergone physical therapy and oral drug treatment. Her physical examination demonstrated tenderness over the right inferomedial buttocks area, posterior thigh, and upper popliteal region. The skin and muscle bulk were normal. She underwent 3-Tesla MR neurography of the lumbosacral plexus, which demonstrated a healed, previously divided right sacrotuberous ligament with extension of small fibrous strands to the subjacent pudendal nerve, but no frank scar encasement. The right pudendal nerve was normal in size and signal intensity. The CBPFCN demonstrated mild enlargement and increased signal intensity compared with the left side, suggestive of right neuropathy (Fig. 2). Differential MR-guided diagnostic blocks of the CBPFCN and pudendal nerves with long-acting local anesthetic resulted in complete temporary pain relief with concordant pre-procedural pain and post-procedural numbness maps only after the CBPFCN block and no substantial pain relief after the pudendal nerve block. Two subsequent therapeutic blocks of the PFCN with long-acting local anesthetic and long-acting steroids each provided pain relief for 1 month, with recurrence of symptoms afterward. The patient underwent cryoablation for longer lasting analgesic therapy of PFCN-mediated pain.
Diagnostic MR neurography. a Axial high spatial resolution intermediate-weighted and b fat-suppressed T2-weighted MR images show the cutaneous branch of the posterior femoral cutaneous nerve (white arrows), which demonstrates abnormal signal hyperintensity (white arrow, b). The black arrows indicate the normal sciatic nerve
The procedure was performed using a clinical, wide-bore 1.5-T MRI system (MAGNETOM Espree, Siemens Healthcare, Erlangen, Germany) in prone position. Axial intermediate-weighted MRI (turbo spin echo; repetition time [TR], 6,500; echo time [TE], 24; slice thickness [SL], 4 mm; echo train length [ETL], 11; field of view [FOV], 348 x 300 mm; base resolution [BR], 448; phase resolution [PR], 70%; receiver bandwidth [BW], 200 Hz, acquisition time [TA], 3:51 min) were used to identify the cutaneous branch of the PFCN using a multi-channel body surface coil and table element coils in parallel (Fig. 3a). The body surface coil was then exchanged with a 19-cm loop coil. Following determination of the skin entry point using skin markers, the interventional field was prepped and draped in standard fashion. Conscious sedation was initiated. Superficial local anesthesia was employed using 1% lidocaine. The procedure was performed in a co-axial fashion using an 18-G introducer needle of 5 cm in length, which served as an access sheath. First, a nerve block was performed using a 22-G needle of 10 cm in length (MReye®, G11583, Cook Medical, Bloomington, IN, USA), which was displayed on axial intermediate-weighted MR images (turbo spin echo; TR, 2,480; TE, 23; SL, 3 mm; ETL, 17; FOV, 338 x 300 mm; BR, 384; PR, 70%; BW, 356 Hz; TA, 28 s) (Fig. 3b). Then, the injection needle was replaced with a cryoablation probe (IceSeed 1.5 MRI Needle, Galil Medical, St Paul, MN, USA), which was placed and visualized using the same technique and pulse sequence as for the injection needle. Following verification of contact of the cryoablation needle with the CBPFCN and sufficient distance from the sciatic nerve, cryoablation was performed with two 3-min freeze/3-min thaw cycles. The ice ball growth was monitored using continuous MRI acquisition at a frame rate of 21 s using axial intermediate-weighted MRI (turbo spin echo; TR, 1,800; TE, 23; SL, 3 mm; ETL, 17; FOV, 338 x 300 mm; BR, 384; PR, 70%; BW, 356 Hz; Fig. 3c). The distance between the outer margin of the ice ball and the sciatic nerve was monitored visually and with manual measurements on the continuously acquired MRI. The total procedure time from first to last image was 81 min. There were no complications and the patient tolerated the procedure very well. The MRI workflow is shown in Table 1.
Interventional MRI. a Axial intermediate-weighted MRI shows the cutaneous branch of the posterior femoral cutaneous nerve (white arrow) between the proximal hamstring muscles medially and the gluteus maximus muscle laterally. The black arrow shows the sciatic nerve. b Axial intermediate-weighted MRI shows a 22-G injection needle (white arrow) near the cutaneous branch of the posterior femoral cutaneous nerve, which was used to perform a nerve block before the cryoablation. c Axial intermediate-weighted MRI shows the cryoablation probe with the ice ball (white arrow) ablating the cutaneous branch of the posterior femoral cutaneous nerve. There is a 5-mm margin to the sciatic nerve (black arrow), which was sufficient to protect the sciatic nerve from thermal injury
Following cryoablation of the PFCN, the patient had substantial pain relief with only minimal symptoms remaining. Following a week, she developed paresthesia on the skin innervated by the CBPFCN, which gradually improved over time with oral anti-inflammatory pain medication. At the 5-month follow-up, she remains free of her posterior thigh symptoms.
Discussion
We report the technique and short-term result of successful MR-guided cryoablation of the CBPFCN for the treatment of chronic neuropathy-mediated sitting pain.
Nerve ablation is an evolving therapeutic option for the treatment of neuropathic pain. Techniques that have been described include radiofrequency ablation [8, 9] and cryoablation [4]. The mechanism of action of cryoablation of the nerves is not fully understood. It involves disruption of both axons and myelin sheath with subsequent Wallerian degeneration, but may leave layers such as the peri- and endoneurium structurally intact [10]. This results in a neurotmesis-type state and long-lasting interruption of the nerves’ ability to conduct pain [10–12]. Owing to less structural damage than with chemodenervation, heat-generating thermal ablation, and surgical nerve division, the formation of neuromas is less likely with cryoablation [13, 14]. Because of the remaining intact neural layers, the nerve may regenerate and symptoms may recur after months of analgesia.
