Abstract
Purpose
Intravascular papillary endothelial hyperplasia (IPEH) is a soft tissue, tumor-like, benign, reactive, vascular proliferation that, although not rare, is uncommonly imaged. We report the imaging findings of intravascular papillary endothelial hyperplasia in 13 patients, highlighting characteristic imaging features.
Materials and methods
We retrospectively reviewed 13 patients with IPEH who had corresponding MR and/or ultrasound imaging. MR imaging studies were evaluated for lesion location, shape, size, signal intensity, signal heterogeneity, and enhancement. Ultrasound studies were assessed for lesion shape, size, echogenicity, heterogeneity, and vascularity. Demographic data, including patient age, gender, and clinical history were also reviewed.
Results
Most patients (11 of 13) presented with an enlarging mass. The age range was 10–72 years (mean 46) with ten females and three males. Eleven of the 13 lesions were primary IPEH without an associated preexisting vascular lesion. Ten of 13 lesions were in the superficial soft tissues, all of which were primary IPEH. Two of the three lesions in the deep tissues were secondary IPEH, arising within a preexisting vascular lesion. Lesions were small (mean 1.4 cm) and had a rounded shape. All of the primary lesions demonstrated high T2 signal peripherally and variable T2 signal centrally, with most demonstrating superficial location (91 %), peripheral enhancement (89 %) and associated dominant vessel (73 %). The five lesions evaluated by ultrasound were all hypoechoic with either scattered or peripheral vascularity on Doppler.
Conclusions
Primary papillary endothelial hyperplasia is commonly seen in the superficial soft tissues when captured on imaging and has a characteristic imaging appearance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intravascular papillary endothelial hyperplasia (IPEH) is a benign, reactive, vascular proliferation of endothelial cells occurring within the vascular lumen associated with organized thrombus, which may be best described as “an exuberant form of organizing thrombus” [1]. Why some thrombi undergo this form or organization is unknown, although, this process may occur in any vessel within the body [1]. Also known as Masson’s tumor, IPEH was originally described by Masson in 1923 as a vegetant intravascular hemangioendothelioma [2]. The designation IPEH was introduced by Clearkin and Enzinger in 1976, following their review of 44 cases from the archives of the Armed Forces Institute of Pathology [3]. IPEH is generally the preferred designation as it is a more accurate description of the histological features [3, 4]. Although IPEH is not rare, accounting for up to 3.7 % of the vascular lesions present in the soft tissues [4], it is rarely imaged due to its small size and superficial location. In a pathology database search at our own institution, the vast majority of the pathologically proven IPEH cases were resected as superficial skin lesions by dermatology, without any imaging.
In addition to the classic or pure form, IPEH may occur in a dilated vessel within a pre-existing vascular lesion [1]; with these two forms designated primary and secondary IPEH respectively [4]. Primary IPEH usually occurs in the superficial soft tissues of the digits or head and neck, arising in dilated vascular spaces [3, 4]. Secondary IPEH, also termed the mixed form, is diagnosed when the lesion develops within a pre-existing vascular lesion, usually within a hemangioma or vascular malformation [1]. Simple excision is typically curative for pure lesions while recurrences are generally associated with secondary cases, in which IPEH is superimposed on other vascular lesions [1, 4].
Accurate characterization of IPEH is important in clinical practice since its imaging findings may share features with other, more common lesions, such as a tenosynovial giant cell tumor or neurogenic tumor. More importantly, its histology can be confused with that of angiosarcoma [1]. Although the imaging findings in IPEH have been previously described in case reports and small case series, we report a series of 13 patients, highlighting the characteristic imaging appearance of the primary form of this uncommonly imaged lesion.
Methods
This investigational protocol was conducted with the approval of the Institutional Review Boards of the contributing institutions and in accordance with the requirements of a retrospective review as well as HIPAA; informed consent was not required.
A retrospective review of the pathology database from the authors’ institutions was performed to identify all patients having a pathologically proven diagnosis of IPEH from January 1, 1990 through June 8, 2015. A total of 48 cases were identified. This list was reviewed and 13 (27 %) patients with MR and/or ultrasound imaging formed the initial study group. Two cases of secondary IPEH were excluded because they were incidentally found by surgical pathology in previously radiated scar tissue. Both of these lesions had imaging available, but the location of the IPEH could not be identified within the scar tissue on MR imaging. Two additional lesions were excluded upon a second review by pathology because they demonstrated insufficient pathologic evidence for definitive diagnosis. The initial nine cases were supplemented by four additional pathologically proven cases from one author’s institutional teaching database.