The current treatment for PFCN-mediated neuralgia consists of perineural injections with long-acting local anesthetics and steroids [2], and surgical resection [3]. In our patients, perineural steroid injections only provided short-term pain relief. Cryoablation could represent an alternative to surgical treatment and therapeutic option in patients with incomplete pain relief or recurring pain after surgery. A patient study is needed to fully assess the role of cryoablation for the treatment of PFCN-mediated pain.
Owing to the small size of the CBPFCN, cryoablation is best performed under direct imaging visualization and monitoring of the ice ball during the ablation process. For this purpose, MR guidance can be advantageous for several reasons. The high spatial and soft-tissue resolution of MRI permits the direct visualization and targeting of the CBPFCN, and its distance the sciatic nerve to ensure a sufficient ablation margin to avoid sciatic injury. In addition, MRI monitoring directly visualizes the cryoablation zone as an area of signal void that resembles the ice ball. This allows for verification that the ice ball reaches the CBPFCN and also that a safe distance to the sciatic nerve remains. In our case, a 5-mm distance between the outer margin of the ice ball and sciatic nerve was sufficient to avoid thermal injury. Ultrasound-guided CBPFCN blocks have been reported and may represent an alternative technique [15], if monitoring of the relationships of ice ball, PFCN, and sciatic nerve can be ensured. Similarly, the procedure may be possible under computed tomography guidance using anatomical landmarks.
Our patient had diminishing skin discomfort after the procedure in the distribution of the ablated CBPFCN, which resolved over the course of a few weeks. The symptoms seemed similar to a not uncommonly reported side effect of nerve ablation [16], which is not well understood, but may constitute a healing response during Wallerian degeneration.
In conclusion, MRI guidance enables cryoablation of the CBPFCN, which seems a promising, minimally invasive technique for the treatment of neuropathy-mediated PFCN pain.
References
Tubbs RS, Miller J, Loukas M, Shoja MM, Shokouhi G, Cohen-Gadol AA. Surgical and anatomical landmarks for the perineal branch of the posterior femoral cutaneous nerve: implications in perineal pain syndromes. laboratory investigation. J Neurosurg. 2009;111(2):332–5.
Fritz J, Bizzell C, Kathuria S, Flammang AJ, Williams EH, Belzberg AJ, et al. High-resolution magnetic resonance-guided posterior femoral cutaneous nerve blocks. Skeletal Radiol. 2013;42(4):579–86.
Dellon AL. Pain with sitting related to injury of the posterior femoral cutaneous nerve. Microsurgery. 2015;35(6):463–8.
Trescot AM. Cryoanalgesia in interventional pain management. Pain Physician. 2003;6(3):345–60.
Filler AG, Haynes J, Jordan SE, Prager J, Villablanca JP, Farahani K, et al. Sciatica of nondisc origin and piriformis syndrome: diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome study of resulting treatment. J Neurosurg Spine. 2005;2(2):99–115.
Fritz J, Chhabra A, Wang KC, Carrino JA. Magnetic resonance neurography-guided nerve blocks for the diagnosis and treatment of chronic pelvic pain syndrome. Neuroimaging Clin N Am. 2014;24(1):211–34.
Fritz J, Dellon AL, Williams EH, Belzberg AJ, Carrino JA. 3-Tesla high-field magnetic resonance neurography for guiding nerve blocks and its role in pain management. Magn Reson Imaging Clin N Am. 2015;23(4):533–45.
Chua NH, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications-a review. Acta Neurochir (Wien). 2011;153(4):763–71.
Diederich CJ. Thermal ablation and high-temperature thermal therapy: overview of technology and clinical implementation. Int J Hyperthermia. 2005;21(8):745–53.
Savastano LE, Laurito SR, Fitt MR, Rasmussen JA, Gonzalez Polo V, Patterson SI. Sciatic nerve injury: a simple and subtle model for investigating many aspects of nervous system damage and recovery. J Neurosci Methods. 2014;227:166–80.
Alaqeel A, Alshomer F. High resolution ultrasound in the evaluation and management of traumatic peripheral nerve injuries: review of the literature. Oman Med J. 2014;29(5):314–9.
Rosen A, Tardast A, Shi TJ. How far have we come in the field of nerve regeneration after trigeminal nerve injury? Curr Oral Health Rep. 2016;3(4):309–13.
Campos NA, Chiles JH, Plunkett AR. Ultrasound-guided cryoablation of genitofemoral nerve for chronic inguinal pain. Pain Physician. 2009;12(6):997–1000.
Koethe Y, Mannes AJ, Wood BJ. Image-guided nerve cryoablation for post-thoracotomy pain syndrome. Cardiovasc Intervent Radiol. 2014;37(3):843–6.
Byas-Smith MG, Gulati A. Ultrasound-guided intercostal nerve cryoablation. Anesth Analg. 2006;103(4):1033–5.
Stolzenberg D, Gordin V, Vorobeychik Y. Incidence of neuropathic pain after cooled radiofrequency ablation of sacral lateral branch nerves. Pain Med. 2014;15(11):1857–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The data of this case report were obtained under an internal review board-approved protocol for prospective data collection and with informed consent.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Joshi, D.H., Thawait, G.K., Del Grande, F. et al. MRI-guided cryoablation of the posterior femoral cutaneous nerve for the treatment of neuropathy-mediated sitting pain. Skeletal Radiol 46, 983–987 (2017). https://doi.org/10.1007/s00256-017-2617-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-017-2617-6