MR imaging studies were evaluated for lesion location, shape, size, signal intensity, signal heterogeneity and enhancement, and the presence or absence of a dominant associated vein. MR imaging parameters and magnet strength varied, but MR imaging generally included multiplanar sequences with T1-weighted, fluid-sensitive and/or STIR imaging, and fat-suppressed contrast enhanced T1-weighted images. Ultrasound studies were assessed for lesion location, shape, size, echogenicity, heterogeneity, and Doppler vascularity. Location was characterized as superficial or deep, relative to deep fascia overlying muscle. Size was reported as the longest axis in any dimension. MR signal intensity was evaluated relative to that of muscle, fat, and fluid. The lesions were defined as heterogeneous if they had any variation of signal internally beyond the expected uniformity seen in a cyst. Heterogeneity was further categorized subjectively as mild, moderate, or marked. All imaging features were by consensus interpretation of two MSK radiologists. On ultrasound, echogenicity was determined relative to that of adjacent fat and muscle. Demographic data including patient age, gender, and clinical presentation were also reviewed and summarized, as was the presence or absence of recurrence following resection.
Results
We identified a total of 13 patients for review, 11 of which had MR imaging and five of which had ultrasound evaluation. Ten of the 11 cases evaluated by MR imaging included post-contrast assessment. Three of five of the ultrasound cases included Doppler evaluation.
The average patient age was 46 years with a range of 10–72 years. The study group included ten females and three males. Two of the 13 cases were recurrent lesions following resection. Eleven of the lesions presented as a palpable mass, three of which were noted as painful. One lesion was incidentally discovered. History was unavailable in the remaining patient.
The lesions ranged in size from 0.5 to 3.5 cm (mean, 1.4). All of the lesions had an ovoid, rounded, or lobulated shape. Eleven of the lesions were primary IPEH and two of the lesions were secondary IPEH. Patients with primary lesions were older, having a mean age of 51 years (range, 15–72 years), in comparison to those with secondary lesions, who had a mean age of 16 years (range, 10–22 years). Primary IPEH had a predilection for the superficial soft tissues with ten (91 %) of 11 lesions located superficially. Of the 11 primary lesions, seven (64 %) were located in the digits (5) or wrist (2) (Figs. 1 and 2). Single lesions were noted in the forearm (Fig. 3), arm, and breast. One occurred (9 %) in the deep soft tissues of the thigh (Fig. 4). This lesion was intermuscular and just deep to the fascia. In addition, a prominent or dilated vein was identified in eight (73 %) of 11 primary lesions (Figs. 2 and 3). A breakdown of size and location based on IPEH type is seen in Table 1.
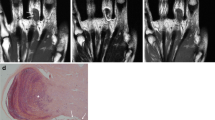
Primary IPEH in the subcutaneous tissue of the thumb. a, b AP radiograph (a) and corresponding clinical photograph (b) show a non-mineralized mass along the radial aspect of the thumb. c Sagittal fat-suppressed T2-weighted SE MR image of the thumb shows a well-defined lobulated lesion with intense peripheral increased T2 signal. The high signal extends centrally but the central region shows predominantly low to intermediate signal intensity. d Corresponding T1-weighted SE MR image shows the mass to be moderately heterogeneous with intermediate signal intensity, with areas of somewhat increased (black asterisks) and decreased (white asterisk) signal intensity. e Similar fat-suppressed T1 image following contrast shows the enhancing vascular proliferation with predominantly peripheral enhancement (black arrows), with central extension (white arrow). A large amount of the lesion shows no significant enhancement (asterisks). f Intraoperative image shows the nodular contour of the lesion (arrows) as well as an area of prominent thrombus (asterisk)
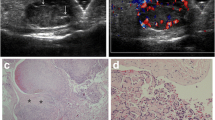
Primary (pure) IPEH in the subcutaneous tissue of the wrist in a 45-year-old woman. a Lateral radiograph shows an ovoid mass along the dorsal aspect of the wrist (arrow). b Clinical photograph at the time of surgery shows the dorsal mass (purple dots). c, d Corresponding T1-weighted SE MR images without (c) and with (d) fat-saturation shows the mass overlying the fourth dorsal compartment with central thrombus (asterisks) having increased signal indicative of subacute blood. The thrombus is more conspicuous on fat-suppressed image (d). e Fat-suppressed T2-weighted image shows a heterogeneous appearance with a peripheral lobulated high signal intensity (arrows) and central low-to-intermediate signal (asterisk). f Fat-suppressed enhanced T1-weighted image shows prominent peripheral enhancement (arrows). The central lower signal (asterisk) corresponds to previously identified thrombus. g, h Transverse conventional (g) and (h) Doppler ultrasound images show an ovoid hypoechoic mass with prominent peripheral vascularity. i, j Intraoperative image (i) following exposure of the purple-red multicystic mass (asterisk) containing clotted blood and the dominant associated superficial vessel (arrow). Photograph of the resected tumor (j) shows these features to better advantage. k Low-power photomicrograph shows well-circumscribed organizing thrombus with central papillary endothelial hyperplasia within a dilated vessel. l Higher magnification of the papillae within the central papillary endothelial hyperplasia illustrates hyalinized fibrin cores (asterisks) surrounded by a single layer of endothelium (arrows)
Similar findings in primary subcutaneous forearm IPEH in a 66-year-old woman. a Fat-suppressed T2-weighted image shows a heterogeneous appearance with a peripheral high signal intensity (arrow) and central low-to-intermediate signal (asterisk). b Fat-suppressed enhanced T1-weighted image shows prominent peripheral enhancement (arrow) with non-enhancing central thrombus (asterisk) in a dilated superficial vein. c, d Axial T1-weighted images proximal to the lesion show the normal size superficial vein (c, arrow), and its dilatation at the margin of the lesion (d, arrow)
Primary IPEH in the deep soft tissue (deep to the deep fascia) of the thigh in a 38-year-old woman. a, b Axial T1 (a) and fat-suppressed T1-weighted (b) SE MR images show a small deep lesion in the lateral aspect of the left thigh showing slightly increased signal intensity as compared to the adjacent skeletal muscle. c Corresponding axial fat-suppressed enhanced T1-weighted image shows peripheral (arrow) and central (asterisk) enhancement around and within the associated thrombus. d Non-fat-suppressed T2-weighted image prior to contrast shows heterogeneous intermediate signal within the mass
When primary IPEH was subdivided by location, the lesions in the digits had similar imaging characteristics (Table 2). The digit lesions all demonstrated peripheral intermediate T1 signal and peripheral T2 hyperintensity, with varying degrees of central nodular heterogeneity. Contrast-enhanced imaging in the digits also demonstrated peripheral enhancement demonstrating varying degrees of central enhancement. One out of the five of the ultrasound cases were in the digits. This lesion had ultrasound findings complimenting the MRI findings of primary IPEH in the digits. This included a round/ovoid shape, superficial location, with peripheral and varying central vascularity.
Secondary IPEH lesions were larger than primary IPEH lesions (2.2 vs. 1.2 cm) and were seen exclusively in the deep soft tissues of the foot and chest wall. The two cases of secondary IPEH arose in association with a hemangioma and an arteriovenous malformation and were both recurrent. Generalization of imaging findings was difficult due to the small number of cases. As would be expected, the imaging features of the two secondary IPEH lesions mirrored those of the underlying preexisting soft tissue vascular lesion.
Discussion
This study represents the largest report of the imaging findings in IPEH to date. Our imaging findings in the digits, accounting for almost half of the primary lesions, had characteristic imaging features including superficial location, small size (mean = 1.2 cm), ovoid or lobulated shape, with peripheral hyperintense T2 signal and enhancement, as well as more variable central enhancement and signal intensity. These features (Figs. 1 and 2) are similar to those seen in smaller case reviews [5, 6] of digital lesions. More importantly, we note a strikingly similar appearance of primary (pure) IPEH lesions in other extremity locations (Figs. 3 and 4). One could note that IPEH is much more common in the mixed (secondary) form, as this form accounted for 65 % of the 85 classifiable cases that were reported by Hashimota et al. [4]; however, secondary cases comprised only 15 % of the cases in this report.
It is more difficult to infer the imaging characteristics of secondary IPEH from the small number of cases included in this report, but in general, these lesions mimicked the imaging characteristics of the associated vascular lesions. Lee and colleagues [7] reported four mixed IPEH cases associated with hemorrhagic/thrombotic venous hemangiomas, also noting that their imaging features may be determined by the underlying lesion.
The reported MR and US imaging appearance correlate well with the underlying pathological features (Fig. 2). As previously described, the peripheral enhancement likely represents vascular proliferation along the vessel wall. Variable central signal reflects a combination of internal organized thrombus (low T2 and absent enhancement) and intravascular proliferation (high T2 signal and enhancement) [6, 7].
Though we only had a small number of cases, the demographics, clinical presentation, and lesion location were consistent with previously reported larger pathology studies. We found that primary IPEH was most common in the digits and in a superficial location [3, 4, 8]. It was more commonly seen in females, often presenting as a palpable mass [1, 3, 4, 8]. The recurrence rate in our study was 15 %, close to the 10 % reported in the literature [8]. Both recurrences occurred in secondary lesions.
We noted a prominent vessel in association with eight (73 %) of 11 cases of primary IPEH. The association of a dilated vessel and IPEH has been well established pathologically, with Hashimoto et al. defining the pure form as occurring “within dilated vascular spaces” [1, 4, 9]. Earlier reports had noted this as well, noting the occurrence of IPEH was not uncommonly seen in association with hemorrhoids [10]. Although the association of primary IPEH and dilated blood vessels on imaging studies has not been emphasized, Clifford et al. [11] reported a case of a deep primary lesion in the triceps muscle noting the gross specimen consisting of a “segment of dilated blood vessel and adjacent skeletal muscle.” This association was well demonstrated in this series (Figs. 2 and 3).
Extravascular papillary endothelial hyperplasia has been previously reported, but is quite rare. In a 1993 review of the literature, Pin et al. [12] reported a case extensively involving a large, traumatic, deep soft-tissue hematoma, identifying 13 (4 %) previously reported examples among 314 cases of papillary endothelial hyperplasia reported in the literature. We have not addressed extravascular papillary endothelial hyperplasia in this report.
As with any retrospective study, there were limitations to this study. MRI parameters varied widely due to the referral nature of the institutions involved and a wide date range of presentation. Additionally, some of the descriptors used to characterize the lesions were subjective.
Although uncommonly imaged, primary IPEH should be considered in a differential for a nodular, superficial, soft tissue mass. Primary IPEH is most common in the finger. In this anatomic location, especially when near a tendinous structure, it may suggest the diagnosis of tenosynovial giant cell tumor or fibroma of tendon sheath. The presence of intralesional thrombus, especially when long standing, may add to this confusion. Small superficial subcutaneous nodular lesions outsides the digits or hand may suggest a cutaneous neuroma or focal hematoma with thrombus.
The diagnosis of primary IPEH should be suggested when the lesion shows high peripheral and variable central signal on fluid-sensitive sequences and predominantly peripheral enhancement, especially when associated with a dominant or dilated vein. Ultrasound typically shows a hypoechoic lesion with either scattered or peripheral Doppler vascularity. The mixed form of IPEH should be considered when a vascular lesion such as a hemangioma or vascular malformation is identified with associated hemorrhage/thrombus. Inclusion of this benign entity in the differential diagnosis will assist the pathologists to arrive at the final diagnosis, as the histology of IPEH can mimic angiosarcoma.
References
Weiss SW, Goldblum JR. Enzinger & Weiss’s soft tissue tumors. Philadelphia: Mosby; 2008. p. 633–79.
Masson P. Hemangioendotheliome vegetant intra-vasculaire. Bull Soc Anat Paris. 1926;93:517–23.
Clearkin KP, Enzinger FM. Intravascular papillary endothelial hyperplasia. Arch Pathol Lab Med. 1976;100(8):441–4.
Hashimoto H, Daimaru Y, Enjoji M. Intravascular papillary endothelial hyperplasia. a clinicopathologic study of 91 cases. Am J Dermatopathol. 1983;5(6):539–46.
Sartore L, Voltan A, Tomat V, Bassetto F, Salmaso R. Masson’s disease in hand surgery: a clinicopathologic study of four cases. J Hand Surg Eur Vol. 2011;36(8):694–7.
Kitagawa Y, Tamai K, Kim Y, Hayashi M, Makino A, Takai S. Intravascular papillary endothelial hyperplasia of the digit: MRI features with histological correlation. J Hand Surg Eur Vol. 2013;38(3):306–12.
Lee SJ, Choo HJ, Park JS, Park YM, Eun CK, Hong SH, et al. Imaging findings of intravascular papillary endothelial hyperplasia presenting in extremities: correlation with pathological findings. Skeletal Radiol. 2010;39(8):783–9.
Elder DE, Elenitsas R, Bernett L, Johnson J, Murphy GF, Xu X. Lever’s histopathology of the skin. Philadelphia: Lippincott Williams & Wilkins; 2008.
Kuo T, Sayers CP, Rosai J. Masson’s “vegetant intravascular hemangioendothelioma:” a lesion often mistaken for angiosarcoma: study of seventeen cases located in the skin and soft tissues. Cancer. 1976;38(3):1227–36.
Kauffman SL, Stout AP. Malignant hemangioendothelioma in infants and children. Cancer. 1961;14:1186–96.
Clifford PD, Temple HT, Jorda M, Marecos E. Intravascular papillary endothelial hyperplasia (Masson’s tumor) presenting as a triceps mass. Skeletal Radiol. 2004;33(7):421–5.
Pins MR, Rosenthal DI, Springfield DS, Rosenberg AE. Florid extravascular papillary endothelial hyperplasia (Masson’s pseudoangiosarcoma) presenting as a soft-tissue sarcoma. Arch Pathol Lab Med. 1993;117(3):259–63.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no disclosures and have not received any grants or assistance. This investigational protocol was conducted with the approval of the Institutional Review Board and in accordance with the requirements of a retrospective review as well as HIPAA; informed consent was not required.
Rights and permissions
About this article
Cite this article
Craig, K.A., Escobar, E., Inwards, C.Y. et al. Imaging characteristics of intravascular papillary endothelial hyperplasia. Skeletal Radiol 45, 1467–1472 (2016). https://doi.org/10.1007/s00256-016-2445-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-016-2445-0